Manuscript accepted on :07-06-2023
Published online on: 25-08-2023
Plagiarism Check: Yes
Reviewed by: Dr. Ankur Kumar Arya and Dr. Amit Panaskar
Second Review by: Dr. Anjaneyulu Vinukonda
Final Approval by: Dr. Ricardo Lagoa
Sri Rahayu Santi , I Made Sukadana*
, I Made Sukadana* , I Gusti Agung Gede Bawa
, I Gusti Agung Gede Bawa and Novi Tamauli Herawati Simalango
and Novi Tamauli Herawati Simalango
Chemistry Department, Faculty of Mathematics and Natural Sciences Udayana University, Bali, Indonesia.
Corresponding Author E-mail:im_sukadana@unud.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2743
Abstract
This study aimed to screen the antibacterial compounds of S. aureus and E. coli on the stem bark of Inocarpus fagiferus Fosb. Extraction of active antibacterial compounds by maceration and partitioning, antibacterial tests were carried out by diffusion method, separation of compounds by column chromatography method, and identification of active fractions using LSMS/MS. The results of maceration of 350 g of Inocarpus fagiferus Fosb stem bark produced 22.97 g of methanol viscous extract which was able to strongly inhibit the growth of S. aureus bacteria (14.75mm) and medium inhibit (8.50 mm) towards E. coli. The partition results of the methanol concentrated extract respectively with n-hexane, chloroform, n-butanol, and water yielded 0.01; 0.01; 2.75, and 0.07 g extracts. Based on extract weight only n-butanol extract allows further separation. The result of antibacterial activity has shown a strong inhibition zone toward S. aureus and E. coli (16.50 mm) and with a minimum inhibitory concentration (MIC) of 5%. The result of separation n-butanol extract by gradient column chromatography (silica gel 60; methanol: chloroform (5:5; 6:4; 7:3; 8:4; 9:1; and 10:0)) yielded 4 fractions (FA, FB, FC, and FD) with FC the most active antibacterial toward S. aureus and E. coli with an inhibition zone 7.25 mm and 6.25 mm respectively at 5%. The results of LCMS/MS show 5 identified compounds known are maltol, nicotinamide, bioachanin A, L-proline, and 2,3-diamino propionic acid, as well as one unidentified compound with a molecular weight of 95.8066 g/mol. Maltol, nicotinamide, bioachanin A, and L-proline are compounds potents to inhibit the growth of S. aureus and E. coli bacteria.
Keywords
Antibacterial; Escherichia coli; Inocarpus Fagigerus Fosb; Staphylococcus aureus
Download this article as:| Copy the following to cite this article: Santi S. R, Sukadana I. M, Bawa I. G. A. G, Simalango N. T. H. S. Antibacterial Compounds Towards Staphylococcus Aureus and Escherichia Coli of the Stem Bark of Inocarpus Fagigerus Fosb. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Santi S. R, Sukadana I. M, Bawa I. G. A. G, Simalango N. T. H. S. Antibacterial Compounds Towards Staphylococcus Aureus and Escherichia Coli of the Stem Bark of Inocarpus Fagigerus Fosb. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/45tIvh5 |
Introduction
Staphylococcus aureus (S. aureus) is a gram-positive bacterium. It is facultatively anaerobic, isn’t move, isn’t form spores, is round, and toxic1,2 S. aureus is pathogens bacteria in food commonly isolated from the environment and the foods, skin, nostrils, and respiratory systems of animals and humans3,4 Staphylococcus infection isn’t occur on healthy skin, but if it to enter the bloodstream or internal tissues able to cause of serious infections5, such as pneumonia, sepsis, bacteremia, mastitis, syndrome toxic shock, and arthritis6,7. Coagulase-positive staphylococci produce a variety of extracellular protein toxins and virulence factors to contribute to their pathogenicity and there are involved in hemolysin, toxic shock syndrome toxin-1 (TSST-1), Panton-Valentine leukocidin (PVL), Staphylococcus enterotoxin (SE), exfoliative toxin A (ETA), and exfoliative toxin B (ETB)8. Meanwhile, Escherichia coli (E. coli) is a gram-negative bacterium, in the form of a short straight rod, has no capsule or spore, is facultatively anaerobic, and grows easily on a simple nutrient medium9. E. coli is the main facultative flora in the intestine that resides normally in the intestinal lumen of the host. As it is a normal flora in the body, when the host is in a weak state (immunosuppression) or when the gastrointestinal protective system is disturbed, these normal “nonpathogenic” bacteria can cause infection10. E. coli can produce colisin which functions as a protective agent for the digestive tract from pathogenic intestinal bacteria. It can play an important role in the synthesis of vitamin K, conversion of bile pigments, bile acids, and absorption of food substances, but if the amount exceeds the threshold then this bacterium becomes a pathogen with different virulence mechanisms, such as infectious diseases of the skin, eyelids, breast, urinary tract, dysentery, heart, bone, muscle, diarrheal disease, kidney failure, sepsis, and meningitis11,12.
Gayam (Inocarpus fagiferus Fosb) or in Bali known as gatep is a plant from the Fabaceae family which has the potential as an antibacterial because traditionally the stem bark has been used by the Balinese for a long time as a medicine for dysentery, urinary tract infections, and inflammation due to insect bites13,14 People in Ambon use the stem bark of Gayam as medicine for bloody rhinitis. Preliminary research showed that the methanol extract of Gayam stem bark was able to inhibit the growth of S. aureus and E. coli with diameters 14.75 mm and 8.50 mm respectively at 100% w/v. This research will investigate to antibacterial of stem bark Inocarpus fagiferus Fosb toward S. aureus and E. coli, then determine the compounds contained in active isolate.
Material and Methods
Materials
The material used is the stem bark of Inocarpus fagiferus Fosb obtained from the Klungkung area whose taxonomy has been identified at the Center for Plant Conservation of the Bali Botanical Garden. The bacteria used to research were S. aureus and E. coli which are obtained in Microbiology Laboratory Biology Department. The chemicals used were methanol, n-hexane, chloroform, n-butanol, agar media (Nutrient Agar), silica gel GF254, aluminum foil, filter paper, synthetic cotton, silica gel 60, and silica GF254.
Equipment
The equipment used includes a set of glassware, scissors, blender, sifter, analytical balance, stir bar, micropipette (Nesco), petri dish, test tube, measuring cup, beaker glass, incubator (Memmert), spirit lamp, loop needle, Laminar Air Flow (LAF), autoclave, rotary vacuum evaporator, vials, a set of thin layer chromatography (TLC), and a set of tools column chromatography, and LCMS/MS Agilent type 6120.
Methods
Extraction and Antibacterial Activity Test of Inocarpus fagiferus Fosb Stem Bark
As much as 350 g of dried powder of Inocarpus fagiferus Fosb stem bark was macerated with methanol for ± 24 hours repeatedly to obtain a concentrated extract, then the methanol solvent was evaporated by an evaporator. Furthermore, concentrated methanol extract was suspended in methanol water (7:3), and the methanol solvent was evaporated until only the water extract remained. This water extract was successively partitioned respectively with n-hexane, chloroform, and n-butanol to obtain n-hexane, chloroform, n-butanol, and water extracts. The four extracts were evaporated and weighed, and tested for their antibacterial activity toward S. aureus and E. coli. The diffusion method using to know potent antibacterial activity. Approximately 1 mL of bacterial suspension was added to 20 mL of nutrient agar medium, then vortexed until homogeneous, cooled, and compacted in a sterile petri dish. In a petri dish, a well with a diameter of ± 6 mm is made. Test extract, positive control (antibiotic), and negative control (aqua dest) of 20µL each were put into the diffusion well which had been preincubated at 37°C for 24 hours. The inhibitory diameter was observed after the incubation period.
Separation, Purification, and Identification of Antibacterial Active Fractions
The most active extract with the largest inhibition zone diameter was then separated and purified by gradient column chromatography (silica gel 60; methanol-chloroform (5:5; 6:4; 7:3; 8:4; 9:1; 10:0). The collected eluate was seen for its separation pattern using the TLC technique. The fractions that had the same separation pattern were combined and tested for antibacterial activity. The most active fraction was continued with a purity test, and identification using LCMS/MS
Results and Discussion
Maceration of 350 g of Inocarpus fagiferus Fosb stem bark with 2000 mL of methanol (4 x 500 mL) yielded 22.97 g of concentrated methanol extract. The results of both S. aureus and E. coli antibacterial activity tests against 100% (w/v) methanol extract are presented in Figure 1 and Table 1.
 |
Figure 1: Antibacterial activity test of 100% (w/v) concentrated methanol extract |
Table 1: Results of antibacterial activity test of 100% (w/v) methanol extract toward S. aureus and E. coli
|
Extract/compound/ solvent |
Inhibition zone (mm) |
|
|
S. aureus (category) |
E. coli (category) |
|
|
Methanol extracts |
14.75 (s) |
8.50 (m) |
|
Chloramphenicol (positive control) |
41 (vs) |
32 (vs) |
|
Methanol (negative control) |
0 (ni) |
0 (ni) |
noted: vs= very strong; s= strong; m= medium; w= weak; and ni= not inhibition
Table 1 shows that methanol extracts at 100% (w/v) can strongly inhibit the growth of S. aureus with a zone diameter of 14.75 mm and medium to inhibit E. coli with a zone diameter of 8.50 mm. This inhibitory power is based on the zone diameter caused by the test extract, which is categorized as follows; very strong (vs) for a diameter of ≥ 20 mm, strong (s) for a diameter of 10-20 mm, medium (m) for diameter 5-10 mm, weak (w) for 5 mm, and not inhibition (ni) for diameter £ 5 mm15. Thus, the methanol extract of Inocarpus fagiferus Fosb stem bark has more potential to inhibit S. aureus than E. coli.
The partition of about 15 g concentrated methanol extract which had previously been suspended in water-methanol (7:3) with n-hexane, chloroform, and n-butanol solvents respectively yielded 0.01 g of n-hexane concentrated extract, 0.01 g of chloroform concentrated extract, 2.75 g of n-butanol concentrated extract, and 0.07 g of an aqueous concentrated extract. Antibacterial activity tests for both S. aureus and E. coli were only carried out on n-butanol extract due to the limited number of other extracts.
Determining the minimum inhibitory concentration (MIC) of n-butanol extract at various tests of concentrations i.e.; 50, 10, 5, and 0.5% (w/v) towards both S. aureus and E. coli bacteria are presented in Figure 2 and Table 2.
 |
Figure 2: Test the antibacterial activity of the butanol fraction at concentrations of 50, 10, 5, and 0.5% |
Table 2: Results of Antibacterial Activity Test and Determination of Minimum Inhibitory Concentration of n-butanol Extract Against S. aureus and E. coli Bacteria
|
Concentration (% w/v) |
Zone Inhibition (mm) |
|
|
S. aureus (category) |
E. coli (category) |
|
|
50 |
13.50 (s) |
11.25 (s) |
|
10 |
8.50 (m) |
7.50 (m) |
|
5 |
6.75 (m) |
6.25 (m) |
|
0,5 |
6.25 (m) |
5.875 (w) |
vs= very strong; s= strong; m= medium; w= weak; and ni= not inhibition
Determination of MIC aims to determine the smallest concentration of extract that is still able to inhibit bacterial growth. Table 2 shows that the n-butanol extract of Inocarpus fagiferus Fosb stems bark with 0.5% (w/v) is a minimum inhibitory concentration which is still able to inhibit the growth of both S. aureus and E. coli.
Furthermore, the separation of about 2 g of n-butanol extract by gradient column chromatography obtained 4 fraction groups (FA, FB, FC, and FD) with color, spot number, Rf value, and weight of each fraction as shown in Table 3.
Table 3: Results of separation of n-butanol extract to yield fractions by gradient column chromatography
|
Fractions |
color |
spot |
Rf |
Weight (g) |
|
FA |
Clear yellow |
1 |
0,68 |
0,08 |
|
FB |
Pale yellow |
1 |
0,88 |
0,07 |
|
FC |
Dark reddish yellow |
1 |
0,73 |
0,24 |
|
FD |
Clear yellow |
1 |
0,62 |
0,11 |
The result of test antibacterial activity fourth fractions (FA, FB, FC, and FD) toward both S. aureus and E. coli showed that only FC was active antibacterial with an inhibition zone diameter of 7.25 and 6.25 mm for S. aureus and E. coli respectively at a concentration of 10% which was categorized as medium (m) as shown in Figure 3.
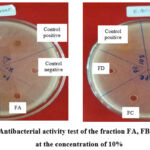 |
Figure 3: Antibacterial activity test of the fraction FA, FB, FC, and FD at the concentration of 10% |
Furthermore, the result identification of FC fraction with LCMS/MS showed that 5 identified compounds namely maltol, nicotinamide, bioachanin A, L-proline, and 2,3-diaminopropionic acid, as well as one unidentified compound with a molecular weight of 95.8066 g/mol as shown in Table 4 as follows:
 |
Table 4: Compounds contained in the FC fraction of the stem bark of Inocarpus fagiferus Fosb |
The compounds maltol, nicotinamide, bioachanin A, and L-proline are known to be antibacterial compounds against both S. aureus and E. coli. Maltol or 3-Hydroxy-2-methyl-4H-pyran-4-one is a phenolic compound derivative that is medium until strong as an antibacterial toward E. coli with an inhibition zone of 10 mm16,17. The mechanism of phenol-derived compounds to inhibit bacterial growth through denaturing cell wall proteins and cytoplasmic membranes, therefore, caused the formation of hydrogen bonds between phenols and proteins which results in disrupted permeability of the cell walls and cytoplasmic membranes, causing an imbalance of macromolecules and cell ions so that it become lysed. Nicotinamide or pyridine-3-carboxamide has antibacterial activity toward S. aureus at a concentration of 0.0625µl/mL but it is not yet known how its mechanism inhibits bacterial growth18. Biochanin A is a compound of the flavonoid group that is active as an antibacterial toward both S. aureus and E. coli at a concentration of 0.84 mg/ mL. It has a mechanism for inhibiting the nucleic acid synthesis in bacteria through the formation of hydrogen bonds between flavonoid compounds and the nitrogenous base of nucleic acid. This formation will also inhibit the formation of DNA and RNA bacteria19. The interaction of flavonoids will also inhibit the function of membrane cells through complex compounds of extracellular and dissolved proteins so that the cell membrane will be damaged and intracellular compounds will come out20. The L-proline compound in a complex with Cu metal known as Cu(L-prolinate)2 was able to inhibit the growth both of S. aureus and E. coli with diameter zone 16 mm and 15 mm respectively21.
Conclusion
In this study, compounds have been extracted from the stems bark of Inocarpus fagiferus Fosb using a methanol solution. It was found that there were five compounds including maltol, nicotinamide, bioachanin A, L-proline, and 2,3-diamino propionic acid, as well as one unidentified compound with a molecular weight of 95.8066 g/mol, where compounds that have the potential to inhibit the growth both of S. aureus and E. coli are maltol, nicotinamide, bioachanin A, and L-proline.
Conflict of Interest
There is no conflict of interest
Funding Source
There are no funding sources
References
- Wu S, Huang J, Wu Q, Zhang J, Zhang F, Yang, Wu H, Zeng H, Chen M, Ding Y, Wang J, Lei T, Zhang S, Xue L. Staphylococcus aureus isolated from retail meat and meat products in China: incidence, antibiotic resistance, and genetic diversity. Front. Microbiol., 2018; 9: 2767. https://doi.org/10.3389/ fmicb.2018.02767.
CrossRef - Massawe H F, Mdegela R H, Kurwijila L R. Antibiotic resistance of Staphylococcus aureus isolates from milk produced by smallholder dairy farmers in Mbeya Region. Tanzania. Int. J. One Health., 2019; 5: 31–37.
CrossRef - Can H Y, Elmalı M, Karago ̈z A. Molecular typing and antimicrobial susceptibility of Staphylococcus aureus strains isolated from raw Milk, cheese, minced meat, and chicken meat samples. Korean J. Food Sci. Anim. Resour., 2017; 37: 175–180. https://doi.org/10.5851/kosfa. 2017.37.2.175.
CrossRef - Chaalal W, Chaalal N, Bourafa N, Kihal M, Diene S M, Rolain J M. Characterization of Staphylococcus aureus isolated from food products in Western Algeria. Foodborne Pathog. Dis., 2018; 15: 353–360. https://doi.org/10.1089/ fpd.2017.2339.
CrossRef - Taylor T A, Unakal C G. Staphylococcus aureus. 2020; Jan. Available from: In StatPearls [Internet]. StatPearls Publishing, Treasure Island (FL) https://www.ncbi. nlm.nih.gov/books/NBK441868/.
- Li Q, Li Y, Tang Y, Meng C, Ingmer H, Jiao X. Prevalence and characterization of Staphylococcus aureus and Staphylococcus argentus in chicken from retail markets in China. Food Control., 2019; 96: 158–164. https://doi.org/10.1016/j. foodcont.2018.08.030.
CrossRef - Sumit R, Pramod K S, Nirpex T, Sugandh A. Community-Acquired Infection Caused by Small-Colony Variant of Staphylococcus aureus, Indian Journal of Medical Microbiology., 2020; 38: 216-218 https://doi.org/10.4103/ijmm.IJMM_20_250
CrossRef - Wang X, Wang X, Wang Y, Guo G, Usman T, Hao D, Tang X, Zhang Y, Yu Y. Antimicrobial resistance and toxin gene profiles of Staphylococcus aureus strains from Holstein milk. Lett. Appl. Microbiol., 2014; 58: 527–534. https://doi.org/ 10.1111/lam.12221.
CrossRef - Pelczar, Michael J dan Chan E C S. Dasar-Dasar Mikrobiologi Jilid II. 1998; UI Press. Jakarta.
- Nataro J P, Kaper J B, Mobley H L. Pathogenic Escherichia coli. Nature Reviews Microbiology. 2004;2: 123-140.
CrossRef - Madduluri S, Rao K B, Sitaram B. In Vitro Evaluation of Antibacterial Activity of Five Indigenous Plants Extracts against Five Bacteria Pathogens of Humans. International Journal of Pharmacy and Pharmaceutical Sciences. 2013;
- Radji M. Buku Ajar Mikrobiologi Panduan Mahasiswa Farmasi dan Kedokteran. 2011; Penerbit Buku Kedokteran EGC, Jakarta.
- Segatri P. Taru Premana, Khasiat Tanam-tanaman Untuk Obat Tradisional. 1999; Upada Sastra, Denpasar.
- Pauku R L. Inocarpus fagifer (Tahitian chestnut) Species Profiles for Pacific Island Agroforestry. 2006; www.traditionaltree.org., 1-18 (Diakses Pada 20 Maret 2021)
- Balouiri M, Sadiki M, Ibnsouda S K. Method for In Vitro Evaluating Antimicrobial Activity. Journal of Pharmaceutical Analysis. 2016; 6: 71-78.
CrossRef - Patel T and Shrivastava N. Clerodendrum and Healthcare: An Overview, Med. Aromat. Plant Sci. Biotechnol. 2007; 1: 142-150.
- Saud R, Pokhrel S, Ydav P N. Synthesis, Characterization and Antimicrobial Activity of Maltol Funcionalized Chitosan Devirate. Journal of Macromolecular Science, Part A. 2019;
CrossRef - Xu P, Zhang Yuan-Yuan, Sun Yong-Xue, Liu Jian-Hua, Yang B, Wan Yu-Zhong, Wang Yu-Liang. Novel Pleuromutilin Derivatives with Excellent Antibacterial Activity Against Staphylococcus aureus. Chem Biol Drugs. 2009; 73: 655-660.
CrossRef - Nikolic I L, Savic I M, Popsavin M M, Rakic S J, Mihajilov-Krstev T M, Ristic I S, Eric S P, Savic-Gajic I M. Preparation, Characterization, and Antimicrobial Activity of Inclusion Complex of Biochanin A with (2-hydroxypropyl)-β-cyclodextrin. Journal of Pharmacy and Pharmacology. 2018; 70: 1485-1483.
CrossRef - He M, Wu T, Pan S, Xu X. Antimicrobial Mechanism of Flavonoids Against Escherichia coli ATCC 25922 by Model Membran Study. Applied Surface Science. 2014; 305: 515-521.
CrossRef - Iqbal M S, Khurshid S J, Iqbail M Z. Antibacterial Activity of Copper-Amino Acid Complex. 2015; 221-222.







