Manuscript accepted on :06-09-2023
Published online on: 27-09-2023
Plagiarism Check: Yes
Reviewed by: Dr. Nikesh Narang
Second Review by: Dr. Abeer Gatea
Final Approval by: Dr. Patorn Promchai
Jihan Hussein1* , Zakaria El-khayat1
, Zakaria El-khayat1 and Hanan Farouk2
and Hanan Farouk2
1 Medical Biochemistry Department, National Research Centre, 33 El Behouth St., Dokki, Giza, Egypt.
2Therapeutic Chemistry Department, National Research Centre (NRC), 33 El Bohouth St. (Former El- Tahrir St.), Dokki, Cairo, Egypt.
Corresponding Author E-mail: jihan_husein@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2714
Abstract
The most common disease states of chronic liver illnesses include alcoholic liver disease (ALD), and viral hepatitis can progress to hepatocellular carcinoma (HCC). However, the role of selenium-associated tumor management angiogenesis in liver fibrosis and inflammation is yet unknown. As a result, in this current study, cytotoxicity of selenium ( Se) was evaluated against hepatocellular carcinoma cells ( HepG2) to determine IC50 ( in vitro study) and we established a mouse model of Ehrlich Ascites Carcinoma (EAC) to explore the role of selenium in the processing of tumor angiogenesis in liver injury and inflammation ( in vivo study). EAC cells was used to induce ascites tumor in albino mice and studied their consequence role on body weight gain and liver e. In EAC tumor-bearing mice, we discovered a substantial increase in body weight. Furthermore, mice with EAC tumors had higher levels of liver enzymes implicated in the etiology of liver inflammation, as well as biomarkers such as tumor necrosis factor α (TNF-), α fetoprotein (AFP), and caspase-3, Bcl2, and DNA damage.
Keywords
Caspase 3; DNA Damage; In Vitro; Inflammation; IC50
Download this article as:| Copy the following to cite this article: Hussein J, El-khayat Z, Farouk H. Antitumor Activity of Selenium in Ehrlich Ascites Carcinoma Bearing Mice.Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Hussein J, El-khayat Z, Farouk H. Antitumor Activity of Selenium in Ehrlich Ascites Carcinoma Bearing Mice.Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/468Mvns |
Introduction
The liver is the largest and one of the most essential organs in our bodies, serving as the primary regulatory organ for several physiological and biochemical processes ; angiogenesis is one of the fundamental physiological and pathological processes involved in carcinogenesis, progression, and metastasis, according to a large body of evidence1. Several growth hormones and cytokines are important in modulating the physiological process of angiogenesis. 2 In almost all malignancies, tumor angiogenesis, which is the growth of new blood vessels to feed nutrients to tumor cells that are dividing quickly, is a key step in the tumor metastasis route3. The damage and fibrosis of the liver caused by tumor angiogenesis is one of the characteristics of cancer that is connected to liver fibrosis and inflammation in chronic liver disease, and that it also promotes the growth of hepatocellular carcinoma (HCC)4. However, the relationship between tumor angiogenesis and hepatitis remains unknown. As a result, a thorough understanding of the relationship between tumor angiogenesis and inflammatory liver illnesses is required for the development of cutting-edge therapeutic options for tumor-associated hepatitis5. This lethal disease-related syndrome is caused by a combination of growth factors and cytokines5. Hypoxia activates numerous pro-angiogenic pathways, which promote capillary growth by secreting a variety of angiogenic growth factors, including the well-studied vascular endothelial growth factor (VEGF), placental growth factor (PIGF), fibrosis-associated transforming growth factor-beta (TGF-beta), and inflammation-associated tumor necrosis growth factor (TNF-beta), via the mitochondria6. In addition to these angiogenic growth factors, several inflammatory cytokines play an important role in liver inflammation and fibrosis7. Addressing tumor angiogenesis will be a promising therapeutic option for treating individuals with tumor-associated liver fibrosis and inflammation2. Several studies have revealed that angiogenesis is a key characteristic of several malignancies, including HCC8. Under normal physiological conditions, angiogenesis allows immune cells to migrate from one organ to another while delivering food and oxygen. It also stimulates the healing of tissue injury and the repair of damaged tissues during the course of tissue homeostasis9. However, the total tumor cell count and tumor volume increased as a result of the EAC cells’ rapid multiplication in the peritoneal cavity, generating a hypoxic environment in the adjacent microenvironment. As a result, many angiogenic growth factors and cytokines are produced, including VEGF, PLGF, TNF, and TGF, stimulating endothelial cells10. These components have been found to have an important role in the angiogenesis of HCC11, 12. It has been proposed that EAC tumor-induced peritoneal angiogenesis and newly created capillaries can transport angiogenic and inflammatory markers to the liver and may promote the activation of stellate cells, Kupffer cells, and mast cells that are linked to the liver, resulting in liver damage. associated inflammation signalling13. Previous investigations indicated that angiogenesis pathways are crucial to the development of hepatitis, fibrosis, and HCC14.
It has been demonstrated that selenium, an essential trace mineral present in both organic and inorganic chemical forms, is vital for sustaining mammalian cells in their ideal physiological state. It has demonstrated chemopreventive ability against the emergence of tumors as well as different types of environmental stress15. Recent studies suggested that the use of Se in combination with conventional chemotherapy medications and therapeutic hormones can disclose cancer, even though Se is in clinical trials for the chemoprevention of prostate, colon, and lung cancer16. Se’ beneficial and harmful effects have a very narrow window; therefore its long- term supplementation for preventative and therapeutic reasons is constrained16.
As a result, the current study assessed the impact of changing angiogenic regulators on the development of tumors and the indicators of inflammation and liver damage. Ehrlich cells were used as a tumor carcinoma model in mice to study the impact of selenium in vivo.
Materials and methods
Materials
Doxorubicin ( DOX) was purchased from the pharmacy.
culture media was obtained from Invitrogen‐Life Technologies.
selenite (Na2SeO3) was purchased from Merck Chemical Inc. (Darmstadt, Germany).
All other using chemicals were HPLC grade and purchased from Sigma-Aldrich, Germany.
In vitro study
Cytotoxic effect on human cell lines
The mitochondrial dependent reduction of yellow MTT (3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyl tetrazolium bromide) to purple formazan was used to determine cell viability.
Procedure
All procedures were carried out in a sterile environment with the use of a Laminar flow biosafety cabinet Class II A2.HepG2 cells were suspended in DMEM (Dulbecco’s Modified Eagle Medium) with high glucose and stable glutamine, 1% antibiotic, and 5% fetal bovine serum at 37 oC in a CO2 incubator (Sartorius stedium,biotech).
Cells were seeded at a density of 10×103 cells/well in fresh complete growth medium in 96-well plastic plates at 37 oC for 24 h under 5% CO2 either alone (negative control) or with different concentrations of drugs and Doxorubicin (DOX) as a positive control group, yielding a final concentration of (50, 25, 12.5, 6.25, 3.125, 1.5625 mg/ml). After 48 hours, the medium was aspirated, and 20 μl of MTT salt (2.5g/ml) was added to each well before incubating for another four hours at 37oC with 5% CO2. To dissolve the generated crystals and terminate the reaction, 200 μl of 10% Sodium dodecyl sulphate (SDS) in 0.01 mole HCL was added to each well, and the plate was then incubated overnight in a dark environment.17, 18.
The absorbance was then measured using a microplate reader at 595nm and a reference wavelength of 620nm. Viability was calculated as follow:
Viability = absorbance of drug / absorbance of control x 100
Cytotoxicity = 100- viability
IC 50 was calculated from the relation between the different concentration of the drug and cell viability for each drug concentration.
The IC 50 was computed using the relationship between drug concentration and cell viability for each drug concentration18.
LD50 estimation based on in vitro IC50 value
Using the following formula, the in vivo LD50 of acute oral toxicity was calculated from the in vitro IC50: log LD50 = 0.372 log IC50 (g/mL) + 2.02419.
In vivo study
Experimental Animals
Adult female Swiss albino mice (25-30g) were procured from the National Research Centre’s Animal House in Giza, Egypt. They were maintained in stainless steel cages in a controlled environment (temperature 20 ± 2 ºC) with regular laboratory diet and ad libitum water at the National Research Centre’s Animal House in Giza, Egypt. . The National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, updated 1985) was followed for animal procedures, and the experiment followed the recommendations and criteria of the National Research Centre’s (NRC) ethical committee.
Transplantation of a tumor
Dr. Gklien kindly provided an EAC cell line, which was maintained in experimental female Swiss albino mice via intraperitoneal injection of 2.5 x 106 cells per mouse. Fluid tumor was detected after 5-7 days of EAC cell inoculation20, 21.
Experimental design
Thirty-two Swiss albino mice were weighted and their weight volumes were recorded to compare with their weight after the experimental period; they were then randomly assigned to four experimental groups (8 mice per group) as appeared in chart 1 and categorised as follows:
Group 1: healthy mice.
Group II : EAC cell line was administered intraperitoneally into healthy mice.
Group lll : mice with EAC were given selenium (as Na2SeO3 dissolved in water) orally for ten days22, 23 at a dose one-tenth of the LD50.
Group IV : mice with EAC were given DOX (1/20 of the LD50) .
 |
Chart 1: Experimental study appeared groups and duration of the experiment |
Following the 10-day study period, rats were fasted overnight and blood was collected from the orbital vein, centrifuged at 4000 rpm for 20 minutes, and the separated plasma was stored at -80 oC for subsequent examination. The comet assay was performed on blood, and other biochemical parameters such as plasma caspase 3, Alpha fetoprotein (AFP), tumor necrosis factor-alpha (TNF-alpha), and Bcl2 were determined using an ELISA technique with commercial kits from R&D Systems GmbH (Wiesbaden, Germany), according to the manufacturer’s instructions . In addition blood transaminases (ALT and AST) were estimated colorimetrically using spectroUV-VIS Double Beam UVD-3500 .EAC fluid volume was sucked and measured its volume by ml using a syringe.
Comet test
The comet test was created to detect cellular DNA damage24. Lymphocytes were separated and washed in pH 7.4 phosphate buffered saline (PBS). Ten microliters of cells were suspended in 75 l of 0.5% low melting agarose to pipette on microscope slides with a layer of 1% agarose, spread with a cover slip, and solidified for 5 minutes on an ice-cold flat tray. After removing the cover slip, the slides were immersed in cold lysis solution for 1 hour, followed by 40 minutes of electrophoresis at 25 V, 300 mA, before being carefully removed from the tank and washed three times with 0.4 M Trizma base at pH 7.5 for 10 minutes. Each slide received 20 microliters of ethidium bromide (10 g/ml). The slides were examined at 40 magnification with a fluorescence microscope (Leica Microsystems, CMS GM b H, Wetzlar, Germany. Model DM 2500) and power Max. 160 W equipped with a 549 nm excitation filter and a 590 nm barrier filter. The “comet appearance” of damaged cells was seen, with a brilliantly fluorescent head and a tail to one side generated by DNA strand breaks that will draw away during electrophoresis. The percentage of damage was calculated by counting the damaged cell out of 100 cells each slide.
Statistical analysis
The statistical package for the social sciences (SPSS) application, version 16, and Microsoft Excel 2007 were used to conduct data analysis. The data were displayed as means standard error (SE). The significance of the difference in results was calculated using one-way ANOVA and the Student’s t-test. A statistically significant difference was defined as P 0.05.
Results
The in vitro study indicated that , IC 50 for selenium was 11.585 μg/ml as appeared in table, and 0.816 μg/ml for doxorubicin ( table1,2&fig1,2) .
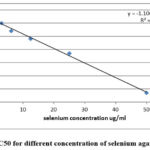 |
Figure 1: IC50 for different concentration of selenium against HepG2 |
Table 1: Cell viability and cytotoxicity of selenium’ different concentrations against HepG2
|
Selenium concentration (μg/ml) |
Cell viability % |
Cytotoxicity % |
IC50 ( μg/ml) |
LD50 ( mg/kg b.w.) |
|
50 |
7 |
93 |
11.585 |
262.887 |
|
25 |
37 |
63 |
||
|
12.5 |
48 |
52 |
||
|
6.25 |
54 |
46 |
||
|
3.125 |
60 |
40 |
||
|
1.562 |
62 |
38 |
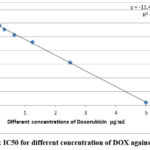 |
Figure 2: IC50 for different concentration of DOX against HepG2 |
Table 2: Cell viability and cytotoxicity of doxorubicin’ different concentrations against HepG2
|
Doxorubicin concentration (μg/ml) |
Cell viability % |
Cytotoxicity % |
IC50 ( μg/ml) |
LD50 ( mg/kg b.w.) |
|
5 |
2 |
93 |
0.816 |
97.982 |
|
2.5 |
31 |
63 |
||
|
1.25 |
46 |
52 |
||
|
0.625 |
51 |
46 |
||
|
0.3125 |
55 |
40 |
||
|
0.156 |
58 |
38 |
Table (3): showed significant increase in body weight gain with a percentage increase of 55.96 % in ehrlich bearing mice related to control .While medication of mice with EAC treated with Se and DOX showed an insignificant change in body weight with percentages reached to -6.12 and- 8.92% respectively from EAC group . The percent of change in the volume of EAC ascites fluid in Se and DOX treated mice were -13.39 and -15.68 respectively (Table 4). Significant elevation in the mortality rate of EAC mice with percentage increase amounted 40% relative to control level . While ,marked reduction in EAC treated mice with Se (30%) was recorded. However ,the mortality rate in DOX –treated EAC mice showed insignificant difference compared to untreated EAC bearing mice (Table 4) . Additionally, Table (6): declared noticeable elevation in ALT, AST in EAC bearing mice relative to control group . Treated EAC mice with Se and DOX revealed an improvement of these values . TNF-α ,caspase 3, AFP and Bcl2 in plasma of EAC bearing mice were significantly increased in EAC compared to control . However, the treatment with either Se or DOX showed marked amelioration in their levels (Table 7). Significant increase in percentage of DNA damage as recorded by the test of comet in EAC bearing mice compared to control mice . Howevere ,treated mice with Se showed marked significant reduction in DNA damage % which is significantly lower compared to the recorded for DOX treated group (Table 8, Fig.3).
Table 3: The mean value of body weight (g) of mice in different groups
|
|
Control |
Ehrlich |
Treated- selenium |
Treated -DOX |
|
Mean ±SE |
26.16±1.10 |
40.80±3.11a |
38.30±2.87a |
37.16±3.18a |
|
% change from control group |
– |
+55.96 |
+46.41 |
+42.10 |
|
%of change from ehrlich |
– |
– |
-6.12 |
-8.92 |
Data are expressed as mean ±SE, where a: indicated significant at P ≤0.05 from the control, and b: indicated significant at P ≤0.05 from ehrlich group
Table 4: The mean Volume (ml) of EAC ascites fluid of mice in different groups
|
|
Control group |
Ehrlich |
Se-treated mice |
DOX -treated mice |
|
Mean ±SD |
0.00 |
15.30 ±0.56 a |
13.25±0.89a,b |
12.90±0.65a,b |
|
%of change from EAC group |
— |
— |
-13.39 |
-15.68 |
Data are expressed as mean ±SE, where a: indicated significant at P ≤0.05 from the control, and b: indicated significant at P ≤0.05 from ehrlich group
Table 5: Mortality rate(%)post 10 days of treatment
|
Control group |
Ehrlich group |
Selenium treated group |
DOX treated group |
|
|
Day 1 |
10 |
10 |
10 |
10 |
|
Day 10 |
9 |
6 |
7 |
6 |
|
Dead animals |
1 |
4 |
3 |
4 |
|
% of death |
10% |
40% |
30% |
40% |
Table 6: Liver functions in different groups
|
Groups |
Control |
Ehrlich group |
Se treated group |
DOX treated group |
|
ALT (U/L) |
11.17±1.00 |
33.50±2.00a |
17.83±1.21ab,c |
21.33± 1.10ab |
|
AST (U/L) % change |
38.17±2.50 |
73.00±4.22a |
40.5±3.11ab,c |
48.50±3.07ab |
Data are expressed as mean ±SE, where a: indicated significant at P ≤0.05 from the control group;b: indicated significant at P ≤0.05 from the ehrlich group;c: indicated significant at P ≤0.05 from the DOX treated group.
Table 7: Serum levels of TNF-α, caspase 3,AFP, and Bcl‐2 in different groups:
|
Groups / Markers |
Control group |
Ehrlich group |
Se- treated group |
DOX t- reated group |
|
TNF-α ( ng/L) |
5.30± 0.70 |
14.72± 1.00 a |
11.22± 0.50 a.b |
12.90± 0.77 a,b |
|
Caspase 3(ng/ml) |
0.49 ± 0.02 |
1.30 ± 0.04 a |
0.72± 0.05 a.b |
0.79 ± 0.05 a,b |
|
AFP(IU/ml) |
0.41± 0.01 |
1.50± 0.07 a |
0.72 ± 0.02 a,b |
0.81 ± 0.03 a,b |
|
Bcl‐2 (ng/ml) |
4.47 ± 0.04 |
9.32 ± 0.05 a |
5.70 ± 0.03 a,b |
6.30± 0.05 a,b |
Data are expressed as mean ±SE, where a: indicated significant at P ≤0.05 from the control group;b: indicated significant at P ≤0.05 from the ehrlich group;c: indicated significant at P ≤0.05 from the DOX treated group.
Table 8: Percentage of DNA damage in different studied groups.
|
groups |
Control group |
Ehrlich group |
Se- treated group |
DOX- treated group |
|
DNA % |
2.20±0.33 |
32.00±3.60a |
16..00±1.03a,b,c |
22.00±2.30a,b |
Data are expressed as mean ±SE, where a: indicated significant at P ≤0.05 from the control group;b: indicated significant at P ≤0.05 from the ehrlich group;c: indicated significant at P ≤0.05 from the DOX treated group.
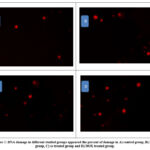 |
Figure 3: DNA damage in different studied groups appeared the percent of damage in A) control group, B) EAC group, C) se treated group and D) DOX treated group. |
Discussion
We seek to describe the novel molecular mechanism of HCC related with liver disease using the EAC mouse model and the therapeutic role of selenium based on this experimental observation. As a result, this model can be utilized to investigate new signaling systems, as well as offer additional insight on how cancer causes hepatitis and related liver illness. Few additional research have revealed that EAC tumor metastasizes to multiple organs, which may be related to the activation of angiogenic pathways, which can cause liver inflammation and fibrosis by various angiogenic growth factors as well as various cytokines2. Tumor angiogenesis and its detrimental consequences in comorbidities are known to be the primary causes of cancer-related liver dysfunction25. Our findings were comparable, and we believe that inflammation-induced hepatitis may be a cause of HCC-related mortality, even after therapeutic recovery26. Cancer cells, as previously stated, activate angiogenesis by raising the expression of AFP, Caspase 3, Bcl2, and
the release of angiogenic growth factors such as VEGF, TNFα, and TGF α, as well as different cytokines27, as evidenced by these findings. Angiogenic factors and previously generated cytokines enter the portal circulatory system and portal vein before reaching the liver, where they bind to particular receptors and activate numerous signal transduction pathways. This pathogenic event then activates hepatic stellate cells, Kupffer cells, and mast cells, which are the mediators of hepatic inflammation, fibrosis, and, in other words, liver damage14. Activation of these hepatic stellate cells, on the other hand, produces chemokines that promote angiogenesis, inflammation, and fibrosis. EGF and EGFR implicated in various biological activities; including mitogenic and angiogenic purposes; EGF-mediated signaling pathway was shown to play a key role in motivating proliferation of microvascular endothelial cells and also lymphatic endothelial cells . EGF can act through both paracrine and autocrine mechanisms to facilitate the expression of key proteases on endothelial cells to remodel surrounding extracellular matrix permitting endothelial cell migration, regulate the expression of VEGF and other growth factors, and induce angiogenesis via PI3k, MAPK, and eNOS pathways in a VEGF-independent 28. The increase in TNFα levels in our results may be related to its participation in the neovascularization process. TNFα is a significant inflammatory mediator that causes a variety of alterations in endothelial cells (EC) , including the activation of adhesion molecules, integrines, and matrix metalloproteinases29. Different types of cancer have altered levels of pro-inflammatory and pro-angiogenic proteins, and TNF-expression has been associated to tumor differentiation, invasiveness, and angiogenesis30. According to the results presented, an increase in TNFα is associated by an increase in tumor volume. The tumor’s ability to develop, as well as its invasiveness and metastatic ability, is enhanced by neovasculaturization15.Furthermore, a rise in tumor volume in mice with EAC is related with an increase in BcL2, AFP, Caspase 3, and DNA damage (Table 7,8).
As we discovered in our in vivo investigation, body weight increases with cancer growth (as seen by the current findings, which reveal a considerable increase in EAC ascitic fluid volumes due to inflammation (infiltration of immune cells) and concomitant fibrosis (collagen fiber accumulation). These pathological alterations damage hepatocytes’ and the liver’s overall physiological function. In support of these findings in liver damage, our biochemical assays demonstrated elevated blood levels of AST and ALT in EAC tumor-bearing mice compared to control mice. Another published study31 backed up our hypothesis. The increased level of liver enzyme activity in serum in EAC tumor-bearing mice definitely suggested liver injury. These new findings further suggest that the EAC tumor promotes peritoneal angiogenesis and is involved in the circulation of proangiogenic substances, which may contribute to the advancement of liver inflammation and fibrosis32. The considerable rise in AFP in the current study suggests that AFP reduces tumor immunity and promotes tumor development, decreasing cancer immunity and increasing tumor 33.Based on our findings, there is a considerable rise in TNFα, BcL2, and Caspse
3 in the serum of EAC-bearing mice compared to the control. These biomarkers are considered key regulators of inflammation and fibrosis. These indicators are thought to be important regulators of inflammation and fibrosis. Overall, elevated expression of these markers in EAC mouse serum can cause various clinical outcomes, such as hepatitis-like symptoms in breast cancer patients. This is the most likely source of liver inflammation and fibrosis in mice with EAC tumors 2. According to the findings, it was discovered that higher tumor volume in EAC- bearing mice is connected with a significant decrease in antioxidant biomarkers SOD, CAT and GSH15 .
Selenium treatment showed a significant improvement in different biomarkers examined in EAC-bearing mice related to control group. These findings can be explained by the fact that Se can inhibit hepatocarcinoma angiogenesis in rats by down-regulating the expression of TNF- and VEGF34, and it also inhibits TNF- in human umbilical vein endothelial cells [HUVEC], which leads to suppression of MMP-2 and MMP-9 activities15. Sodium selenite and other different forms of selenium might be able to inhibit cancer metastasis and primary tumor growth in several cancers in animals 15. Many researchers have observed that inhibiting antioxidant mechanisms in rat blood and tissues after irradiation35 and in irradiated carrier mice by EAC15 is associated with an increase in lipid peroxide products. Numerous investigations, on the other hand, have revealed that tumor growth can generate antioxidant disruptions in some tumor host tissues15. In fact, tumor growth may be to blame for the depletion of antioxidants in the liver as well as an increase in the quantity of lipid peroxidation products36. Past lipid peroxidation appears to be begun by the extraction of hydrogen from lipid molecules by lipid radiolysis products, resulting in permeability disruptions due to variations in membrane proteins and polysaccharides. It has also been shown that the rate of LPx increase following irradiation is proportional to the radiation dose and time37.When oxidative damage caused by tumor development is severe, ROS scavenging enzymes (SOD & CAT) and GSH are degraded15. In turn, free radicals and oxidative stress enhance the expression of TNF- and AFP, which are involved in the angiogenesis process and contribute to tumor formation. Furthermore, the anticancer efficacy of several Se compounds in the EAC mouse model has previously been described16. Se as adjuvant therapy with cyclophosphamide shown significant anticancer and antioxidant benefits in EAC-bearing mice16.Thus, Se played an important role in the reduction of Bcl2 in EAC cells, which may lead to the induction of mitochondria-mediated apoptosis by altering mitochondrial permeability and releasing specific apoptotic proteins such as cytochrome c 38; this, in turn, increases the cascade of caspases in EAC cells, particularly caspase-3, known as the death protein. 39, 40. These pathways have been approved in tumor cell execution in vitro in human colon cancer cells and human oral squamous cell carcinoma, respectively. Furthermore, our findings support the findings of Jin et al41, who discovered a strong apoptotic effect on colorectal cancer cells in vitro and in vivo related with Bcl-2/Bax/Caspase-3 signaling. These findings were supported by a recent experiment42 that validated these apoptotic processes in human prostate cancer cells in vitro. The most recent data also revealed higher DNA damage in EAC mice, as well as the modulatory action of Se. This could be because Se can impact the level of p53 in EAC cells; also, the P53 gene is recognized as the tumor suppressor gene and is in charge of DNA repair or damage in cells38. In cancer cells, the p53 gene is mutated, and the p53 proteins are changed into mutant p53 proteins that supply energy and dietary antioxidants to cancer cells, making them more resistant to chemotherapy medicines. and growing their numbers38. Finally, the data reported here show the establishment of a novel evidence-based mechanism for analyzing Ehlish-induced tumor angiogenesis in mice. In this mouse EAC model, we also demonstrated how tumor angiogenesis contributes to the activation of liver enzymes, the elevation of TNF-α, AFP, Bcl2, caspase 3, as well as an increase in DNA damage, and ultimately leads to the development of hepatitis due to inflammatory cell infiltration and collagen deposition. As a result, our findings may give more evidence that inhibiting tumor angiogenesis may be a promising therapeutic strategy in the treatment of liver impairment associated with advanced hepatitis. Furthermore, Se was able to lower inflammatory markers as well as Bcl2 activity, which supported an increase in caspases, particularly caspase-3, which caused cancer cells to be executed.
Conclusion
From the in vitro study (using HepG2 cell line) we can calculate LD50 that was used in determination of using selenium dose. This work appeared that Ehrlich Ascites Carcinoma (EAC) elevated liver functions and the inflammatory markers as appeared by measuring TNF-α, AFP in addition to increasing DNA damage, BcL2 and AFP; whereas treatment with selenium could attenuate these disturbances . We can consider selenium as a promising agent that can regulat angiogenesis and the development of tumors in liver inflammation; and we recommended more studies on different types of cancer.
Conflict of Interest
There is no conflict of Interest
References
- Longatto Filho, A.; Lopes, J. M.; Schmitt, F. C., Angiogenesis and breast cancer. Journal of oncology 2010, 2010.
- Gowda, N. G. S.; Shiragannavar, V. D.; Prabhuswamimath, S. C.; Tuladhar, S.; Chidambaram, S. B.; Santhekadur, P. K., Ehrlich Ascites carcinoma mice model for studying liver inflammation and fibrosis. Advances in Cancer Biology-Metastasis 2022, 4, 100029.
CrossRef - Ziyad, S.; Iruela-Arispe, M. L., Molecular mechanisms of tumor angiogenesis. Genes & cancer 2011, 2 (12), 1085-1096.
CrossRef - Novotný, J.; Zikán, M., Tumor angiogenesis. Klin Farmakol a Farm 2010, 24, 124-126.
- Kukla, M.; Gabriel, A.; Sabat, D.; Liszka, Ł.; Wilk, M.; Petelenz, M.; Musialik, J.; Dzindziora-Frelich, I., Association between liver steatosis and angiogenesis in chronic hepatitis C. Polish Journal of Pathology 2010, 61 (3).
- Zuazo-Gaztelu, I.; Casanovas, O., Unraveling the role of angiogenesis in cancer ecosystems.Frontiers in Oncology 2018, 8, 248.
- Liu, Y.; Kong, X.; Li, X.; Li, B.; Yang, Q., Knockdown of metadherin inhibits angiogenesis in breast cancer. International Journal of Oncology 2015, 46 (6), 2459-2466.
CrossRef - Li, J.; Hu, K.; He, D.; Zhou, L.; Wang, Z.; Tao, Y., Prognostic value of PLXND1 and TGF-β1 coexpression and its correlation with immune infiltrates in hepatocellular carcinoma. Frontiers in oncology 2021, 10, 604131.
CrossRef - Moustafa, A. H. A.; Ali, E. M. M.; Moselhey, S. S.; Tousson, E.; El-Said, K. S., Effect of coriander on thioacetamide-induced hepatotoxicity in rats. Toxicology and industrial health 2014, 30 (7), 621-629.
CrossRef - Zhu, X.-D.; Tang, Z.-Y.; Sun, H.-C., Targeting angiogenesis for liver cancer: past, present, and future. Genes & Diseases 2020, 7 (3), 328-335.
CrossRef - Mossenta, M.; Busato, D.; Baboci, L.; Di Cintio, F.; Toffoli, G.; Dal Bo, M., New insight into therapies targeting angiogenesis in hepatocellular carcinoma. Cancers 2019, 11 (8), 1086.
CrossRef - Tousson, E.; Hafez, E.; Abo Gazia, M. M.; Salem, S. B.; Mutar, T. F., Hepatic ameliorative role of vitamin B17 against Ehrlich ascites carcinoma–induced liver toxicity. Environmental Science and Pollution Research 2020, 27, 9236-9246.
CrossRef - Tsuchida, T.; Friedman, S. L., Mechanisms of hepatic stellate cell activation. Nature reviews Gastroenterology & hepatology 2017, 14 (7), 397-411.
CrossRef - Santhekadur, P. K.; Akiel, M.; Emdad, L.; Gredler, R.; Srivastava, J.; Rajasekaran, D.; Robertson, C. L.; Mukhopadhyay, N. D.; Fisher, P. B.; Sarkar, D., Staphylococcal nuclease domain containing-1 (SND1) promotes migration and invasion via angiotensin II type 1 receptor (AT1R) and TGFβ signaling. FEBS open bio 2014, 4, 353-361.
CrossRef - Mohamed, M. R.; Osman, S. A.; El Fateh, N. M.; Refaat, M. M., Antitumor activity of resveratrol in combination with selenium in Ehrlich ascites carcinoma bearing and/or irradiated mice. Egy J Pure Appl Sci 2015, 53, 27-39.
CrossRef - Kumari, M.; Ray, L.; Purohit, M.; Patnaik, S.; Pant, A.; Shukla, Y.; Kumar, P.; Gupta, K., Curcumin loading potentiates the chemotherapeutic efficacy of selenium nanoparticles in HCT116 cells and Ehrlich’s ascites carcinoma bearing mice. European Journal of Pharmaceutics and Biopharmaceutics 2017, 117, 346-362.
CrossRef - Thabrew, M. I.; Hughes, R. D.; McFarlane, I. G., Screening of hepatoprotective plant components using a HepG2 cell cytotoxicity assay. Journal of pharmacy and pharmacology 1997, 49 (11), 1132-1135.
CrossRef - Hussein, J.; El Bana, M.; Latifa, Y. A.; Saleh, S., Wound Healing Activity of Cotton Fabrics Loaded with Silver Nanoparticles in Experimental Model of Diabetes. Biomedical and Pharmacology Journal 2023, 16 (1), 53-65.
CrossRef - Wong, T.; Hashim, Z.; Zulkifli, R.; Ismail, H.; Zainol, S.; Rajib, N.; Teh, L.; Majid, F. A. A., LD 50 Estimations for Diabecine TM Polyherbal Extracts Based on In Vitro Diabetic Models of 3T3-L1, WRL-68 and 1.1 B4 Cell Lines. Chemical Engineering Transactions 2017, 56, 1567-1572.
- El-Gawish, M., Antitumor activity of inositol hexaphosphate (Phytic acid) in mice loaded with solid tumor. Egypt. J. Biomed. Sci 2003, 11, 106-121.
- Medhat, D.; Hussein, J.; El-Naggar, M. E.; Attia, M. F.; Anwar, M.; Latif, Y. A.; Booles, H. F.; Morsy, S.; Farrag, A. R.; Khalil, W. K., Effect of Au-dextran NPs as anti-tumor agent against EAC and solid tumor in mice by biochemical evaluations and histopathological investigations. Biomedicine & Pharmacotherapy 2017, 91, 1006-1016.
CrossRef - Milošević, M. D.; Paunović, M. G.; Matić, M. M.; Ognjanović, B. I.; Saičić, Z. S., The ameliorating effects of selenium and vitamin C against fenitrothion-induced blood toxicity in Wistar rats. Environmental toxicology and pharmacology 2017, 56, 204-209.
CrossRef - Hussein, J.; Farouk, H.; El-khayat, Z., Therapeutic Efficacy of Selenium in Management of Hyperhomocytenemia in Cisplatin-Induced Nephrotoxicity. Biomedical and Pharmacology Journal 2022, 15 (4), 1905-1915.
CrossRef - Hussein, J.; Rasheed, W.; Ramzy, T.; Nabeeh, M.; Harvy, M.; El-Toukhy, S.; Ali, O.; Raafat, J.; El- Naggar, M., Synthesis of docosahexaenoic acid–loaded silver nanoparticles for improving endothelial dysfunctions in experimental diabetes. Human & Experimental Toxicology 2019, 38 (8), 962-973.
CrossRef - Wang, J.; Zhang, Q.; Wang, D.; Yang, S.; Zhou, S.; Xu, H.; Zhang, H.; Zhong, S.; Feng, J., Microenvironment-induced TIMP2 loss by cancer-secreted exosomal miR-4443 promotes liver metastasis of breast cancer. Journal of Cellular Physiology 2020, 235 (7-8), 5722-5735.
CrossRef - Diamond, J. R.; Finlayson, C. A.; Borges, V. F., Hepatic complications of breast cancer. The lancet oncology 2009, 10 (6), 615-621.
CrossRef - Yoo, B. K.; Emdad, L.; Lee, S.-G.; Su, Z.-z.; Santhekadur, P.; Chen, D.; Gredler, R.; Fisher, P. B.; Sarkar, D., Astrocyte elevated gene-1 (AEG-1): A multifunctional regulator of normal and abnormal physiology. Pharmacology & therapeutics 2011, 130 (1), 1-8.
CrossRef - Ma, R.; Feng, Y.; Lin, S.; Chen, J.; Lin, H.; Liang, X.; Zheng, H.; Cai, X., Mechanisms involved in breast cancer liver metastasis. Journal of translational medicine 2015, 13 (1), 1-10.
CrossRef - Song, K.; Zhu, F.; Zhang, H.-z.; Shang, Z.-j., Tumor necrosis factor-α enhanced fusions between oral squamous cell carcinoma cells and endothelial cells via VCAM-1/VLA-4 pathway. Experimental cell research 2012, 318 (14), 1707-1715.
CrossRef - Feng, C.-c.; Wang, P.-h.; Ding, Q.; Guan, M.; Zhang, Y.-f.; Jiang, H.-w.; Wen, H.; Wu, Z. In Expression of pigment epithelium-derived factor and tumor necrosis factor-α is correlated in bladder tumor and is related to tumor angiogenesis, Urologic Oncology: Seminars and Original Investigations, Elsevier: 2013; pp 241-246.
CrossRef - KunduSen, S.; Gupta, M.; Mazumder, U. K.; Haldar, P. K.; Saha, P.; Bala, A., Antitumor activity of Citrus maxima (Burm.) Merr. leaves in ehrlich’s ascites carcinoma cell-treated mice. International Scholarly Research Notices 2011, 2011.
CrossRef - El-Kenawi, A. E.; El-Remessy, A. B., Angiogenesis inhibitors in cancer therapy: mechanistic perspective on classification and treatment rationales. British journal of pharmacology 2013, 170 (4), 712- 729.
CrossRef - Belyaev, N. N.; Bogdanov, A.-Y.; Savvulidi, P.-G.; Krasnoshtanov, V.-K.; Tleulieva, R.-T.; Alipov, G.- K.; Sekine, I.; Bae, J.-S.; Lee, J.-B.; Min, Y.-K., The influence of alpha-fetoprotein on natural suppressor cell activity and Ehrlich carcinoma growth. The Korean Journal of Physiology & Pharmacology 2008, 12 (4), 193-197.
CrossRef - Liu, J.-G.; Zhao, H.-J.; Liu, Y.-J.; Liu, Y.-w.; Wang, X.-L., Effect of two selenium sources on hepatocarcinogenesis and several angiogenic cytokines in diethylnitrosamine-induced hepatocarcinoma rats. Journal of Trace Elements in Medicine and Biology 2012, 26 (4), 255-261.
CrossRef - Oliĭnyk, B.; Baraboĭ, V.; Oliĭnyk, S.; Horchakova, N., Effect of splenosid on lipid peroxidation process and glutathione antioxidant system in rats exposed to fractionated radiation. Ukrains’ kyi Biokhimichnyi Zhurnal (1999) 2001, 73 (1), 73-77.
CrossRef - Gupta, A.; Bhatt, M. L.; Misra, M. K., Lipid peroxidation and antioxidant status in head and neck squamous cell carcinoma patients. Oxidative medicine and cellular longevity 2009, 2 (2), 68-72.
CrossRef - Abu-Zeid, M.; Hori, H.; Nagasawa, H.; UTO, Y.; INAYAMA, S., Studies of Methyl 2-Nitroimidazole- 1-acetohydroxamate (KIN-804) 2: effect on certain antioxidant enzyme systems in mice bearing Ehrlish ascites carcinoma. Biological and Pharmaceutical Bulletin 2000, 23 (2), 195-198.
CrossRef - Elhagrasi, A.; Mahmoud, A.; Foda, D.; Ibrahim, N.; Yousef, O., Secondary metabolites and biological activities of Allium porrum L. in attacking Ehrlich ascites carcinoma in mice. Egyptian Journal of Chemistry 2019, 62 (Special Issue (Part 1) Innovation in Chemistry), 211-227.
- Ahmed, M. M.; Elmenoufy, G. A., Quince polysaccharides induced apoptosis in human colon cancer cells (HCT-116). Res In Can Tumor 2016, 5, 1-9.
- Farhadi, F.; Jahanpour, S.; Hazem, K.; Aghbali, A.; Baradran, B.; Pakdel, S. M. V., Garlic (Allium sativum) fresh juice induces apoptosis in human oral squamous cell carcinoma: The involvement of caspase-3, Bax and Bcl-2. Journal of dental research, dental clinics, dental prospects 2015, 9 (4), 267.
CrossRef - Jin, S. J.; Yang, Y.; Ma, L.; Ma, B. H.; Ren, L. P.; Guo, L. C.; Wang, W. B.; Zhang, Y. X.; Zhao, Z. J.; Cui, M., In vivo and in vitro induction of the apoptotic effects of oxysophoridine on colorectal cancer cells via the Bcl-2/Bax/caspase-3 signaling pathway. Oncology Letters 2017, 14 (6), 8000-8006.
CrossRef - Russo, A.; Cardile, V.; Graziano, A. C.; Avola, R.; Bruno, M.; Rigano, D., Involvement of Bax and Bcl- 2 in induction of apoptosis by essential oils of three Lebanese Salvia species in human prostate cancer cells. International journal of molecular sciences 2018, 19 (1), 292.
CrossRef







