Manuscript accepted on :27-01-2023
Published online on: 26-07-2023
Plagiarism Check: Yes
Reviewed by: Dr. Mahdy Assafi
Second Review by: Dr. Sinai W. Mohammed
Final Approval by: Dr. H Fai Poon
Saloni , Sarika Suresh
, Sarika Suresh and Ramya Premanath*
and Ramya Premanath*
Nitte (Deemed to be University), Nitte University Centre for Science Education and Research, Paneer Campus, Deralakatte, Mangaluru, Karnataka, India
Corresponding Author E-mail: ramya@nitte.edu.in
DOI : https://dx.doi.org/10.13005/bpj/2750
Abstract
The rapid increase in drug resistance in nosocomial pathogens has warranted the search for novel treatment strategies. Targeting quorum sensing (QS) found in bacteria is opined as an alluring method as many pathogenic bacteria employ QS to modulate their virulence. Plants with an enchanting repertoire of phytochemicals can serve as the source for anti-QS compounds. Ferns, the vascular plants have been reported to be used for treating various ailments in traditional systems of medicine. Although some studies have described the antibacterial activity of ferns, to the best of our knowledge there are no investigations carried out to explore their anti-QS potential. Against this background, the current investigation aimed at identifying the anti-QS activity of ferns in inhibiting biofilm formation in selected nosocomial pathogens. Of the several ferns tested, hexane extract of Psilotumnudum leaf and ethanol extract of Cheilanthestenuifolia leaf exhibited significant anti-biofilm activity against S. aureus and E. coli respectively. There was a marked reduction in biofilm formation of approximately 80%. The presence of anti-QS compounds in these ferns paves way for further research to isolate and identify them.
Keywords
Anti-quorum sensing activity; Biofilm; Ferns; Nosocomial pathogens
Download this article as:| Copy the following to cite this article: Saloni S, Suresh S, Premanath P. Assessment of Anti-biofilm Activity of Ferns Against Nosocomial Pathogenic Bacteria. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Saloni S, Suresh S, Premanath P. Assessment of Anti-biofilm Activity of Ferns Against Nosocomial Pathogenic Bacteria. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/43CSLSB |
Introduction
Nosocomial bacterial infections are a serious health concern around the world, leading to increased morbidity, mortality, treatment costs, and hospital admissions. These nosocomial pathogens are well known to have acquired resistance to a large number of antibiotics in current use. The increase of multidrug resistant strains has necessitated the development of novel treatment strategies. Quorum sensing (QS) inhibition is considered one of the attractive targets as many pathogenic bacteria employ QS to modulate their pathogenicity1. Bacterial QS is a mechanism that relies on the release and uptake of small signaling molecules called auto-inducers (AI), the production of which is dependent on the density of the bacterial population in the surrounding medium2.The produced AIs play a major role in regulating the gene expression, which in turn controls various bacterial responses such as bio-film formation, production of virulence factors, motility, the establishment of genetic competence, and fluorescence3. With the use of QS inhibitors antimicrobial resistance, persistence, and virulence could be addressed in pathogenic bacteria. The major advantage of using the QS inhibitors is that they do not kill bacteria but rather control bacterial virulence factor production without encouraging the development of bacterial resistance4.
Synthetic QS inhibitors have been shown to successfully suppress QS in bacteria. However, due to the presence of certain compounds, they are considered to be hazardous to human health and have found limited application5.Because of their high reactivity and instability, the majority of the synthetic anti-QS compounds have yet to be qualified as chemotherapeutic agents5. Considering this, there is a compelling need for discovering novel anti-QS compounds with low toxicity and high reactivity. Natural sources are being investigated for potential therapeutic and antipathogenic compounds that could operate as non-toxic QS inhibitors, allowing infections to be controlled without the development of bacterial resistance. Plants are appraised to possess a wide range of phytochemicals exhibiting various kinds of activities against microorganisms. Ferns, a group of vascular plants found in abundance in the tropics have been used in Ayurveda and other traditional medicine to treat different ailments6. Although a few researchers have worked on the antimicrobial activity of these ferns, studies on QS modulators are lacking. Considering this, the current study intends to identify ferns that have anti-QS efficacy against a few nosocomial bacterial pathogens.
Methods
Ferns used and preparation of crude extracts
Fresh plant material from eight different ferns was collected from Mangaluru and neighbouring districts, situated in South Karnataka. The ferns were washed with distilled water and dried at 40ºC for 48-72 h in a hot air oven, pulverized, and subjected to extraction. The list of ferns used for the current study is provided in Table 1.
Table 1: List of ferns used in the study.
|
Sl. No. |
Common name |
Scientific name |
|
1. |
Black maidenhair |
Adiantumphilipense |
|
2. |
Whisk fern |
Psilotumnudum |
|
3. |
Oak leaf fern (leaf and rhizome) |
Drynariaquercifolia |
|
4. |
Sheevothi |
Sellaginelladelicatula |
|
5. |
Rock fern |
Cheilanthestenuifolia |
|
6. |
Lady fern |
Athyriumhohenackerianum |
|
7. |
Starry spike moss |
Sellaginellawildtype |
|
8. |
Dixie silverback fern |
Pityrogrammacalomelanos |
Sequential extraction of pulverized fern material was performed according to a previously described method by Dharajiyaet al. (2017)7 with minor modifications. To 100 ml of hexane, 10g of the pulverized material was mixed and incubated in a shaker for 24 h at 37ºC. Filtration of the obtained extract was done using a clean muslin cloth. Second and third extractions were carried out using an additional 100ml of solvent. A rotary flash evaporator was used to concentrate the extracts under reduced pressure. The plant material used for the extraction was allowed to dry and once completely dried was further used for extraction with other solvents such as acetone, ethanol, and water. The extracts thus obtained were weighed and stored at 4ºC until further use.
Bacterial strains
Chromobacteriumviolaceum MTCC 2656, ChromobacteriumviolaceumMCC 2216 Acinetobacter baumannii ATCC 19606, Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 700603, Staphylococcus aureus ATCC 25923, and Pseudomonas aeruginosa ATCC 15692. All the ATCC strains were procured from Himedia, the authorized distributors of ATCC products and C. violaceum cultures were purchased from MTCC, Chandigarh.
Assessment of antibacterial activity
The antibacterial activity of different solvent extracts was assessed against the test bacteria by the disc diffusion method as per the method of Alva et al. (2019)8.1ml of DMSO was used for dissolving 50mg of the dried extracts. Approximately 1×106 CFU/ml cultures of A. baumannii, E. coli, K. pneumoniae, S. aureus, and P. aeruginosa were used to lawn nutrient agar plates. The plates were allowed to dry and sterile discs impregnated with 50μl of extract were placed. 50μl of DMSO served as the negative control and ciprofloxacin at a concentration of 50μg/disc served as the drug control. The plates were incubated for 24 h at 37ºC. The zone of inhibition around the sample extract in comparison to the control was considered to be positive.
Determination of minimum inhibitory concentration (MIC)
Various concentrations of extracts ranging from 0.25mg/mL – 40mg/mL in DMSO were freshly prepared. Approximately 1×106 CFU of 24h culture of A. baumannii, E. coli, K. pneumoniae, S. aureus, P. aeruginosa, and C. violaceum were used to lawn the agar plates onto which discs impregnated with different concentrations of the extracts were positioned. 50μl of the solvent served as the negative control. The plates were incubated overnight and a clear zone of inhibition around the sample extract at different concentrations was noted down. The MIC values were ascertained to the least concentration where there was no visible growth.
Determination of anti-QS activity using C. violaceumMTCC2656
The method of Alva et al. (2019)8 was used to determine the anti-QS activity of essential oils.Sterile discs were soaked in different concentrations of the extracts (50 µl) at their sub-MIC levels. Such discs were placed on previously inoculated nutrient agar plates. After overnight incubation, the plates were examined for the pigment inhibition surrounding the disc, and the diameter of the pigment inhibition was measured (mm). C. violaceum MCC 2216 a mutant strain for violacein production was maintained as the negative control.
Biofilm quantification
Biofilm formed by the bacterial strains was quantified by the microtitre plate method according to the method of O’Toole (1998)9 with minor modifications. 1×106CFU/mL of the test bacteria grown in Luria Bertani broth was diluted with fresh medium in the ratio of 1:100. In each well of the 96 well microtitre plate 100μl of the diluted culture was taken and incubated for 24 h at 37°C. After incubation, the plate was inverted, unattached cells were discarded, and adherent cells in the medium were washed thrice with PBS of pH 7.4. The pellet was mixed with 100µl of 0.1% freshly prepared crystal violet and incubated for 10 min. The stain removal was carried out by washing the pellet with PBS three times. For stain solubilization, 125µl of 30% acetic acid was added to the stained and rinsed pellet and incubated for 15 minutes. From each well 100µl was transferred to a fresh plate and optical density was measured at 550 nm in an ELISA reader. The percent reduction in biofilm formation in the presence of plant extracts was measured as (OD of control – OD of treated) / (OD of control) x100
Statistical analyses
The data were subjected to one sample t-test using SPSS, 16.0 software and was considered to be significant at P ≤ 0.05.
Results
Antibacterial activity
Variation in the zones of inhibition was observed against the selected bacterial pathogens. The zones of inhibition were in the range of 8-17mm with different extracts. None of the bacterial strains showed susceptibility to water extracts. The solvent control (DMSO) exhibited a zone of 0.7±0.1 mm. Of the selected pathogens used in the study, S. aureus was found to be more susceptible to extracts of different ferns and K. pneumoniae showed the highest resistance. The highest activity was observed with hexane extract of C. tenuifoliaagainst K. pneumoniae followed by hexane extract of S. delicatulaagainst E. coli. A. hohenackerianum, Selaginellawildtype, P. calomelanos, and D. quercifolia(rhizome) were excluded from the further investigation as they did not exhibit any antibacterial activity. The remaining 5 fern extracts were considered for MIC determination. Table 2 illustrates the zones of inhibition of different fern extracts against the selected pathogenic bacteria.
Table 2: Zones of inhibition (mm) against the selected bacterial pathogens
|
Extracts |
Fern |
E. coli |
A. baumannii |
K. pneumoniae |
S. aureus |
P. aeruginosa |
|
Hexane |
A. philippense |
8±0 |
– |
– |
12.5±0.7 |
11±0.1 |
|
P. nudum |
– |
– |
– |
12.5±0.7 |
10±0 |
|
|
D. quercifolialeaf |
13.5±0.7 |
– |
– |
12±1.4 |
12±0.2 |
|
|
S. delicatula |
16.5±0.7 |
13.5±0.7 |
– |
12±0 |
11±0.4 |
|
|
C. teneifolia |
12.5±0.7 |
8±0 |
17±1.4 |
11±1.4 |
10±0.7 |
|
|
A. hohenackerianum |
– |
– |
– |
– |
– |
|
|
Sellaginella wildtype |
– |
– |
– |
– |
– |
|
|
P. calomelanos |
– |
– |
– |
– |
– |
|
|
D. quercifolia rhizome |
– |
– |
– |
– |
– |
|
|
Acetone |
A. philippense |
11.5±0.7 |
– |
– |
10.5±0.7 |
15.5±2.12 |
|
P. nudum |
10±2.8 |
– |
– |
10.5±2.1 |
14.5±0.7 |
|
|
D. quercifolialeaf |
– |
10.5±0.7 |
– |
11±1.4 |
8.5±0.7 |
|
|
S. delicatula |
– |
9±0 |
– |
11.5±0.7 |
– |
|
|
C. teneifolia |
12.5±0.7 |
9.5±0.7 |
12±1.4 |
11.5±0.7 |
– |
|
|
A. hohenackerianum |
– |
– |
– |
– |
– |
|
|
Sellaginella wildtype |
– |
– |
– |
– |
– |
|
|
P. calomelanos |
– |
– |
– |
– |
– |
|
|
D. quercifolia rhizome |
– |
– |
– |
– |
– |
|
|
Ethanol |
A. philippense |
10±0 |
– |
– |
11±2.8 |
– |
|
P. nudum |
9±1.4 |
9.5±0.7 |
– |
10.5±3.5 |
– |
|
|
D. quercifolialeaf |
– |
9±0 |
– |
10±4.2 |
– |
|
|
S. delicatula |
– |
– |
– |
12±2.8 |
– |
|
|
C. teneifolia |
13±1.4 |
– |
– |
9±1.4 |
– |
|
|
A. hohenackerianum |
– |
– |
– |
– |
– |
|
|
Sellaginella wildtype |
– |
– |
– |
– |
– |
|
|
P. calomelanos |
– |
– |
– |
– |
– |
|
|
D. quercifolia rhizome |
– |
– |
– |
– |
– |
|
|
Drug Control (Ciprofloxacin) |
22±1.4 |
24±1.4 |
27.5±3.5 |
23.5±0.7 |
26±2.8 |
MIC determination
Fernextracts from the initial antibacterial screening that showed activity against selected pathogens were assessed for their MIC values. The concentration of the extracts in the range of 0.25-40 mg/mL was used (Table 3). The least MIC values for the test bacteria ranging from 3.0-10.0 mg/mL and 0.50-2 mg/ml for C. violaceum were considered for further anti-QS activity studies.
Table 3: MIC determination of fern extracts against selected pathogens
|
Extract |
Ferns |
E. coli |
K. pneumoniae |
S. aureus |
A. baumannii |
P. aeruginosa |
C. violaceum |
|
Hexane |
A. philippense |
10mg |
– |
10mg |
– |
20mg |
0.50mg |
|
P. nudum |
– |
– |
10mg |
– |
3mg |
0.75mg |
|
|
D. quercifolia |
10mg |
– |
|
– |
20mg |
0.75mg |
|
|
S. delicatula |
10mg |
– |
20mg |
8mg |
6mg |
0.50mg |
|
|
C. teneifolia |
10mg |
4mg |
10mg |
8mg |
10mg |
1mg |
|
|
Acetone |
A. philippense |
10mg |
– |
10mg |
– |
|
0.50mg |
|
P. nudum |
10mg |
– |
4mg |
– |
20mg |
0.75mg |
|
|
D. quercifolia |
– |
– |
10mg |
10mg |
10mg |
2mg |
|
|
S. delicatula |
– |
– |
30mg |
10mg |
10mg |
0.75mg |
|
|
C. teneifolia |
4mg |
10mg |
10mg |
10mg |
– |
0.75mg |
|
|
Ethanol |
A. philippense |
10mg |
– |
10mg |
– |
– |
0.75mg |
|
P. nudum |
10mg |
– |
10mg |
10mg |
– |
0.75mg |
|
|
D. quercifolia |
|
– |
10mg |
– |
– |
0.75mg |
|
|
S. delicatula |
|
– |
10mg |
– |
– |
0.75mg |
|
|
C. teneifolia |
10mg |
– |
10mg |
– |
– |
2mg |
Determination of anti-QS activity
Fern extracts with concentrations lower than their MIC values were used for assessing the anti-QS activity using C. violaceum. The diameter of the zone of pigment inhibition surrounding the discs was measured. Hexane extract of S. delicatula at a concentration of 0.25mg, acetone extract (0.25mg) of A. philippense, and ethanol extract of C. teneifolia (0.75mg) produced the highest pigment inhibition (Table 4).
Table 4: Anti-QS activity of the extracts against C. violaceum
|
Extract |
Fern |
Concentration (mg/mL) |
Zone of pigment Inhibition (mm) |
|
Hexane |
A. philippense |
0.25 |
10±1.4 |
|
P. nudum |
0.50 |
10 |
|
|
D. quercifolia |
0.50 |
11 |
|
|
S. delicatula |
0.25 |
11.5±2.1 |
|
|
C. teneifolia |
0.75 |
9.5±0.7 |
|
|
Acetone |
A. philippense |
0.25 |
11.5±0.7 |
|
P. nudum |
0.50 |
9±1.4 |
|
|
D. quercifolia |
1.00 |
10 |
|
|
S. delicatula |
0.25 |
9.5±0.7 |
|
|
C. teneifolia |
0.25 |
10 |
|
|
Ethanol |
A. philippense |
0.25 |
9±1.4 |
|
P. nudum |
0.25 |
10.5±2.1 |
|
|
D. quercifolia |
0.50 |
10±1.4 |
|
|
S. delicatula |
0.50 |
10±1.4 |
|
|
C. teneifolia |
0.75 |
11±1.4 |
Biofilm quantification
Although all 5 ferns used for assessing the anti-QS activity against C. violaceum were found to be effective in inhibiting violacein production at their sub-MIC values, only P. nudum and C. teneifolia were selected for evaluating the biofilm inhibition. The wide spectrum of activity of these two ferns against the selected pathogens made us select them for biofilm assay. There was a significant variation in the biofilm formed by bacteria in the presence of fern extracts at their sub-MIC values. The highest biofilm inhibition was found with hexane extract of P. nudum against S. aureus followed by ethanol extract of C. tenuifoliaagainst E. coliat concentrations of 9.75mg/mL. The percent reduction in biofilm formation by the test bacteria in the presence of plant extracts is shown in table 5.
Table 5: Percent reduction in biofilm formation in the presence of plant extracts
|
Organism |
Fern |
Extract |
% reduction |
|
|
3.75mg/mL |
9.75 mg/mL |
|||
|
E. coli |
P. nudum |
Hexane |
– |
33.89 |
|
C. tenuifolia |
Acetone |
27.45 |
– |
|
|
C. tenuifolia |
Ethanol |
– |
80.39 |
|
|
S. aureus |
P. nudum |
Hexane |
– |
82.07 |
|
C. tenuifolia |
Acetone |
– |
37.25 |
|
|
C. tenuifolia |
Ethanol |
– |
75.35 |
|
|
P. aeruginosa |
C. tenuifolia |
Hexane |
– |
79 |
|
K. pneumoniae |
C. tenuifolia |
Hexane |
28.29 |
– |
|
A. baumannii |
C. tenuifolia |
Acetone |
– |
57.61 |
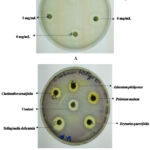 |
Figure 1: Representative images for MIC determination against A. baumannii using acetone extract of Drynaria quercifolia (A) and anti-QS activity against C. violaceum using different extracts (B). |
Discussion
Over the last two decades, drug resistance has tremendously increased and resistant bacteria are considered one of the critical threats to human health. The development of novel approaches to tackle drug resistance is the need of the hour. Inhibition of QS is one such alternative strategy that can be effectively employed for managing illnesses caused by resistant bacteria. According to the World Health Organization, plants are regarded as the best source of antimicrobial compounds10. In India, herbal medicines have long been used to treat a number of ailments. The use of pteridophytes has been mentioned in ancient Indian systems of medicine such as Ayurveda, Unani, and Siddha11. Although very few researchers have worked on its antibacterial activity, to the best of our knowledge, there are no reports on the anti-QS activity of ferns. The lacuna of studies in this area prompted us to undertake this investigation with the aim of screening ferns for their anti-QS activity against multidrug resistant bacteria.
Initially, sequential extraction of the dried powder of ferns with solvents of increasing polarity was carried out. Dried powder of the extracts was used to prevent the interference of water during extraction. Researchers usually employ different solvents for initial extraction as the target compound remains to be unknown and different polarities of solvent help in extracting different classes of phytochemicals12. Similarly in the present study, different solvents varying in their polarities were used for sequentially extracting the anti-QS compounds. The study utilized five strains of bacteria that are reported as the causative agents of serious nosocomial infection13,14. The difference in the antibacterial activity in fern extracts might be ascribed to the phytochemicals found in the ferns and the bacteria tested. It can also be attributed to the polarity of the extractable compounds found in ferns, polarity of solvents used, and propensity of the extracted compound to diffuse in the media used for the assay15. In the current investigation, none of the aqueous extracts showed antibacterial activity indicating that the compounds of interest have a different polarity than water.
The study employed C. violaceum as the biomonitor strain for screening the anti-QS compounds from ferns as demonstrated by the reduction in the violacein production. Earlier reports on medicinal plants have indicated a positive association between antibacterial and anti-QS activity 16,17. Contemplating this hypothesis, the current study selected the ferns with antibacterial activity to assess the anti-QS potential. We could establish a positive correlation between the antibacterial and anti-QS activity as reported in the earlier studies.
Not many studies have been carried out to evaluate the antibacterial and anti-QS activity of ferns against pathogenic bacteria. In a study byAdnan et al. (2020)18, the crude extract of A. philippenseshowed the antibacterial activity against selected strains of E. coli, S. aureus, and P. aeruginosa at concentrations ranging from 31-1000 μg/mL. Although, A. philippensein our investigation did show the antibacterial activity against E. coli, S. aureus, and P. aeruginosa, the concentration at which the activity was seen was higher as compared to the study by Adnan. In the current investigation, none of the D. quercifolia rhizome extracts showed antibacterial activity against the tested pathogens. On the contrary, studies by Irudayaraj and Senthamarai (2004) and Kandhasamyet al. (2008)19,20 observed antibacterial activity of D. quercifolia rhizome against E. coli, P. aeruginosa, S. aureus, and K. pneumoniae. The reason for this difference in the activity could be ascribed to the bacterial strains used and the extraction procedures employed 21.
Microbial biofilms are regarded as one of the important virulence factors of pathogenic bacteria by the virtue of which they resist antimicrobial agents. Such biofilms formed on biotic and abiotic surfaces comprises of microbial assemblages with considerable changes in their gene expression along with the metabolic activity conferring them resistance to antimicrobial treatment22. More importantly, in a number of pathogenic bacteria causing chronic infection, biofilm formation is indicated as an important factor that aids in their survival23. The hexane extract of P. nudum and ethanol extract of C. tenuifolia showed a remarkable reduction in QS activity as indicated by the reduction in biofilm formation at a concentration of less than 10mg/mL in S. aureus and E. coli. Notably, the concentrations used represent that of the crude extracts, and hence, the concentration would be much lower when the presumed anti-QS compound is isolated and purified from these ferns. Although, studies do not exist revealing the biofilm inhibition ability of P. nudum and C. tenuifolia extracts, a study by Adnan et al. (2020)18 has disclosed the anti-biofilm activity of a fern, A. philippense at a much lesser concentration.The anti-biofilm activity of the P. nudum and C. tenuifolia extracts could be attributed to the presence of tannins, phenolics, saponins, flavonoids, and terpenoids either alone or in combinations23. The mechanism of anti-biofilm activity of the extracts can be speculated due to the degradation or modification of the signaling molecules or a decrease in the synthesis of the signaling molecules24.
Conclusion
The quest for alternative therapeutic resources is the resultant effect of drug resistance in pathogenic bacteria. The use of QS inhibitors could prove to be an effective and appealing antipathogenic therapy to combat the emergence of antibiotic resistance in bacteria. The study draws special attention to investigate the uncharted anti-QS potential of ferns besides the usual assessment of traditional medicinal plants. The present investigation examines the use of ferns, the compounds of which could be successfully used as anti-QS inhibitors in future in treating infections caused by multidrug resistant strains of bacteria. The presence of QS inhibitory compounds in the leaves of P. nudum and C. tenuifoliaeffectively inhibit the biofilm formation in S. aureus and E. coli respectively thus reducing the virulence of these resistant pathogens. Further studies on these plants are required to isolate and identify the anti-QS compounds which could serve as a promising therapeutic option in controlling the virulence of pathogens.
Acknowledgment
The authors are thankful to Nitte (Deemed to be University) for all the facilities provided.
Conflict of interest
There is no conflict of interest by all the authors.
Funding sources
The work was supported by the Nitte University grant (N/RG/NUSR2/NUCSER/ 2020/12).
References
- Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist. 2019;12:3903.
CrossRef - Zhong L, Ravichandran V, Zhang N, Wang H, Bian X, Zhang Y, Li A. Attenuation of Pseudomonas aeruginosa quorum sensing by natural products: Virtual screening, evaluation and biomolecular interactions. Int. J. Mol. Sci.2020;21:190.
CrossRef - Verbeke F, De Craemer S, Debunne N, Janssens Y, Wynendaele E, Van de Wiele C, De Spiegeleer B. Peptides as quorum sensing molecules: measurement techniques and obtained levels in vitro and in vivo. Front. Neurosci. 2017; 11:183-201.
CrossRef - Asfour HZ. Anti-quorum sensing natural compounds. J MicroscUltrastruct. 2018; 6: 1- 10.
CrossRef - Zhao X, Yu Z, Ding T. Quorum-sensing regulation of antimicrobial resistance in bacteria. Microorganisms. 2020;8:425.
CrossRef - Baskaran X.R, Geo Vigila A.V, Zhang S.Z, Feng S.X, Liao W.B. A review of the use of pteridophytes for treating human ailments. J. Zhejiang Univ. Sci. B. 2018;19:85-119.
CrossRef - Dharajiya D, Pagi N, Jasani H, Patel P. Antimicrobial activity and phytochemical screening of Aloe vera (Aloe barbadensis Miller). Int J CurrMicrobiol App Sci. 2017;6:2152-62.
CrossRef - Alva P.P, Suresh S, Gururaj M.P, Premanath R. Evaluation of anti-quorum sensing activity of indigenous dietary plants against Pseudomonas aeruginosa. Eur. J. Integr. Med. 2019;30: 100931.
CrossRef - O’Toole G.A, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 1998;28:449-61.
CrossRef - World Health Organization. WHO traditional medicine strategy 2002–2005. 2002. WHO, Geneva. 2002.
CrossRef - Singh SK, Rajkumar SD. Biodiversity and indigenous use of medicinal ferns in Chandraprabha wildlife sanctuary, Chandauli, Uttar Pradesh. Int. J. Biosci.. 2017;5: 19-25
CrossRef - Koffi, E.; Sea, T.; Dodehe, Y.; Soro, S. Effect of solvent type on extraction of polyphenols from twenty three Ivorian plants. J. Anim. Plant Sci. 2010; 5:550–558.
- Premanath R, Suresh S, Alva P.P, Akash S.K. Biofilm Forming Abilities of Microorganisms Associated with Diabetic Wound Infection: A Study from A Tertiary Care Hospital. Biomed Pharmacol J 2019;12(2).
CrossRef - Nouri F, Kamarehei F, Asghari B, Hazhirkamal M, Abdollahian A.R, Taheri M. Prevalence and drug resistance patterns of bacteria isolated from wound and bloodstream nosocomial infections in Hamadan, West of Iran. All Life. 2022;15:174-82.
CrossRef - Sampathkumar P, Dheeba B, Vidhyasagar V, Arulprakash T, Vinothkannan R. Potential antimicrobial activity of various extracts of Bacopamonnieri (Linn.). Int. J. Pharmacol. 2008;4(3):230-2.
CrossRef - Damte D, Gebru E, Lee S.J, Suh J.W, Park S.C. Evaluation of anti-quorum sensing activity of 97 indigenous plant extracts from Korea through bioreporter bacterial strains Chromobacteriumviolaceum and Pseudomonas aeruginosa. J. Microb. Biochem. Technol. 2013;5:42-6.
CrossRef - Namasivayam S.K, Vivek J.M. Screening of quorum sensing (qs) modulatory effect of medicinal plant extracts against quorum sensing mediated virulence factors of human pathogenic gram negative bacteria. Int J Pharm Phytochem Res. 2016;8:263.
- Adnan M, Patel M, Deshpande S, Alreshidi M, Siddiqui AJ, Reddy MN, Emira N, De Feo V. Effect ofAdiantumphilippense extract on biofilm formation, adhesion with its antibacterial activities against foodborne pathogens, and characterization of bioactive metabolites: an in vitro-in silico approach. Front. Microbiol. 2020;11:823.
CrossRef - Irudayaraj V, Senthamarai R. Pharmacognostical studies on amedicinal fern, Drynariaquercifolia(L.) J. Sm. (Polypodiaceae:Pteridophyta). Phytomorphology 2004; 54: 193-200.
- Kandhasamy M, Arunachalam K.D, Thatheyus A.J. Drynariaquercifolia (L.) J. Sm.: a potential resource for antibacterialactivity. Afr J Microbiol Res 2008; 2: 202-205.
- Mithraja M.J, Irudayaraj V, Kiruba S, Jeeva S. Antibacterial efficacy of Drynariaquercifolia (L.) J. Smith (Polypodiaceae) against clinically isolated urinary tract pathogens. Asian Pac. J. Trop. Biomed. 2012;2:S131-5.
CrossRef - Sanchez C.J, Mende K, Beckius M.L, Akers K.S, Romano D.R, Wenke J.C, Murray C.K. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect. Dis. 2013;13:1-2.
CrossRef - Limoli D.H, Jones C.J, Wozniak D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015; 3(3), 10.1128/microbiolspec.MB-0011-2014.
CrossRef - Ranjan K.P, Ranjan N, Bansal S.K, Arora D.R. Prevalence of Pseudomonas aeruginosa in post-operative wound infection in a referral hospital in Haryana, India. J Lab Physicians. 2010; 2: 74 -77.
CrossRef







