Manuscript accepted on :12-06-2023
Published online on: 15-09-2023
Plagiarism Check: Yes
Reviewed by: Dr. Dito Anurogo and Dr. Revathi Shenoy
Second Review by: Dr. Pandurangan Annamalai
Final Approval by: Dr. Jihan Seid Hussein
Maslichah Mafruchati1* and Wan Iryani Wan Ismail
and Wan Iryani Wan Ismail
1Department of Veterinary Anatomy, Faculty of Veterinary Medicine (60115), Universitas Airlangga, Mulyorejo, C Campus, Surabaya, Indonesia
2Science and Marine Environment, Universiti Malaysia Terengganu, Terengganu Malaysia
Corresponding Author E-mail: maslichah-m@fkh.unair.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2735
Abstract
Background: In mammals, birds, and amphibians, the B lineage of lymphoid cells first arise during embryogenesis and are distinguished by their capacity to produce immunoglobulin. For the purpose of researching the development of the B-cell repertoire and the development of self-tolerance, these early B-cell precursors are of utmost importance. The genetic and/or microenvironmental variables that control the beginning of immunoglobulin synthesis in embryonic haemopoietic cells are, however, poorly understood. Purpose: The ontogeny of B-cell precursors in chicken embryos from day three of incubation was examined in this work. Research methods: The terms "ontogeny, B-cell, precursors, chicken embryos, incubation" were used in a thorough literature search in the PubMed, NCBI, and Google Scholar databases. After all articles were picked based on the inclusion and exclusion criteria, 38 papers that satisfied the criteria for inclusion were collected. Result: The study's findings show that clglarge basophilic hemopoietic stem cells and cIg+ small lymphoid B-cell precursors are two types of migrant cells that appear to enter the embryonic bursa of Fabricius. Hence, B lymphopoiesis does not only take place in the bursa of Fabricius in the avian embryo. Although the yolk sac and the hemopoietic tissues around the dorsal aorta are strong candidates, the identity of the extra-bursal location remains unknown. Conclusion: Hence, general haemopoietic organs may serve as the initial site of B lymphopoiesis in both birds and mammals. Only later in the course of avian development do the bursal follicles become accessible and take over.
Keywords
B-Cell; Chicken Embryos; Incubation; Precursors; Public Health
Download this article as:| Copy the following to cite this article: Mafruchati M, Ismail W. I. W, Analysis of the Development of B-Cell Precursors in Day Three Incubated Chicken Embryos. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Mafruchati M, Ismail W. I. W, Analysis of the Development of B-Cell Precursors in Day Three Incubated Chicken Embryos. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/3LowMbN |
Introduction
In mammals1 birds 2,3 and avian embryos, lymphoid cells of the B lineage, which are known for their capacity to synthesize immunoglobulin, first emerge. For research on the development of the B-cell repertoire and the establishment of self-tolerance, these early B-cell precursors are crucially important4–7. However, little is known about the genetic and/or microenvironmental factors that control the onset of immunoglobulin synthesis in embryonic haemopoietic cells. Additionally, it appears that B-cell differentiation begins in such structurally and functionally unrelated organs as mammalian fetal liver4,5. There is disagreement over the exact role the avian bursa plays in B-cell differentiation. The development of the humoral immune system undoubtedly involves the embryonic bursa, as demonstrated by bursectomy studies2.
The earliest immunoglobulin-positive cells that can be found in the chicken embryo are also said to be present there. As a result, IgM-containing cells were first discovered in the follicles of the 14-day embryonic bursa8. A long-lasting and significant reduction of cellular and circulating IgM, IgA, and IgG was also produced by the in vivo injection of anti-p antibodies at day 13 in combination with surgical bursectomy at hatching9. These details were used to show that, in the chicken embryo, IgM biosynthesis begins exclusively in the bursal microenvironment, and that B-cell precursors eventually “switch” from IgM to IgG to IgA synthesis9 though a “switch” from IgM to either IgG or IgA cannot be completely ruled out10. Researchers who discovered residual B-cell activities in bursaless birds have refuted this widely held belief11.
Therefore, they suggested that B-cell differentiation could take place outside of the Fabricius bursa, at least in part. Given this debate and our prior findings of CLG cells in 12 day bursal mesenchyme12. In the 11th and 12th day’s blood as well as the 11, 12, and 13th day’s bursal mesenchyme, lymphoid cells were discovered that contained trace amounts of cytoplasmic immunoglobulin. In bursal follicles that were 14 days old, clg large basophilic cells first emerged.
Prebursal stem cells that express the sialyl Lewis X (sLex) carbohydrate epitope and surface IgM reach the bursal anlage epithelium and multiply between embryonic days (ED) 8 and 14, resulting in the formation of nascent lymphoid follicles. The process of immunoglobulin (Ig) heavy- and light-chain diversification by Ig-gene conversion is started by a differentiation event by ED15-17. A change in the growing B-cell surface expression of sLex to a structurally related carbohydrate known as Lewis X (Lex) has been demonstrated to coincide with the commencement of Ig-gene conversion. At ED15/16 or ED17/18, respectively, developing B-cells expressing sLex or Lex were isolated from the bursa for functional investigations. Next, PCR amplification of the rearranged Ig light-chain gene was performed in both groups. Ig-gene conversion was started in 76% of the Lex population, but in just 29% of the sLex cells throughout the course of 4 independent trials, according to the DNA sequence of the PCR-amplified Ig light chain. As a result, both phenotypic and functional studies revealed that the embryonic bursa played a significant role in the beginning of repertoire formation through a significant B-cell differentiation event that occurred on or around ED17/18.20-22 The purpose of these research was The ontogeny of B-cell precursors in chicken embryos from day three of incubation was examined in this work.
Method
Using the search terms “ontogeny, B-cell, precursors, chicken embryos, incubation,” a thorough literature search was carried out in the PubMed, NCBI, and Google Scholar databases. The publications that were discovered using these keywords were first selected using a variety of inclusion criteria, including journals that do not accept payments for their articles and research findings that focus on “ontogeny, B-cell, precursors, chicken embryos, incubation.” Articles that do not meet the inclusion requirements are ignored, and those that do will be subjected to data analysis.
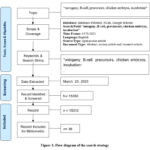 |
Figure 1: Flow diagram of the search strategy. |
Result
Ontogeny, B-cell precursors, chicken embryos, and incubation were the search terms used to find 15350 articles in the databases of PubMed, NCBI, and Google Scholar. After a second round of selection based on the inclusion and exclusion criteria, 38 articles that met the inclusion criteria were retrieved.
Blood from 11 and 12 day embryos had a small number of lymphoid cells that stained for both cytoplasmic and membrane-bound light chains (figure 2). At different phases of development, blood was not examined.
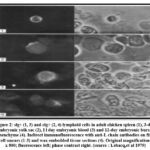 |
Figure 2: slg+ (1, 3) and clg+ (2, 4) lymphoid cells in adult chicken spleen (1), 3-day embryonic yolk sac (2), I l day embryonic blood (3) and 12-day embryonic bursal mesenchyme (4). |
Daily samples of ten to twenty-one day bursas (fourteen to nineteen at each stage) were taken. Indirect immunofluorescence was used to check for L chain +, IgG +, IgM +, and IgA + cells in at least two sections per bursa. Phase contrast was used to locate labeled cells, and some of them were moved following Giemsa staining. Tables 1 and 2 provide summaries of the findings.
Table 1: Frequency of lymphoid cells expressing Ig in the 3–8-day embryonic yolk sac. (source: Lebacq, et al 1979)
|
Developmental age (days) |
Ig-containing cells in yolk sac* (%) |
Red blood cells in yolk sac (%) |
Ig-containing cells in non-red blood cells of yolk sac (%) |
|
|
DIF |
IIF |
|||
|
3 |
NT§ |
0-04 |
95 |
0-8 |
|
6 |
0 |
NT |
|
|
|
|
0 |
NT |
|
|
|
|
<0-01 |
NT |
|
|
|
|
0-01 |
0-02 |
87-5 |
0-15 |
|
7 |
0-03 |
NT |
|
|
|
|
0-02 |
NT |
|
|
|
|
0-02 |
NT |
|
|
|
|
0-03 |
0-03 |
76 |
0-12 |
|
8 |
0-02 |
NT |
|
|
|
|
0-01 |
NT |
|
|
|
|
0-02 |
0-03 |
86 |
0-21 |
|
10 |
0-03 |
NT |
|
|
|
|
NT |
0-04 |
NT |
NT |
Table 2: Ig+ cells’ emergence, distribution, and frequency during the development of the embryonic bursa (source: Lebacq,et al 1979).
|
Age of embryo (days) |
Location of Ig + cells* |
|||||
|
Outer M |
Inner M |
Touching E |
Plain E |
Thickened E |
Foll |
|
|
9 |
-+ |
– |
– |
– |
– |
– |
|
10 |
– |
– |
– |
– |
– |
– |
|
11 |
+ |
– |
– |
– |
– |
– |
|
12 |
+ |
++ |
+ |
– |
– |
– |
|
13 early |
– |
++ |
+ |
+ |
+ |
– |
|
13 late |
|
+ |
+ |
+ |
+ |
+ |
|
14 |
|
+ |
+ |
|
|
++ |
|
16 |
|
++ |
|
|
|
+++ |
|
18 |
|
++ |
|
|
|
++++ |
|
21 |
|
++ |
|
|
|
+++++ |
Table 3. When, where, and how frequently IgM+, IgA+, and IgG+ cells emerge throughout the development of the embryonic bursa (source: Lebacq,et al 1979).
|
Age of embyro (days) |
Location of Ig + cells |
Class of cellular Ig+ |
|||
|
µ |
ý |
αb |
αs |
||
|
11 |
M |
-± |
– |
– |
+ |
|
|
E |
– |
– |
– |
– |
|
12 |
M |
– |
– |
– |
++ |
|
|
E |
– |
– |
– |
– |
|
13 |
M |
+ |
+ |
+ |
++ |
|
|
E-F |
+ |
+ |
+ |
+ |
|
14 |
M |
+ |
+ |
+ |
++ |
|
|
F |
++ |
+ |
+ |
+ |
|
16 |
M |
++ |
+ |
NT |
++ |
|
|
F |
+++ |
+ |
NT |
++ |
|
21 |
M |
++ |
++ |
NT |
++ |
|
|
F |
++++ |
++++ |
NT |
++++ |
At day 14, five to ten percent of the follicles had big cIg+ basophilic cells that were vividly labeled by anti-L and anti-,u antibodies (Fig. 2). However, sIgM + lymphoid cells were also discovered outside of the follicles. In follicles and mesenchyme, the anti-y, anti-a, and anti-ab reagents labeled sporadic cells (Fig. 3).
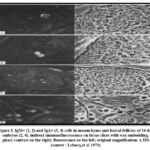 |
Figure 3: IgM+ (1, 2) and IgA+ (3, 4) cells in mesenchyme and bursal follicles of 14-day embryos (2, 4). indirect immunofluorescence on tissue slices with wax embedding. |
Discussion
Ontogeny, B-cell precursors, chicken embryos, and incubation were the search terms used to find 15350 articles in the databases of PubMed, NCBI, and Google Scholar. After a second round of selection based on the inclusion and exclusion criteria, 38 articles that met the inclusion criteria were retrieved. The study described here was designed to investigate the potential for extra-bursal B-cell development in the early avian embryo. Using purified antibodies to chicken Ig chains in a sensitive indirect immunofluorescence assay on fixed cell smears and wax-embedded tissue sections, we were able to identify in 3 day and older embryonic yolk sac, 11 and 12 day blood, and 11, 12 and 13 day bursal mesenchyme a limited population of putative B-cell precursors characterized by the presence of small amounts of cytoplasmic Ig and a lymphoid morphology. No early chicken embryos without clg lymphoid cells are known to exist at this time. As a result, the earliest sIgA and sIgM cells were found in the yolk sac on day 5, the bursa and spleen on day 10, and the follicles of the Fabricius embryonic bursa on day 148. Incubation began in 1976.
The following technical factors were crucial for identifying the weakly labelled cIg lymphoid cells in the chicken embryo: (1) the specificity of the anti-Ig reagents; (2) the affinity of the anti-Ig reagents, which allowed their use at 15 ug/ml and made the labeling by unidentified contaminating antibodies extremely unlikely; (3) the sensitivity of the assay, which allowed the detection of minute amounts of labelled Igs; and (4) the. Identifying whether the intracellular Ig in the early clg yolk sac cells came from the mother or the embryo is crucial. Considering that the yolk contains a significant amount of maternal IgG13.
Albumin contains very small amounts of IgM and IgA14. Although the vitelline membrane and eventually an extension of the chorion continue to separate the yolk and albumin, it is unlikely that these macromolecules will contaminate early yolk sac cells15. Large lymphoid cells with IgM or possibly IgA were found in the early yolk sac (3 days of incubation and onward). The remaining labelled material was made up of yolk spheres, vacuolated macrophage-like cells, and entodermal cells containing IgG. The Ig in the clg lymphoid cells, in contrast to these, was uniformly distributed throughout the cytoplasm, making it unlikely that it was obtained extrinsically through pinocytosis. To formally prove that IgM and possibly IgA in early yolk sac cells have embryonic origins, allotypic markers would be required.
Large pre-B cells described in murine are similar to the large cIgM cells found in the yolk sac of a three-day-old mouse16. In general, membrane-bound IgM7 the validity of which is currently under investigation. Since the primordium of the bursa has not yet begun to form on day three of incubation, it can be formally excluded17. The size of the small pre-B cells found in the blood, lymph nodes, liver, and spleen of human fetuses is comparable to that of the small clg lymphoid cells found in I1 to 13 day bursal mesenchyme6. Similar to human development, they were examined at later stages while being stained. Interesting studies using anti-H chain reagents showed that the majority of these cIg lymphoid cells, indicating the existence of a second antibody specificity in the former reagent. The two other reagents that were said to have labeled the earliest sIg cells found in the yolk sac, thymus, and bursa of chicken embryos may also have fit into this category18.
Our antibody preparations were identical in immunoelectrophoresis and indirect immunofluorescence on adult chicken spleen and jejunum sections19 with the exception of when tested on embryonic bursa sections. The reagent obviously needs to be studied further in order to fully understand this additional specificity. What relationships exist between the clg large lymphoid cells of the early yolk sac (days 3), blood (days 11 and 12), bursal follicles (day 14), and the temporally intermediate cIg small lymphoid cells of bursal mesenchyme (day 11) Over time, the circumstances surrounding what occurs to Ig cells in the embryonic bursa become more complicated. Starting on day 14 of incubation, small numbers of cIgA and cIgG cells were present in the bursal follicles in addition to the large basophilic cIgM cells.Lawton et al. states the following, the percentage of bursa cells that switch IgM biosynthesis to IgA or IgG biosynthesis10.
The widely held dogma states that the generation of B cells with a highly diverse receptor repertoire occurs in the bone marrow continuously throughout a person’s life. This is unquestionably true for humans and mice, but many higher vertebrate species use a different approach, which incorporates gut-associated lymphoid tissue (GALT) structures, to broaden the B cell repertoire. The chicken, which has the bursa of Fabricius as its principal B cell organ, is the best illustration of this option. Pre-bursal, bursal, and post-bursal phases are the three separate phases of B cell development in chickens. Hematopoietic precursor cells, which come from para-aortic foci of intra-embryonic mesenchyme, commit to the B cell lineage during the pre-bursal phase and move through the blood to numerous lymphoid organs like the spleen and bone marrow. The bursal phase begins between embryonic days 9 (ED9) and 12 (ED12), when a limited number of pre-bursal stem cells go to the bursa anlage, where only 2–5 B cell precursors colonize each of the 10,000–12,000 lymphoid follicles. The proliferative expansion of cells takes place inside the follicles. B cells must produce a wide range of distinct B cell receptors (BCRs) in order to recognize the enormous number of potential antigens. As a result, starting around ED15, the bursal environment causes gene conversion, rather than gene rearrangement, as it does in humans and mice, to cause the BCR to diversify. Around hatch, the post-bursal phase starts, and it lasts until bursa involution. When exposed to antigen, immature bursal B cells with functioning and diverse BCR begin to migrate to the periphery and spread to the secondary lymphoid organs, where they can develop into cells that secrete antibodies. The percentage of migratory cells is incredibly modest, and more than 90% of bursal B-cells undergo apoptosis before dying. After hatching, the formerly homogeneous bursa follicles separate into a dense, highly growing B-cell cortex and a more heterogeneous medulla comprising B-cells, a variety of myeloid cells, and stroma cells, including bursal secretory dendritic cells. Highly specialized sequential cell migration procedures are required to produce initially proliferative and then compartmentalized B cell follicles in the bursa and to seed peripheral lymphatic systems with B cells from the bursa. B cell precursors must initially migrate into the bursal anlage’s mesenchyme in the first stage of their migration. Those first bursal B cells must then move from the bursal mesenchyme into forming follicular buds during a second migration stage. These bursal secretory dendritic cells (BSDC), which are of hematopoietic origin and cross the basement membrane under the surface epithelium to generate a so-called dendro-epithelial tissue, are what cause the formation of these follicular buds. B cells move to the follicular border in a third migratory stage as the once homogeneous follicles divide into cortex and medulla. As a result, close to hatch, some B cells cross the line and move into a layer of mesenchymal reticular cells that are desmin and vimentin positive, where they continue to multiply. A tiny percentage of B cells finally emigrate in a fourth migration step, mostly from the follicular cortex of the bursa to the peripheral lymphoid organs 23-30
Conclusion
After 15350 items were discovered using keyword searches in the PubMed, NCBI, and Google Scholar databases, 38 papers met the criteria for inclusion. According to the study’s findings, the embryonic bursa of Fabricius appears to receive two different types of migrant cells: clglarge basophilic hemopoietic stem cells and cIg small lymphoid B-cell precursors. Therefore, the bursa of Fabricius is not the only location where B lymphopoiesis occurs in the avian embryo. Although the yolk sac and the haemopoietic tissues surrounding the dorsal aorta are strong candidates, the identity of the extra-bursal site is unknown. Thus, general haemopoietic tissues may serve as the initial site of B lymphopoiesis in both birds and mammals. Only later in the course of avian development do the bursal follicles become accessible and take over.
Funding Statement
There is no funding for this research/text. Everything is done personally by the author.
References
- Nossal GJ V, Pike BL. Differentiation of B lymphocytes from stem cell precursors. In: Microenvironmental Aspects of Immunity: Proceedings of the Fourth International Conference on Lymphatic Tissue and Germinal Centers in Immune Reactions Held in Dubrovnik, Yugoslavia, June 26–30, 1972. Springer; 1973:11-18.
- Thorbecke GJ, Warner NL, Hochwald GM, Ohanian S. Immune globulin production by the bursa of Fabricius of young chickens. Immunology. 1968;15(1):123.
- Warner NL. The immunological role of the avian thymus and bursa of fabricius. Folia Biol (Praha). 1967;13(1):1-17.
- Owen JJT, Cooper MAXD, Raff MC. In vitro generation of B lymphocytes in mouse foetal liver, a mammalian ‘bursa equivalent.’ Nature. 1974;249:361-363.
CrossRef - Moore MAS, Owen JJT. Stem-cell migration in developing myeloid and lymphoid systems. Lancet. 1967;290(7517):658-659.
CrossRef - Gathings WE, Lawton A, Cooper MD. Immunofluorescent studies of the development of pre‐B cells, B lymphocytes and immunoglobulin isotype diversity in humans. Eur J Immunol. 1977;7(11):804-810.
CrossRef - Raff MC, Megson M, Owen JJT, COOPER MAXD. Early production of intracellular IgM by B-lymphocyte precursors in mouse. Nature. 1976;259(5540):224-226.
CrossRef - Kincade PW, Cooper MD. Development and distribution of immunoglobulin-containing cells in the chicken: An immunofluorescent analysis using purified antibodies to µ, γ and light chains. J Immunol. 1971;106(2):371-382.
CrossRef - Lawton AR, Kincade PW, Cooper MD. Sequential expression of germ line genes in development of immunoglobulin class diversity. Biol Aging Dev. Published online 1975:59-71.
CrossRef - Martin LN, Leslie GA. IgM-forming cells as the immediate precursor of IgA-producing cells during ontogeny of the immunoglobulin-producing system of the chicken. J Immunol. 1974;113(1):120-126.
CrossRef - Claflin AJ, Smithies O, Meyer RK. Antibody responses in bursa-deficient chickens. J Immunol. 1966;97(5):693-699.
CrossRef - Ritter MA, Lebacq A. Embryonic bursa development in vitro. Eur J Immunol. 1977;7(7):468-475.
CrossRef - Rose ME, Orlans E. Normal immune responses of bursaless chickens to a secondary antigenic stimulus. Nature. 1968;217:231-235.
CrossRef - Rose ME, Orlans E, Buttress N. Immunoglobulin classes in the hen’s egg: their segregation in yolk and white. Eur J Immunol. 1974;4(7):521-523.
CrossRef - Romanoff AL. The avian embryo. Structural and functional development. avian embryo Struct Funct Dev. Published online 1960.
- Melchers F, Von Boehmer H, Phillips RA. B-lymphocyte subpopulations in the mouse. Organ distribution and ontogeny of immunoglobulin-synthesizing and of mitogen-sensitive cells. Transplant Rev. 1975;25:26-58.
CrossRef - Ackerman GA, Knouff RA. Lymphocytopoiesis in the bursa of Fabricius. Am J Anat. 1959;104(2):163-205.
CrossRef - Albini B, Wick G. Ontogeny of lymphoid cell surface determinants in the chicken. Int Arch Allergy Immunol. 1975;48(4):513-529.
CrossRef - Lebacq-Verheyden A-M, Vaerman J-P, Heremans JF. A possible homologue of mammalian IgA in chicken serum and secretions. Immunology. 1972;22(1):165.







