Manuscript accepted on :13-09-2023
Published online on: 29-09-2023
Plagiarism Check: Yes
Reviewed by: Dr. Moumita Hazra and Dr. M Mohan Varma
Second Review by: Dr. Hanefi ÖZBEK
Final Approval by: Dr. Patorn Promchai
Nehal E. Refaay 1* , Noha M. Halloull 2
, Noha M. Halloull 2 and Nehal A. Amer 1*
and Nehal A. Amer 1*
1Human Anatomy and Embryology Department, Faculty of Medicine, Zagazig University, Egypt.
2Forensic medicine and Clinical toxicology, Faculty of Medicine, Zagazig University, Egypt.
Corresponding Author E-mail:nehal.roaa@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2737
Abstract
Amiodarone (AMR) is a very powerful and efficient anti-arrhythmic agent since it outperforms other treatments in preventing and treating ventricular and supraventricular dysrhythmias. Melatonin is produced by a variety of organs, including the pineal gland. It has anti-oxidant and anti-inflammatory properties. Curcumin provides a variety of different health benefits and has been demonstrated to have considerable antioxidant action. The purpose of this study was to look into and evaluate the preventive benefits of melatonin and curcumin against AMR-induced lung damage.60 adult male albino rats were utilized in this study divided equally into 5 main groups: (control with no drugs, sham divided into 2 subgroups sham1 receiving 500Ug/kg body weight (BW) of melatonin and sham2 receiving 200 mg/kg BW of curcumin, AMR treated group receiving 40 mg/kg BW of AMR orally, AMR with melatonin group receiving500Ug/kg body weight (BW) of melatonin and40 mg/kg BW of AMR, and AMR with curcumin group receiving 200 mg/kg BW of curcumin and 40 mg/kg BW of AMR). this study was continued for 6 weeks. The lung tissue was processed for histopathological and biochemical evaluation at the end of the experiment and revealed significant elevations in inflammatory cytokine (il6) and oxidative parameters, lung alterations with fibrosis and marked cellular infiltration in the AMR-treated group. Yet treatment with melatonin and curcumin improved fibrosis detected by decreased area of positive TGF-β1 staining and lower number of stained macrophages by CD68 along with improving the antioxidant status of the tissue. Thus melatonin and curcumin had a protective effect over AMR-induced fibrosis.
Keywords
Antioxidant; Amiodarone; Curcumin; Fibrosis; Melatonin
Download this article as:| Copy the following to cite this article: Refaay N. E, Halloull N. M, Amer N. A. A Comparative Study of Lung-Protective Effects of Melatonin Versus Curcumin in Amiodarone Treated Adult Male Albino Rats. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Refaay N. E, Halloull N. M, Amer N. A. A Comparative Study of Lung-Protective Effects of Melatonin Versus Curcumin in Amiodarone Treated Adult Male Albino Rats. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/3PUj7fa |
Introduction
Amiodarone (AMR), a byproduct of iodine benzofuran HCl, is sometimes referred to as Cordarone or Pacerone 1. It is one of the most successful oral antiarrhythmic medications ever developed 2, in addition to being the more widely prescribed antidysrhythmic medication ever created3. Although the use of AMR as a therapeutic agent is very common ,Yet it is sometimes limited because of serious undesirable consequences that impact the majority of important organs4.
Amiodarone (AMR), tends to build up in the thyroid and lungs causing these tissues to become dysfunctional because of its iodine-containing composition 3. According to clinical studies, relatively long serious pulmonary fibrosis caused by amiodarone-related lung injury is a common factor in increasing mortality and morbidity 5. It is believed that approximately 6% of amiodarone users have lung toxicity, which manifests as intra-alveolar or interstitial inflammation or pulmonary fibrosis, with a death rate of 5-10%. Also, It was observed that using AMR for an average of 56 months lowers overall lung capacity and can develop lung fibrosis6,7.
Melatonin is a neurohormone released via the pineal gland during nighttime. It was lately discovered to have the ability of antioxidant activity, anti-tumor genesis, sleep control, and immunological modification8. Melatonin exerts anti-fibrotic actions via inhibition of the etiology of fibrosis. It also inhibits leptin-induced increase of collagen type 1. Additionally, it modulates connective tissue growth factor and fibronectin 9.
In addition, Various investigations have shown that melatonin has anti-inflammatory properties in various model systems and contexts. It inhibits the release of pro-inflammatory cytokines and adhesion molecules with the resultant ability of regulation of inflammation according to experiments and clinical studies 10. Furthermore, it has been demonstrated that, it can protect against aging-related inflammatory response as well as alcoholic hepatic impairment 11,12.
Curcumin (diferuloyl methane), an essential ingredient of the rhizomes of the plant Curcuma longa, gives Curry its distinctive flavor and yellow appearance when used as spice 13. Curcuminoids are native phenols which are fundamental for turmeric’s yellowish color 14. Curcumin possesses antioxidant, antimicrobial, antifungal, cancer prevention, and anti-inflammatory effects 15,16,17
The current study aimed to look into the apparent beneficial influence of melatonin and curcumin on Amiodarone-induced inflammatory and histological changes in the lungs of adult male albino rats. Based on our current knowledge, such research is the earliest research that analyze and compare the preventive effects of melatonin and curcumin against Amiodarone-induced pulmonary damage.
Materials and methods
Chemicals.
Amiodarone
(amiodarone hydrochloride) 200 mg tablets were obtained from the Global Napi for Pharmaceuticals Products company in Egypt (under license of Sanofi Aventis, France). Its brand name is Cordarone tablets. The amiodarone solution was generated fresh through disintegrating each tablet in 10 ml purified water.
Melatonin
It was received from Nature’s Bounty, USA Company, each tablet contains 3 mg of melatonin, which was crumbled then soaked in 1% corn oil.
Curcumin
Curcumin was purchased from Elhawag factory for raw oils Bader city -Cairo–Egypt
Animals
The Institutional Animal Care and Use Committee at Zagazig University (ZU-IACUC) approved all experimental procedures with approval number (ZU-IACUC/3/F/418/2022), and all procedures were carried out in compliance with ARRIVE guidelines.60 healthy adult male albino wistar rats (180-250 gm) were utilized in this study. The animals were taken from the animal house at the faculty of medicine at Zagazig University. The rats were kept in plastic cages with free access to commercial food pellets and water at room temperature (20-26°C) and normal relative humidity for two weeks prior to the commencement of the experiment. Throughout the experiment, the rats were kept in cages with 12-hour light/dark cycles. The animals were handled in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Experiment protocol
The rats were classified into five main groups, each with 12 rats:
Group 1 (Control group): This group was split into three subgroups (CN1, CN2, and CN3), each of which contained four rats. Rats in CN1 received no treatment throughout the experiment, CN2 only received 0.15 ml of saline per day intraperitoneal (i.p.) for six weeks., and CN3 received corn oil via gastric tube for six weeks.
Group 2 (Sham group) was split into two subgroups (Sh1, Sh2)., each with six rats. Melatonin was given to the rats in Sh1 once daily via intraperitoneal route for 6 weeks at a dose of 500Ug/kg body weight (BW) 12. Rats in the Sh2 group were received 200 mg/kg BW of curcumin dissolved in corn oil orally by gastric gavage 18 for 6 weeks.
Group 3 (Amiodarone treated group): The rats were given amiodarone (AMR) in the form of a single oral dosage (40 mg/kg BW) every day. A dose equivalent to the predicted therapeutic levels for adult albino rats 19 through gastric tube for 6 weeks in order to elicit lung toxic effects 18.
Group 4 (Amiodarone and Melatonin): The rats were given both; 40 mg/kg BW of amiodarone(AMR) as a single oral dosage through gastric tube 20 and 500ug/kg of Melatonin (given 2 hours before AMR dose) as a single intraperitoneal dose. These medications were continued daily for 6 weeks 12.
Group 5 (Amiodarone and Curcumin): The rats were given both; 40 mg/kg BW of amiodarone (AMR) as a single oral dose 20. and 200 mg/kg BW curcumin 18 Both medications were given through gastric gavage and continued daily for 6 weeks.
After the end of the experiment, the following procedures were performed
Lung and Body Weight were reported
Body weight was reported before giving the anesthetic to the rat, After the chest was opened, the lungs were extracted and weighted. Calculations were made for total body weight and wet lung weight in accordance with earlier research by Chen 21.
The total leukocyte counts in the Broncho alveolar lavage fluid (BALF):
Rats were put under anesthesia with ketamine (90 mg/kg/i.p.) and xylazine (10 mg/kg/i.p.). A catheter needle was used to slowly inject 5 mL of phosphate buffer solution (PBS) at 37 °C into the trachea after making an incision in it. Two additional times were performed accordingly, yielding roughly 15 mL, which was then maintained on ice. Estimation was used to asses
Total leukocyte counts: using lavage samples 22. The Barbara and Stanley method was used to calculate total leukocyte counts 23. In summary, the vitality of lung cells has been established with the trypan blue dye excluding technique. A drop of lung tissue preparation stained with trypan blue (0.047%) was placed on the hemocytometer in equal parts. A low power light microscope was utilized to count the viable cells (unstained).
IL6: BALF was centrifuged (2000 g for 10 minutes at 4 °C). The supernatant was subsequently serially diluted and kept at -80 °C for interleukin (IL)-6 testing. The evaluation was carried out in accordance with the manufacturer’s procedure using commercially available Abcam (IL-6: ab100772, UK) Camarillo, USA enzyme-linked immunosorbent assay (ELISA) kits. The findings were presented as (pg/mL) 24.
Lung samples extraction and homogenization
After BALF was removed, the chest was opened and the lungs were removed, submerged in saline and immediately deposited in liquid nitrogen, and then preserved at -80 C for additional examination. The homogenization process was fresh. The newly excised lung segments were preserved in 10% formalin for histopathological preparation, and the homogenate was utilized to determine biochemical parameters.
Estimation of oxidative stress markers
A 10% (w/v) lung homogenate was produced by homogenizing fresh lung tissues in Tris-HCl buffer (5 mmol/L containing 2 mmol/L EDTA, pH 7.4). Following centrifugation of the homogenates at 1000 rpm for 10 mins at 4°C, the resulting supernatant was subsequently utilized to assess the oxidant-antioxidant status. Applying the manufacturer’s guidelines, Biodiagnostic Company (biodiagnostic_eka@lycos.com and info@bio-diagnostic.com) measured the levels of malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT).
Histopathological examination for lung
The extracted lung samples were preserved in 10% formalin, bathed in distilled water and dried in a grading sequence of ethanol (70–100%) before embedding in paraffin (Merck Millipore, Darmstadt, Germany) and the following stains were applied:
Hematoxylin and Eosin (H & E) 25
Masson trichrome staining used for detection of collagen fibers in tissues 25,26
Immunostaining: Ten fields across three sections of every rat were encoded and photographed, allowing for blind observation and assessment utilizing a light microscope (Anatomy Department, Zagazig University).
For CD68
CD68 mouse monoclonal antibody (procured from Novocastra labs, UK, at a dilution of 1: 20) was used for immunostaining alveolar macrophages using an avidin biotin-peroxidase technique. This antibody was discovered to bind to a unique cytoplasmic glycoprotein found in mononuclear phagocytes, microglia, and epidermal langerhans cells 27. Biotinylated antimouse antibody (diluted 1: 200) and avidin biotin-conjugated peroxidase complex (Vector Lab. Inc., USA) were applied to paraffin sections of the lung. The procedure was carried out using 0.05% diaminobenzidine (Dakopatts Glostrup, Denmark) as the peroxidase substrate, and the slides were counterstained with Meyer’s hematoxylin 28 .The cytoplasm’s reaction was seen brown, while the nucleus was blue. The immunological response’s selectivity was investigated through substituting phosphate-buffered saline for the primary antiserum as a negative control 29.
For TGF-β1
Sections were dewaxed, mixed with water, and adjusted in 0.3% Triton X-100 Tris buffered saline (TBS) at 58 °C for immunohistochemical examination (Sigma). 0.3% H2O2 (Sigma) in methanol was used to suppress endogenous peroxide activity for 30 minutes. After that, slices were given a 0.1% trypsin treatment in 0.05M Tris and 0.02M CaCl2 (pH 8.0). Non-specific binding (BSA) was eliminated by inhibiting with 1.5% normal goat sera (Santa Cruz) in TBS with 0.5% bovine serum albumin. Sections were then mixed gently with rabbit polyclonal IgG to rat TGF-β1 (Santa Cruz) in TBS with 0.5% BSA for an overnight incubation at 4 °C, followed by an overnight incubation with a diluted version of the secondary antibody (Santa Cruz) against TGF-β1 in TBS with 0.5% BSA, and finally an hour-long incubation with avidin-biotin complex (Santa Cruz) in TBS with 0.1% For 5 minutes, development was carried out using 0.5% 3, 3-diaminolunzidine (Sigma) in a solution of 0.1% H2O2, 0.05M Tris, and 0.85% NaCl (pH 7.4). The sections were then dried, counterstained with Gill’s hematoxylin, and fixed using Permont (Sigma) 30.
Image analysis
10 fields from five sections were recorded from one rat within every group, allowing for blind assessments and evaluations. according to the results of H&E; morphological alterations was reported and lung injury score was calculated as follow, normal lung was graded as 0, mild alteration as 1, moderate alteration as 2, and severe alteration as 3.for example, it was stated that fibrosis was absent, mild, moderate or severe. The suggested guidelines for the histological examination of the lung in a study written by Kubiak et al. served as the foundation for histopathological grading 31. Also, the mean number of positive alveolar macrophages was determined utilizing immunostained slices with anti-CD 68 monoclonal antibodies from sections stained with anti CD68, quantity of the TGF B1 positively tagged areas from TGF B1 immunostained slides. Image analyzer software (Leica Qwin 500 Image Analyzer, England) was used to analyze the values in the image Analyzing unit of the Human Anatomy &Embryology Department, Faculty of Medicine, Zagazig University, Egypt. Calibration automatically converted the measurement units (Pixels) to micrometers.
Statistical analysis
The findings of the data analysis were displayed as graphs and tables using the Statistical Package of Social Science version 22 (SPSS). Weight and other quantitative factors were given as mean ±SD. Following a normality check, the proper statistical tests of significance were run. These findings were deemed significant provided the significance probability was less than 0.05 (P<0.05). P-values more than 0.05(P>0.05) were deemed to be statistically insignificant (NS).
Ethics Approval
All experimental methods were carried out in accordance with the required rules and regulations of Zagazig University’s Institutional Animal Care and Use Committee (ZU-IACUC/3/F/418/2022).
Results
The anthropometric, biochemical, and morphometrical measures did not significantly differ between all control subgroups and Sham subgroups (p > 0.05). Therefore, the negative control subgroup was chosen throughout the whole study for the sake of statistical analysis compared to other groups.
Results of anthropometric measures
Regarding Body weight
There is no significant difference (p > 0.05) between all studied groups. Regarding lung weight results, in group received only AMR, there is a significant rise (p < 0.05) in lung weight when compared with control and sham groups. Concomitant treatment of AMR with Melatonin resulted in insignificant change (p > 0.05) in lung weight when compared with AMR only. On the other hand, administration of Curcumin with AMR resulted in a significant (p < 0.05) decrease in lung weight when compared with AMR only treated group (table 1).
Table 1: Effect of AMR ± either Melatonin or curcumin on total body weight, lung weight in different studied groups.
|
Group |
Body weight (gm) Mean± SD |
Lung weight (gm) Mean± SD |
|
|
control |
CN1(n=4) |
240.6±36.4 |
2.25±0.085 |
|
CN2(n=4) |
253±38 |
2.17±0.01 |
|
|
CN3(n=4) |
239±25.7 |
2.26±0.12 |
|
|
Sham |
Sham1 (n=6) |
255±36.9 |
2.25±0.102 |
|
Sham2 (n=6) |
248±36.4 |
2.21±0.12 |
|
|
AMR |
(n=12) |
201.66±22.5 |
2.997±0.2 a b c f |
|
AMR+ Melatonin |
(n=12) |
241±10.1 |
2.55±0.106 |
|
AMR+ Curcumin |
(n=12) |
255±13.2 |
2.45±0.099 d |
|
P value |
.00 |
.00 |
Results of BALF analysis
Regarding total leucocyte count, there is a significant increase (p < 0.05) in total leucocyte count in AMR treated rats when matched with other groups. concomitant administration of AMR with Melatonin resulted in a significant (p < 0.05) decrease in leucocytic count when compared with control, Sham, AMR groups. There is no significant difference (p > 0.05) between the group received both AMR with Curcumin and control group on other hand, there was significate difference (p < 0.05) between this group and AMR and both Sham groups (table 2).
Regarding levels of (IL- 6), there is a significant increase (p < 0.05) in il-6 level in AMR treated group when compared with control and Sham groups. Also receiving Melatonin or Curcumin with AMR resulted in a significant change (p < 0.05) in il-6 levels when compared to AMR alone or with control or Sham groups (table 2).
Table 2: Effect of AMR ± either Melatonin or curcumin on total leucocytic count and level of (IL- 6) in BALF of different studied groups
|
Group |
CN1(n=4) |
Leucocyte count *105 Mean± SD |
Interleukin-6 (IL- 6) (pg/ml) Mean± SD |
|
control |
CN1(n=4) |
30.67±3.05 |
1.89±0.11 |
|
CN2(n=4) |
27.1±1.67 |
1.87±0.12 |
|
|
CN3(n=4) |
25±1.66 |
1.79±0.32 |
|
|
Sham |
Sham1 (n=6) |
23.3±2.08 |
1.55±0.12 |
|
Sham2 (n=6) |
23±1.5 |
1.41± 0.03 |
|
|
AMR |
(n=12) |
121.67±7.6 a b c e f |
5.8±0.23 a b c e f |
|
AMR+ Melatonin |
(n=12) |
56.3±17.6 a b c d |
3.38±0.54 a b c d |
|
AMR+ Curcumin |
(n=12) |
69.3± 3.6 b c d |
3.25±0.27 a b c d |
|
P value |
.00 |
.00 |
mean± standard deviation (SD), One-way ANOVA, and Tukey HSD Post-hoc Test, p > 0.05: no significant differences, p < 0.05: significant differences. a significant versus control, b significant versus sham1, c significant versus sham2, d significant versus AMR, e significant versus AMR+ Melatonin, f significant versus AMR+ Curcumin.
Biochemical results
Regarding MDA levels
AMR treatment resulted in a significant elevation (p < 0.05) in levels of MDA versus other studied group. When AMR administered with either Melatonin or curcumin, the results demonstrated a significant change (p < 0.05) in MDA when compared to AMR, control and both sham groups (table 3).
Regarding CAT levels
AMR treatment resulted in a significant reduction (p < 0.05) in levels of CAT versus other studied group. When AMR administered with Melatonin, there was a significant change (p < 0.05) in CAT levels when compared to AMR, AMR with curcumin, control and both sham groups. On the other hand, co-treatment of AMR with curcumin denied a significant change (p < 0.05) versus AMR only and AMR with melatonin groups only (table 3).
Regarding SOD levels
There was asignificant reduction (p < 0.05) in SOD levels in AMR group versus control, both sham and AMR with curcumin groups.co-treatment of AMR with melatonin resulted in nonsignificant change (p >0.05) in SOD level versus other groups while coadminstration of curcumin with AMR resulted in significant change (p < 0.05) in SOD levels versus AMR only without significant change with other studied groups (table 3).
Table 3: Effect of AMR ± either Melatonin or curcumin on oxidative stress markers in different studied groups.
|
Group |
MDA nmol /mg protein Mean± SD |
CAT nmol/min /mg protein Mean± SD |
SOD (U/mg protein) Mean± SD |
|
|
Control |
CN1(n=4) |
1.2±0.18 |
1.18± 0.18 |
80.1±6.3 |
|
CN2(n=4) |
1.1±0.1 |
1.3±0.1 |
82.3±7.1 |
|
|
CN3(n=4) |
.99±0.13 |
.987± 0.21 |
80.3±4.8 |
|
|
Sham |
Sham1 (n=6) |
1.029±0.4 |
1.2±0.15 |
79.5±11.8 |
|
Sham2 (n=6) |
.96±0.06 |
0.96±0.05 |
77.5±11.2 |
|
|
AMR |
(n=12) |
6.08 ±0.15 a b c e f |
0.4±0.08 a b c e f |
42.2±6.7 a b c f |
|
AMR+ Melatonin |
(n=12) |
4.6±0.1 a b c d f |
1.82±0.07 a b c d f |
65.05±8.2 |
|
AMR+ Curcumin |
(n=12) |
2.3±0.1 a b c d e |
1.2±0.06 d e |
66.25±5.2 d |
|
P value |
.00 |
.00 |
.00 |
mean± standard deviation (SD), One-way ANOVA, and Tukey HSD Post-hoc Test, p > 0.05: no significant differences, p < 0.05: significant differences. MDA Malondialdehyde, CAT Catalase, SOD Superoxide dismutase a significant versus control, b significant versus sham1, c significant versus sham2, d significant versus AMR, e significant versus AMR+ Melatonin, f significant versus AMR+ Curcumin
light microscopic results
H&E stained sections
All control subgroups and sham groups showed that, the lung has a typical spongy histopathological structure. It is formed of healthy patent alveoli, normal alveolar sacs separated with thin interalveolar septa at some sites and thick ones at other sites. The bronchioles were normal and healthy. Also, the blood vessels were seen normal in between the alveoli (Figs 1 A&B). In AMR treated lungs substantial alveolar injury was detected. there was collapsing alveoli, thick interalveolar septa, dilated distended bronchi along with inflammatory cellular infiltrates and tissue exudate between the alveoli. The pulmonary blood vessels showed thickening of their walls (Fig. 1 C). Red blood corpuscles (RBCs) were seen extravasating inside the lumens of alveoli from congested blood capillaries. In most bronchioles, RBCs and alveolar macrophages were found with intrabronchial cellular debris (Fig. 1 D).
Concomitant administration of AMR with melatonin showed moderate reduction of alveolar changes. Some alveoli regained degree of inflation however the intervening interalveolar septa were mildly thickened in most sites with mild inflammatory cellular invasion along with tissue exudate, the blood vessels were congested (Figs 1 E). Concomitant administration of amiodarone with curcumin showed evident reduction of pathological changes; with the exception of a minimal amount of inflammatory cellular invasion and clogged blood vessels, the bronchi were healthy and the alveoli were largely patent (Figs 1 F).
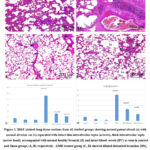 |
Figure 1: H&E-stained lung tissue sections from all studied groups showing normal patent alveoli (a) with normal alveolar sac (s) separated with intact thin interalveolar septa (arrows), thick interalveolar septa (arrow head), accompanied with normal healthy bronchi (B) and intact blood vessels (BV) as seen in control and Sham groups (A, B) respectively. |
Masson᾽s trichrome stained sections
Collagen fibers were evenly distributed into tiny fibers surrounding the blood vessels of the lungs and, to a smaller degree, throughout interalveolar septa in the lung stroma., and around the pulmonary bronchioles in all control and sham subgroups’ lung tissue (Fig. 2 A&B). lung sections from rats treated with AMR showed an increase in collagen deposition especially around the bronchus and bronchioles (Fig. 2 C). Lung sections from rats treated with melatonin and AMR showed moderate collagen fibers within the interalveolar septa, round the bronchioles, and lung blood vessels (Fig. 2 D). Lung sections from rats treated with curcumin and AMR showed mild collagen fibers within interalveolar septum, round the bronchioles, and around lung blood vessels (Fig. 2 E).
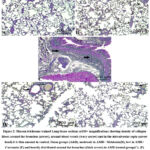 |
Figure 2: Masson trichrome stained Lung tissue sections at100× magnifications showing density of collagen fibers around the bronchus (arrow), around blood vessels (wavy arrow) and in the interalveolar septa (arrow head). |
Immunohistochemical staining with anti CD68
It’s used for immunostaining of alveolar macrophages. The control and sham groups’ lung tissue had normal few sporadic brown stained alveolar macrophages (Fig. 3A &B). Although the combination of melatonin and AMR revealed a moderate distribution of these cells within the lung stroma (Fig. 3 D), the combination of both AMR and curcumin produced a mild dispersion of these cells in the lung stroma (Fig. 3 E). Nevertheless, the lung treated with AMR displayed noticeably enhanced brown positive colored cells in interalveolar septa (Fig. 3C)
Morphometric analysis of lung tissue stained with CD68 revealed that, there was a significant increase (p < 0.05) in number of macrophages stained with CD68 in lung tissue in AMR treated rats versus other tested groups. Treatment with Melatonin as well as Curcumin resulted in significant change (p < 0.05) in macrophage number when compared with AMR only treated group without other tested groups (Fig 3F).
 |
Figure 3: CD 68 stained lung issue sections at 100, 400 magnifications showing stained macrophage cells in lung tissue(arrow). there is heavy infiltration of macrophage cells in interalveolar septa in AMR group(C), |
Immunohistochemical staining with TGF- ß1
In lung slices from the control group (Fig.4A) as well as Sham group (Fig.4 B), the histochemical identification of TGF-ß1 was nearly negative with very low reaction. low reaction in Amiodarone + curcumin group (Fig.4 E), and moderate reaction in amiodarone + melatonin group (Fig. 4D). But there was strong reaction for TGF- ß1 in lung sections of AMR treated group (Fig. 4 C).
Morphometrical analysis of Area percent stained positive with TGF- ß1 revealed that, it was significantly increased (p < 0.05) in AMR group versus other tested groups. Treatment with either Melatonin or curcumin with AMR resulted in significant change (p < 0.05) versus AMR only group with nonsignificant change (p > 0.05) versus other tested groups (Fig 4F)
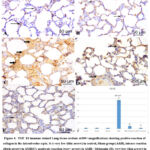 |
Figure 4: TGF- ß1 immune stained Lung tissue sections at100× magnifications showing positive reaction of collagen in the interalveolar septa. it is very low (thin arrow) in control, Sham groups(A&B), intense reaction (thick arrow) in AMR(C), |
Morphometrical results
Thickness of interalveolar septa was significantly increased (p < 0.05) in AMR treated group versus other studied groups. co-treatment with Melatonin as well as curcumin significantly (p < 0.05) changed this thickness versus other studied groups (Fig 1G).
Lung injury score was significantly increased (p < 0.05) in AMR treated group when compared to control, sham groups. co-treatment with Melatonin significantly (p < 0.05) changed it versus all other studied groups. However, treatment with curcumin significantly (p < 0.05) changed the condition with AMR, AMR with melatonin and with both sham groups only without control group (Fig 1E).
Discussion
Amiodarone was a relatively efficient type III antiarrhythmic medication for management of refractory arrhythmias 32. A significant increased dosage of amiodarone was linked to an increased risk of harmful effect on lung and other tissues. Amiodarone seemed to have a half-life of 30-60 days and was highly fat soluble. It has a bioavailability of 35-65%. Also it is metabolized in the liver, and its primary metabolite, desethylamiodarone, is active. Medications that suppress the hepatic cytochrome CYP3A4 enzyme raise its serum levels. It was stated that, because amiodarone has significant inhibitory effects on phospholipase, it promotes the buildup of phospholipids in inclusion bodies of alveolar macrophages as well as type II pneumocytes in the pulmonary tissue, resulting in cytotoxicity following a full month of its usage33.
In the current study, Administration of AMR to rats wasn’t accompanied by significant change in total body weight. This was not the same result obtained with 5 this may be because different dose or mode of administration; The previous authors used 80 mg/kg/day of AMR via an intraperitoneal route. On the other hand, results of the previous research matched results of this research in significant increase in lung weight following AMR administration. An initial edema with accompanied inflammation caused by AMR may be the cause of increased lung weight 5. Coming with these results, total WBCs cell count in BALF was significantly high in AMR only treated rats. However, this result improved to some extent with concomitant administration of either melatonin or curcumin.
In addition, in this research, AMR leads to significant increase in iL-6 in lung tissue. This was previously detected by34 using ELISA in C57BL/6 mice treated with AMR. However, the use of melatonin and curcumin suppresses this rise. This was in line with the finding of 12 that reported that melatonin administration decreased inflammatory marker expression in the hepatic and adipose cells of induced Diabetes Mellitus (DM) in rats and in liver of mice underwent common bile duct ligation (CBDL) in an experimental study performed by 35. IL-6 is a pro-inflammatory cytokine, and its excessive generation implies inflammation. Inflammation is the first step in the progression of fibrosis. Whenever iL-6 levels rise, it has an effect on acute-phase protein (APP) production36. Coming with this explanation, applying of Masson’s trichrome stain to the pulmonary tissue of AMR treated rats in this research demonstrated severe pulmonary collagen accumulation within interalveolar septa, around bronchioles and around pulmonary blood vessels. This was in harmony with 37 in rats treated with AMR for 7 days by gastric intubation. The morphometric data obtained with TGF‐ß1in this study emphasized this increase in average area percentage of collagen fibers in AMR treated group in comparison with the control group.
In the current study, the level of MDA was significantly higher in the AMR-treated group compared to the control and Sham groups. This was the same result evidenced by39 in an experiment performed on rats treated with AMR via an intra-tracheal dosage. Also CAT and SOD levels measured in this research were significantly reduced in AMR treated rats which matched results obtained with 37,39 respectively. However, the use of melatonin and curcumin improved this result. 40 proposed that oxidative stress played a role in how lung toxicity developed., attributing such results to the ability of AMR to elicit enormous injury to the blood air barrier with resultant rise of free radical production along with mitochondrial hydrogen peroxide 41. Furthermore,39 linked these alterations to amiodarone’s toxic effect, hypersensitivity, and increased oxidant indicators.
Histological findings of this study showed that AMR therapy resulted in extensive interstitial edema with capillary congestion, deteriorated alveolar structures, and considerable inflammatory invasion of cells in the lung parenchyma along with localized bronchiolar epithelial atrophy. In 2022, 42 found the same findings in rats treated with 100 ml AMR by gavage for 7 days only. According to 5, AMR-induced pulmonary damage is histologically visible one week following exposure, involving capillary congestion, interstitial capillary dilatation, and lymphocyte invasion. The researchers additionally discovered that the length of exposure affects the degree of lung injury.
According to some researchers, pulmonary damage caused by AMR can result in lung fibrosis. It is regarded as a chronic and irreversible respiratory disorder characterized by an abnormal accumulation of collagen fibers that leads to tissue damage38. Disruption to the epithelial cells and basement membranes is thought to be the primary cause of pulmonary fibrosis. afterwards, various types of cells move towards the site of this damage, including immunological cells, inflammatory cells, and fibroblasts, which trigger multiple cytokines secretion, increasing the inflammatory process and subsequent matrix remodeling. At the end of all of these processes, there is a significant increase in the formation of collagen fibers which is characteristic of pulmonary fibrosis 4,43.
In this study, the histological analysis of the AMR-treated rats yielded severe lung inflammation disrupting the bronchiolar architecture including both the bronchiolar lumen and the walls. It was invaded with neutrophils and pus cell. This was the same previously reported by 5 in rats given AMR by different doses via an intraperitoneal route.39 found also the same histopathological findings in rats treated with AMR for the same period. This was confirmed by CD68 staining that proved excessive number of macrophages in AMR treated rats in this study.44 explained the presence of macrophages as these cells consumed the extravascular RBCs as well as the produced lamellar bodies with their contained surfactant. Also,45 stated that such clues were caused by a non-specific interstitial pneumonitis constituted primarily of mononuclear cells, alveolar macrophages along with collagen deposition. This was the same recorded also by46.
Macrophages are cells of the immune system. They are found throughout body tissues with prevalence in the lung and liver, act as immunological sentries, guarding the body from invaders and injuries. Inflammatory macrophages implicated with pulmonary fibrosis induction47, M1-proinflammatory cytotoxic macrophages and M2-anti-inflammatory/reparative macrophages grow in reaction to cues in the tissues 48. In this study, the lung alveoli in AMR treated rats were collapsed. This was the same reported by 49 who stated that, collapsed alveoli reported in AMR administered rats could be a result of disequilibrium in surfactant synthesis and breakdown. Surfactant synthesis by hyperplastic pneumocytes type II appeared to outstrip alveolar macrophages’ ability to breakdown it.
Melatonin is widely recognized for its anti-inflammatory and antioxidant properties 50. In this study Melatonin therapy -to some extent- improved lung histology induced by AMR and decreased number of invading cells. This was coped with51 through a research employed on rats to prove the Melatonin’s antioxidant properties on lung injury produced by chest trauma. In addition, it diminished edema, exudate, neutrophil infiltration and inflammatory cytokines in lung tissue as evidenced by 52 in a rat model of subsequent lung damage following liver ischemia. Furthermore,53 found that melatonin decreased the inflammatory mediators and macrophages in mice which were subjected to cigarette smoke (CS) and explained that as melatonin acts as a SIRT1 enhancer so it can actively treat the airway inflammation brought on by CS. Another study on the function of melatonin in the treatment of Covid 19 virus respiratory disorders discovered the vital function of it in lowering pro-inflammatory cytokines generation, allowing this to eradicate a variety of severe inflammations 54
Melatonin, also in this study, improved AMR associated fibrosis in rats. This was originally agreed upon research of 9 and 55 in mice treated with cytotoxic drugs. The previous authors stated that melatonin suppresses endoplasmic reticulum tension and the transformation from epithelial to mesenchymal cells in treated mice lungs. In addition, it reduced significantly the area stained positive with TGF- β as stated by 9 who claimed that through stimulating the Hippo system, melatonin prevented TGF-induced fibrogenesis in lung fibroblasts, boosting nuclear translocation, and enhancing YAP1 inhibition and destruction in the cytoplasm [56]. Also, melatonin caused an improvement in the biochemical profile of lung tissue; it mitigated the antioxidant enzymes in AMR treated tissue. This is compatible to the findings of 39, which demonstrated melatonin’s suppression of reactive oxygen species generation and that a non-selective melatonin receptor antagonist inhibited apoptosis in mice lungs treated with Bleomycin. Moreover, 57 stated that rats receiving melatonin had higher antioxidant activities including superoxide dismutase (SOD)and catalase (CAT).
Curcumin has a number of biological properties, it is used for prevention of cancer, atherosclerosis and diabetes in addition to being capable to decrease oxidative stress and inflammation58. It had the ability to suppress inflammation and acute lung injury in animal models caused by cyclophosphamide59. In this study, Curcumin therapy to some extent, improved the histological findings when combined with AMR. Curcumin improved the pulmonary architecture with restoring normal histology of alveoli, bronchi and interalveolar septa with improvement of the general condition of pulmonary blood vessels along with suppressing polymorphonuclear cell invasion. This was the same findings of 60 in a research conducted on rats receiving 300 mg/kg/day curcumin daily for one week as an antioxidant against cyclophosphamide. Furthermore, the identified negative effects of AMR on rat lung tissue were related mainly to oxidative stress and subsequent fibrosis as confirmed by higher levels of MDA along with and low levels of CAT detected in this study, co-treatment with curcumin improved MDA and CAT levels as evidenced in previous studies of 61 in liver of rats and 62 in different cancer cell lines treated with curcumin.
In this study, curcumin significantly reduced amiodarone associated increase in TGF- ß percentage. This was the same recorded by 63. The previous authors stated that, this reduction in TGF-ß is probably via a decrease in c-Jun expression. However, 64 didn’t find the same result in mice, this may be due to difference in animal species. Curcumin’s supression of collagen accumulation resembles hepatic damage, according to a research that found curcumin suppress collagen accumulation by suppressing collagen mRNA production, proposing that curcumin can decrease collagen gene transcription in rats with lung fibrosis caused by AMR [64]. Also, in this study, rats treated with curcumin beside AMR had low cellular content in BALF which may be due to curcumin’s membrane-stabilizing and anti-inflammatory properties65. In addition, lung of Curcumin and AMR treated group reported decreased levels of il6 in lung tissue. This was in harmony with 64 in in a previous research conducted on induced acute viral pneumonia in an infected mice model (reovirus 1/L) where it was found that curcumin modified the production of key cytokines linked with ARDS progression, such as IL-6 and TNF.
Conclusion
In conclusion, after regular ingestion of AMR, oxidative stress could be a contributory factor of lung injury in rat, as shown by the inflammatory response along with extensive fibrosis in the lung tissue. As a result of their anti-inflammatory and antioxidant characteristics, the results showed that melatonin or curcumin significantly reversed the pulmonary toxicity caused by amiodarone in rats with preference of curcumin over melatonin.
Acknowledgements
Special thanks to Anatomy Department, Faculty of Medicine, Zagazig University. Many thanks to Zagazig University Animal House Department and Scientific Medical Research Center.
Conflict of Interests
There is no conflict of Interest.
Funding Sources
There is no Funding Sources
References
- Schwaiblmair, M., et al., Amiodarone-induced pulmonary toxicity: an under-recognized and severe adverse effect? Clinical Research in Cardiology, 2010. 99(11): p. 693-700.
- Kawabata, M., et al., Role of oral amiodarone in patients with atrial fibrillation and congestive heart failure. Journal of cardiology, 2011. 58(2): p. 108-115.
- Lafuente‐Lafuente, C., et al., Amiodarone concentrations in plasma and fat tissue during chronic treatment and related toxicity. British journal of clinical pharmacology, 2009. 67(5): p. 511-519.
- Sarg, N. and K.M. Kamal, Protection against lung toxicity induced by Amiodarone in Albino rats by fish oil. Journal of American Science, 2019. 15(12).
- Al-Shammari, B., et al., A mechanistic study on the amiodarone-induced pulmonary toxicity. Oxidative medicine and cellular longevity, 2016. 2016.
- Kudenchuk, P.J., et al., Prospective evaluation of amiodarone pulmonary toxicity. Chest, 1984. 86(4): p. 541-548.
- Young, R., R. Hopkins, and T. Eaton, Potential benefits of statins on morbidity and mortality in chronic obstructive pulmonary disease: a review of the evidence. Postgraduate medical journal, 2009. 85(1006): p. 414-421.
- Hu, W., et al., Melatonin: the dawning of a treatment for fibrosis? Journal of Pineal Research, 2016. 60(2): p. 121-131.
- Zhao, X., et al., Melatonin protects against lung fibrosis by regulating the Hippo/YAP pathway. International Journal of Molecular Sciences, 2018. 19(4): p. 1118.
- MacDonald, I.J., et al., Reconsidering the role of melatonin in rheumatoid arthritis. International journal of molecular sciences, 2020. 21(8): p. 2877.
- Shang, B., et al., Protective effect of melatonin on myenteric neuron damage in experimental colitis in rats. Fundamental & Clinical Pharmacology, 2016. 30(2): p. 117-127.
- Yapislar, H., et al., Anti-Inflammatory Effects of Melatonin in Rats with Induced Type 2 Diabetes Mellitus. Life, 2022. 12(4): p. 574.
- Kumar, A., et al., Curcumin (diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-κB activation. Biochemical pharmacology, 1998. 55(6): p. 775-783.
- Kolev, T.M., et al., DFT and experimental studies of the structure and vibrational spectra of curcumin. International Journal of Quantum Chemistry, 2005. 102(6): p. 1069-1079.
- Perrone, D., et al., Biological and therapeutic activities, and anticancer properties of curcumin. Experimental and therapeutic medicine, 2015. 10(5): p. 1615-1623.
- Jurenka, J.S., Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Alternative medicine review, 2009. 14(2).
- Gardare, M., et al., A REVIEW ON ANTIBACTERIAL, ANTIVIRAL, AND ANTIFUNGAL ACTIVITY OF CURCUMIN. 2021.
- Sudjarwo, S.A., K.E. Sudjarwo, and G.W. Sudjarwo, Mechanisms of endothelial cell protection by curcumin in hypercholesterolemia. Journal of Applied Pharmaceutical Science, 2011(Issue): p. 32-35.
- El Sayed, O.A., et al., Histopathological and biochemical toxic effect of amiodarone on thyroid gland in albino rat. The Egyptian Journal of Hospital Medicine, 2007. 29(1): p. 463-474.
- Kannan, R., et al., Tissue drug accumulation and ultrastructural changes during amiodarone administration in rats. Toxicological Sciences, 1989. 13(4): p. 793-803.
- Chen, Y., et al., Comparing study of the effect of nanosized silicon dioxide and microsized silicon dioxide on fibrogenesis in rats. Toxicology and industrial health, 2004. 20(1-5): p. 21-27.
- Henderson, R.F., Use of bronchoalveolar lavage to detect respiratory tract toxicity of inhaled material. Experimental and Toxicologic Pathology, 2005. 57: p. 155-159.
- Mishell, B.B. and S.M. Shiigi, Selected methods in cellular immunology. 1980: WH Freeman.
- Gao, H., et al., Levels of interleukin‑6, superoxide dismutase and malondialdehyde in the lung tissue of a rat model of hypoxia‑induced acute pulmonary edema. Experimental and Therapeutic Medicine, 2016. 11(3): p. 993-997.
- Bancroft, J.D. and M. Gamble, Theory and practice of histological techniques. 2008: Elsevier health sciences.
- Drury, R. and E. Wallington, Carleton’s histological technique 5th ed. New York: Churchill Livingstone, 1980.
- Elner, S.G., et al., CD68 antigen expression by human retinal pigment epithelial cells. Experimental eye research, 1992. 55(1): p. 21-28.
- Cattoretti, G., et al., Antigen unmasking on formalin‐fixed, paraffin‐embedded tissue sections. The Journal of pathology, 1993. 171(2): p. 83-98.
- Kiernan, J.A., Histological and histochemical methods: theory and practice. Shock, 1999. 12(6): p. 479.
- Xu, M., et al., Effects of curcumin in treatment of experimental pulmonary fibrosis: a comparison with hydrocortisone. Journal of ethnopharmacology, 2007. 112(2): p. 292-299.
- Kubiak, B.D., et al., Peritoneal negative pressure therapy prevents multiple organ injury in a chronic porcine sepsis and ischemia/reperfusion model. Shock, 2010. 34(5): p. 525-534.
- Van Herendael, H. and P. Dorian, Amiodarone for the treatment and prevention of ventricular fibrillation and ventricular tachycardia. Vascular health and risk management, 2010. 6: p. 465.
- Kaya, S.B., et al., Acute amiodarone toxicity causing respiratory failure. Revista da Associação Médica Brasileira, 2017. 63: p. 210-212.
- Ma, W., et al., Loureirin B attenuates amiodarone-induced pulmonary fibrosis by suppression of TGFβ1/Smad2/3 pathway. Tropical Journal of Pharmaceutical Research, 2020. 19(7): p. 1371-1376.
- Aurellia, N., et al., Effect of Curcumin on Interleukin-6 Expression and Malondialdehyde Levels in Liver Fibrosis. Open Access Macedonian Journal of Medical Sciences, 2022. 10(B): p. 2319-2326.
- Hashessh, E.A.A.G., Clinicopathological studies of Thymus vulgaris Extract Against Cadmium Induced Hepatotoxicity in Albino Rats. IDOSI Publications, 2014.
- Ahmed, D., M.Y. Youssef, and N.M. Emam, Oxidative stress in amiodarone-induced pulmonary toxicity in rats and the protective effect of L-carnitine and vitamin C. Mansoura Journal of Forensic Medicine and Clinical Toxicology, 2020. 28(1): p. 43-53.
- Zaeemzadeh, N., et al., Protective effect of caffeic acid phenethyl ester (CAPE) on amiodarone-induced pulmonary fibrosisin rat. Iranian journal of pharmaceutical research: IJPR, 2011. 10(2): p. 321.
- Gawad, F.A.E.-R., et al., Amiodarone-induced lung toxicity and the protective role of Vitamin E in adult male albino rat. Eur J Anat, 2018. 22(4): p. 332-333.
- Ashrafian, H. and P. Davey, Is amiodarone an underrecognized cause of acute respiratory failure in the ICU? Chest, 2001. 120(1): p. 275-282.
- Rebrova, T.Y. and S. Afanasyev, Free radical lipid peroxidation during amiodarone therapy for postinfarction cardiosclerosis. Bulletin of experimental biology and medicine, 2008. 146(3): p. 283.
- Esin, A., et al., Protective Role of White Cabbage Extract Against Amiodarone-Induced Lung Damage in Rats. Online Türk Sağlık Bilimleri Dergisi. 7(1): p. 143-150.
- Todd, N.W., I.G. Luzina, and S.P. Atamas, Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis & tissue repair, 2012. 5(1): p. 1-24.
- Savani, R.C., et al., Respiratory distress after intratracheal bleomycin: selective deficiency of surfactant proteins B and C. American Journal of Physiology-Lung Cellular and Molecular Physiology, 2001. 281(3): p. L685-L696.
- Larsen, B.T., et al., Lymphoid hyperplasia and eosinophilic pneumonia as histologic manifestations of amiodarone-induced lung toxicity. The American journal of surgical pathology, 2012. 36(4): p. 509-516.
- Li, S., J. Shi, and H. Tang, Animal models of drug-induced pulmonary fibrosis: An overview of molecular mechanisms and characteristics. Cell Biology and Toxicology, 2022. 38(5): p. 699-723.
- Meziani, L., et al., CSF1R inhibition prevents radiation pulmonary fibrosis by depletion of interstitial macrophages. European Respiratory Journal, 2018. 51(3).
- Budin, C.E., et al., Pulmonary Fibrosis Related to Amiodarone—Is It a Standard Pathophysiological Pattern? A Case-Based Literature Review. Diagnostics, 2022. 12(12): p. 3217.
- Nagata, N., et al., Characterization of amiodarone pneumonitis as related to inflammatory cells and surfactant apoprotein. Chest, 1997. 112(4): p. 1068-1074.
- Kostoglou-Athanassiou, I., Therapeutic applications of melatonin. Therapeutic advances in endocrinology and metabolism, 2013. 4(1): p. 13-24.
- Ozdinc, S., et al., Melatonin: is it an effective antioxidant for pulmonary contusion? journal of surgical research, 2016. 204(2): p. 445-451.
- Chiu, M.-H., et al. Protective effect of melatonin on liver ischemia-reperfusion induced pulmonary microvascular injury in rats. in Transplantation proceedings. 2012. Elsevier.
- Shin, N.R., et al., Role of melatonin as an SIRT1 enhancer in chronic obstructive pulmonary disease induced by cigarette smoke. Journal of Cellular and Molecular Medicine, 2020. 24(1): p. 1151-1156.
- Hardeland, R. and D.-X. Tan, Protection by melatonin in respiratory diseases: valuable information for the treatment of COVID-19. Melatonin Research, 2020. 3(3): p. 264-275.
- Elkerdasy, H., et al., The possible protective effect of melatonin and coenzyme Q10 on lung injury induced by bleomycin in adult male albino rats. The Egyptian Journal of Hospital Medicine, 2021. 83(1): p. 1536-1543.
- Erdemli, Z., et al., Effects of acrylamide and crocin on rat lung tissue. Annals of Medical of Research, 2022. 29(4).
- Yildiz, A., et al., The protective effect of melatonin in lungs of newborn rats exposed to maternal nicotine. Biotechnic & Histochemistry, 2018. 93(6): p. 442-452.
- Tsuda, T., Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, and future perspectives. Food & function, 2018. 9(2): p. 705-714.
- Venkatesan, N. and G. Chandrakasan, Modulation of cyclophosphamide-induced early lung injury by curcumin, an anti-inflammatory antioxidant. Molecular and cellular biochemistry, 1995. 142(1): p. 79-87.
- Saghir, S.A., et al., Curcumin prevents cyclophosphamide-induced lung injury in rats by suppressing oxidative stress and apoptosis. Processes, 2020. 8(2): p. 127.
- Alhusaini, A., et al., Curcumin ameliorates lead-induced hepatotoxicity by suppressing oxidative stress and inflammation, and modulating Akt/GSK-3β signaling pathway. Biomolecules, 2019. 9(11): p. 703.
- Gabr, S.A., et al., Curcumin Modulates Oxidative Stress, Fibrosis, and Apoptosis in Drug-Resistant Cancer Cell Lines. Life, 2022. 12(9): p. 1427.
- Punithavathi, D., N. Venkatesan, and M. Babu, Protective effects of curcumin against amiodarone‐induced pulmonary fibrosis in rats. British journal of pharmacology, 2003. 139(7): p. 1342-1350.
- Avasarala, S., et al., Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome. PloS one, 2013. 8(2): p. e57285.
- Nirmala, C. and R. Puvanakrishnan, Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Molecular and cellular biochemistry, 1996. 159: p. 85-93.







