Manuscript accepted on :21-03-2023
Published online on: 12-06-2023
Plagiarism Check: Yes
Reviewed by: Dr. Vijay Rekulapally
Second Review by: Dr. Sonal Deshpande
Final Approval by: Dr. Anton R Kiselev
Ratika Rahmasari1 , Galang Reynaldi2, Roshamur Forestrania2
, Galang Reynaldi2, Roshamur Forestrania2 , Nuraini Puspitasari2
, Nuraini Puspitasari2 , Berna Elya2*
, Berna Elya2*
1Laboratory of Microbiology and Biotechnology, Faculty of Pharmacy, Universitas Indonesia, Depok, Indonesia.
2Laboratory of Phytochemistry and Pharmacognosy, Faculty of Pharmacy, Universitas Indonesia, Depok, Indonesia.
Corresponding Author E-mail:berna.elya@ui.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2663
Abstract
Oral and dental problems caused by microbial infections are one of the global health problems which reduce the quality of life for those affected. Enterococcus faecalis is a gram-positive bacterium, linked to oral cavity infections, which are usually managed by chlorhexidine as an oral antiseptic. However, there is a concern related to the occurrence of its resistance. Zanthoxylum acanthopodium has been reported to have antimicrobial activity, but its activity against E. faecalis has not yet been known. This research aims to observe microscopic characteristics by scanning electron microscopic, determining total flavonoid content, and identifying anti-E. faecalis effectiveness of fruit and leaf of Z. acanthopodium ethanol extract. Microscopic evaluation confirmed the presence of the epicarp and mesocarp in fruits, meanwhile three different forms of calcium oxalate existed in its leaf. Further evaluation showed that fruit ethanol extract (flavonoid content of 20.84 mg EQ/g) did not exhibit activity against E. faecalis. However, leaf ethanol extract (flavonoid content of 131.73 mg EQ/g) showed activity against E. faecalis with a coefficient value of 0.4 relative to chlorhexidine. This study demonstrated for the first time, antimicrobial effectiveness of Z. Acanthopodium leaves ethanol extract against E. faecalis.
Keywords
Anti- Enterococcus faecalis; Flavonoid; Microscopic observation; Zanthoxylum acanthopodium
Download this article as:| Copy the following to cite this article: Rahmasari R, Reynaldi G, Forestrania R, Puspitasari N, Elya B. Zanthoxylum acanthopodium: Microscopic, Chemical Characteristic, and Anti- Enterococcus faecalis Effectivity of Fruit and Leaves Ethanol Extract. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Rahmasari R, Reynaldi G, Forestrania R, Puspitasari N, Elya B. Zanthoxylum acanthopodium: Microscopic, Chemical Characteristic, and Anti- Enterococcus faecalis Effectivity of Fruit and Leaves Ethanol Extract. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/3O0zi9M |
Introduction
It was estimated that nearly 3.5 billion people worldwide suffer from oral diseases, with dental caries at the top of the list1. The oral and dental problems are caused by several factors, including sugar from food, interactions between tooth surfaces, and microbial biofilms2. Enterococcus faecalis is one of the microorganisms linked to oral and dental problems usually found in periodontitis and caries lesions3. Currently, chlorhexidine, an antiseptic, is used to manage periodontitis and caries caused by bacterial infection. However, the occurrence of E. faecalis resistance against it has become the main concern4.
Herbs have been used in dentistry to reduce inflammation as antimicrobial, antiviral, and analgesic. Herbs also aid in healing and effectively controlling microbial plaque in gingivitis and periodontitis5.Indonesia has many sources of herbal ingredients such as plants and spices that have antimicrobial activity empirically. Several studies have reported the antimicrobial activity of Z. acantopodium ethanol extract, including fruit and leaf activity against Streptococcus mutans and Candida albicans6. However, not only limited information regarding to phytochemical content of Z. acantopodium, but its antibacterial activity against E. faecalis has not been reported. Therefore, this research aims to evaluate its microscopic characteristics, total flavonoids, chemical content, and its activity against E. faecalis.
Material and Method
Plant materials
Fresh fruit and leaves of Z. acanthopodium were obtained from Dolok Pardamean District, Simalungun Regency, North Sumatra Province, and determined at the Center of Indonesian Institute of Sciences (LIPI) Cibinong, West Java. The fruit and leaves were dried and stored at room temperature for further treatment.
Microorganism
E. faecalis ATCC 29212 was kindly provided by the Indonesian Microbiology Laboratory of the National Food and Drug Testing Development Center. E. faecalis were maintained in Enteroccoci agar, incubated at 37ºC incubator and passaged regularly.
Microscopic Characteristic Scanning Electron Microscope
Microscopic characteristic of fruit and leaves powder of Z. acanthopodium was observed using a Scanning Electron Microscope (Hitachi HT-770) at Indonesia Institute for Science, Bogor, Indonesia.
Preparation of ethanolic extract of Z. acanthopodium
The fruit and leaves were cleaned, then dried for 4 days without exposure to sunlight. The dry Simplicia was ground to powder. The fruit and leaves powder were then extracted with 96% ethanol using the ultrasound-assisted extraction method for three cycles7. The obtained supernatant was concentrated by a rotary evaporator (Buchi Rotavapor-205) to obtain the concentrated extract.
Determination of Total Flavonoid
A total of 50 µL of the test solution and each series of quercetin-comparison solutions were transferred into the different wells of 96 well-plate. 150 µL of ethanol, 10 µL of 10% aluminum chloride, 10 µL of sodium acetate 1M was then added to each well. The plate was shaken for 60 seconds and left to stand for 40 minutes at room temperature, followed by absorption measurement at a wavelength of 415 nm. Blank measurements were carried out for each of the comparison solutions and test solutions without the addition of aluminum chloride. The calibration curves of the quercetin-comparison solution were made with a concentration series of 6.81, 9.09, 11.36, 13.63, 15.91, and 18.18 µg / mL, then the levels of the test solution were calculated.
Chemical Analysis
Chemical compounds were analysed with LCMS-MS Instrument, Waters Acquity UPLC I-Class dan XEVO G2-XS QTof. As for Liquid chromatography system, stationary phase used were ACQUITY UPLC® BEH C18 1.7 μm 2.1 x 50 mm, and mobile phase were applied gradient with solvent A (H2O + 0,1 % Formic Acid (FA)) and Solvent B (Acetonitrile + 0,1% FA). The column temperature was 40.0 °C. Injection volume applied was 1 mL. As for Mass Spectrometry, sample was full scanned (m/z 100 – 1200) with 6eV ESI method. The identification of compound was based on comparison time retention and mass spectra stored in data system.
Antimicrobial effectiveness evaluation
Antimicrobial effectiveness were evaluated using a coefficient value which referred to the phenol coefficient value with some modification8. A series of tubes consisting of chlorhexidine, fruit-, and leaves- ethanol extract each at 1:40 (0.0125%, w/V), 1:80 (0.00625%, w/V), 1: 100 (0.005%, w/V); were prepared. Further, 0.5 mL of 106 E. faecalis cells/mL was added into each solution, shaken homogeneously, and incubated at room temperature. At 5 minutes, 10 minutes, and 15 minutes after incubation, 1 loop of liquid was taken and inoculated into a different tube containing 5 mL of Brain Heart Infusion Broth (Difco). Furthermore, each tube was incubated at 37°C for 24 hours followed by turbidity observation. The coefficient value is the quotient of the highest dilution factor of the test solution with the highest dilution factor of chlorhexidine, each of which kills the test bacteria within 10 minutes but does not kill within 5 minutes9. Two independent experiments were performed, each in duplicate.
Result
Microscopic observation
The microscopic study of Z. acanthopodium fruit revealed the presence of the epicarp and mesocarp layer (Figure 1). Meanwhile, the presence of three different forms of calcium oxalate, which are fibril, spiral fiber, and granule, were also observed at the leaves section (Figure 2).
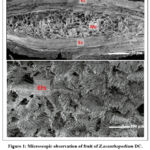 |
Figure 1: Microscopic observation of fruit of Z.acanthopodium DC. using scanning electron microscope.
|
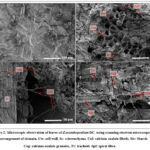 |
Figure 2: Microscopic observation of leaves of Z.acanthopodium DC. using scanning electron microscope.
|
Extraction and Chemical analysis
The ethanol was used as a solvent to attract the polar compounds in the sample. Instead of increasing the temperature, current research used the ultrasound-assisted extraction method. The liquid extract was further evaporated until concentrated extract was obtained. The yield for fruit and leaves of ethanol extract was 11,06% and 14,87% as shown in Table 1, respectively.
Table 1: Extract yield of fruit and leaves ethanol extracts.
|
Z. acanthopodium |
Weight (g) |
Yield (%) |
|
|
Simplicia |
Crude extract |
||
|
Fruit |
60 |
6,64 |
11,06 |
|
Leaves |
16 |
2,38 |
14,87 |
The determination of total flavonoid levels was based on the colorimetric method using aluminum chloride (AlCl3). Total flavonoid levels were expressed as mg equivalent of quercetin per g of sample (mg EQ/g). The quercetin standard curve, y = 0.0442x – 0.0624, was obtained with a correlation coefficient (r) of 0.9972 (supplementary data). The r value indicated that the standard curve fit as a regression line16. After determination, total flavonoids were 20,84 mg QE/ g for fruit ethanol extract and 131, 73 mg EQ/ g for leaves ethanol extract as shown in Table 2, respectively. The leaf ethanol extract showed higher flavonoid content than fruit ethanol extract.
Table 2: Flavonoid total of Z. acanthopodium ethanol extract.
|
Z. acanthopodium ethanol extract |
Flavonoid total (mg EK/ g) |
|
Fruit |
20, 84 |
|
Leaves |
131,73 |
Further, identification of pythochemical components in Z. acanthopodium fruit and leaf, was performed by Liquid Chromatography Mass Spectrometry. Based on the LCMS-MS result analysis, d-Lirioferine (Lirioferine), Lycopodine, and Yuanhunine were main compounds found in Z. acanthopodium leaves. Meanwhile, Isopsoralidin, Kokusaginine, Quercetin, Quercimetrin and Stearidonic Acid were main coumponds found in Z. acanthopodium fruit. Table 3 shows all compounds identified by LCMS-MS. Quercetin and Quercimetrin are flavonoid compounds, which is well known for their antimicrobial activity. Lirioferine, lycopodine, kokusaginine and yuanhunine are alkaloids. Isopsoralidine is fenolic compound while Stearidonic acid is a fatty acid.
Table 3: Main compounds found in leaf and fruit Z. acanthopodium ethanol extract.
|
Sample |
Compound Name |
m/z Observed |
RT (Min) |
Molecular Formula |
|
Leaves |
d-Lirioferine (Lirioferine) |
342.1705 |
2.90 |
C20H23NO4 |
|
Lycopodine |
248.2009 |
7.17 |
C16H25NO |
|
|
Yuanhunine |
356.1856 |
3.37 |
C21H25NO4 |
|
|
Fruit |
Isopsoralidin |
337.1080 |
6.53 |
C20H16O5 |
|
Kokusaginine |
260.0921 |
4.79 |
C14H13NO4 |
|
|
Quercetin |
303.0503 |
3.54 |
C15H10O7 |
|
|
Quercimetrin |
465.1033 |
3.30 |
C21H20O12 |
|
|
Stearidonic Acid |
277.2171 |
7.26 |
C18H28O2 |
Anti E. faecalis effectiveness
 |
Figure 3: Coefficient value determination relative to chlorhexidine based on medium turbidity. Medium turbidity result after contact with E. faecalis for 5, 10, and 15 minutes. M means Medium only without any treatment. |
The effectiveness of ethanol extract against E. faecalis was evaluated using a modified coefficient value. This evaluation will measure the effectiveness of extract in inhibiting E. faecalis after 10 minutes interaction but not at 5 minutes interaction with E. faecalis. Chlorhexidine, a mouth-washed antiseptic for oral disease management was used as a controlled drug17. The evaluation showed that Chlorhexidine inhibited E. faecalis after 10 minutes interaction at concentration 0.005% (w/V) or at 1: 100 dilution. The fruit ethanol extract was not able to inhibit E. faecalis after 10 minutes interaction, in all tested concentration (Figure 3). On the other hand, leaf ethanol extract inhibited E. faecalis after 10 minutes of interaction at 0.0125% (w/V) (1:40 dilution). The coefficient value of all tested extract effectiveness, relative to chlorhexidine against E. faecalis is shown in Table 4, respectively.
Table 4: Z. acanthopodium ethanol extract coefficient value relative to Chlorhexidine
|
Sample |
Dilution |
anti E. Faecalis effectiveness |
||||
|
contact time (minutes) |
Coefficient value* |
|||||
|
5 |
10 |
15 |
||||
|
Chlorhexidine |
1:40 |
– |
– |
– |
1 |
|
|
1:80 |
– |
– |
– |
|||
|
1:100 |
+ |
– |
– |
|||
|
Z. acanthopodiun ethanol extract |
Fruit |
1:40 |
+ |
+ |
+ |
none |
|
1:80 |
+ |
+ |
+ |
|||
|
1:100 |
+ |
+ |
+ |
|||
|
Leaves |
1:40 |
+ |
– |
– |
0.4 |
|
|
1:80 |
+ |
+ |
+ |
|||
|
1:100 |
+ |
+ |
+ |
|||
Note: 1:40 is 0.0125 % w/V; 1:80 is 0.00625% w/V; 1:100 is 0.005% w/V; + means medium were observed turbid; – means medium were observed clear; * relative to chlorhexidine. The results were derived from two independent experiments each in duplicate.
Discussion
Natural products including plants are valuable materials for the drug discovery and development since they possess diversity of metabolites. Zanthoxylum acanthopodium DC is one of the Rutaceae plants which distributes widely in subtropical and tropical areas. Traditionally, Zanthoxylum genus is used for mouth-washed teeth protection and has been reported to exhibit activity against bacterial dan fungi in vitro. However, report regarding to fruit, leaves morphological structure of Z. acanthopodium, growth in Indonesia tropical area, and its activity against E. faecalis is poor. In current study, observation by scanning electron microscopic showed the scattered arrangement of kidney-shaped stomata (Figure 2) on leaves. This is in agreement with its class classification, dicotyledoneae10. This microscopic characteristic of fruit and leaves powder of Z. acanthopodium as presented, is useful for further identification. Further the arrangement of epicarp and mesocarp (figure 1) and scattered kidney-shaped stomata (figure 2) were known related to its secreted metabolites not only to its function11. Bioactive phytochemical was known to accumulated higher in fruit than other tissue. It has been demonstrated that fruit mesocarp contributed in synthesize of hydroxilated metabolites and the thickness of epicarp involves in its resistance against external factors which both involved in production and accumulation of secondary metabolites12,13. Further, the prolong exposure of environment signal or stress at leaves, lead to adjustment of stomata size and density which further affected the shifts of primary to secondary metabolism resulting in elevation of secondary metabolites production11,14. Thus, it is possible to roughly predict the secondary metabolite level production with density of stomata15. To note, the same species plant may have may differ stomata density in response to environmental condition. However, in current study we could only observe the stomata shaped. Briefly, the identification of microscopic characteristic is important not only for plant identification but might indicated the level of metabolites being secreted. However, further histochemical test is needed to evaluate the phytochemical location in the cells.
In current study, extraction were performed by the ultrasound-assisted extraction method which will enhance the interaction of solvent-sample and avoid product degradation because of high temperature7. Flavonoid content evaluation showed that leaves of Z. acanthopodium have higher flavonoid content compared to fruit flavonoid content (Table 2). To note, flavonoids that found in Z. acanthopodium fruit was Quercetin (Table 3) which is well known to exhibit antibacterial such as against K. pneumoniae, B. cereus, A. parasiticus, A. flavus, S. aureus, S. epidermidis, B. subtilis, M. luteus and E. coli18. In the opposite, anti-E.faecalis activity using modified phenol coefficient value, was only shown from the leaves ethanol extract with main alkaloid content. The high flavonoid and main alkaloid content of leaves ethanol extract, indicate the presence of various abundant compounds in the leaves ethanol extract.
It was reported previously, that total flavonoid content is also positively linked to its antimicrobial activity19.This explained why the effectiveness of Z. acanthopodium against E. faecalis using coefficient value method, only observed at leaves ethanol extract (Table 4). It was reported that various flavonoids act as antibacterial by causing cell-membrane damage and inhibition on respiratory chain20. Nonspecific interaction between flavonoid and phospholipid can alter the membrane properties such decrease the fluidity and integrity of cellular membrane. Meanwhile, the binding of flavonoid at the polyphenol binding pocket of ATP synthase was the main reason of respiratory chain inhibition at bacterial21,22.
In addition, LCMS-MS analysis (Table 3) indicated the presence of alkaloids as main compounds on leaves ethanol extract which also could explained its activity against A. faecalis (Table 4). Lirioferine is an phenolic aporphine alkaloids, found in Litsea cubeba and reported to have antimicrobial activity. The presence of phenolic group on Lirioferine has been suggested responsible for its activity23. Aporphine alkaloids also have been reported for its potential antibacterial activity in 2014 by Udvardy. Lycopodine is also an alkaloid and it is known as its antimicrobial activity24. Isopsoralidin is a derivative from psoralidin, which is known for its anticancer, antiosteoporotic, anti-inflammatory, anti-vitiligo, antibacterial, antiviral, and antidepressant-like effects. Psoralidin has been reviewed its antibacterial activity by using diffusion method against S. aureus, S. epidermidis, P. aeruginosa, K. pneumoniae, P. mirabilis, P. vulgaris, and E. coli, with best results was against K. pneumoniae25. Based on chemical identification (table 2 and 3), the presence of flavonoid and alkaloid in the leaves of Z. acanthopodium might responsible as anti E. faecalis with similar mechanism as explained above. However, further antimicrobial activity research using different method including isolation and characterization of active compound(s), is needed to confirm Z. acanthopodium active compound against E. faecalis structure and its mechanism of action.
Conclusion
In the present study, the obtainedZ. Acanthopodium Microscopic characteristics is hopefully useful for plant identification and rough prediction of secreted metabolite level. In addition, the high flavonoid, alkaloid content, and anti E. faecalis activity from leaves ethanol extract indicate its potential to be developed as new antimicrobial or as combine therapy against E. faecalis. However, further research is needed for elucidation of main active compound and its mechanism of action.
Conflict of Interest
The authors have no conflict of interest to declare
Funding Sources
There are no funding sources.
References
- James S, Abate D, Abate K, Abay S, Abbafati C, Abbasi N et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2018;392(10159):1789-1858.
CrossRef - FDI World Dental Federation. The Challenge of Oral Disease. A call for global action. 2015; 2nd ed. Brighton, UK: Myriad Editions.
CrossRef - Castilho A, Silva J, Saraceni C, Díaz I, Paciencia M, Varella A et al. In vitro activity of Amazon plant extracts against Enterococcus faecalis. Brazilian Journal of Microbiology. 2014;45(3):769-779.
CrossRef - Bhardwaj P, Hans A, Ruikar K, Guan Z, Palmer K. Reduced Chlorhexidine and Daptomycin Susceptibility in Vancomycin-Resistant Enterococcus faecium after Serial Chlorhexidine Exposure. Antimicrobial Agents and Chemotherapy. 2017;62(1).
CrossRef - Kumar G, Jalaluddin M, Rout P, Monhanty R, Dileep C. Emerging Trends of Herbal Care in Dentistry. Journal of clinical and diagnostic research. 2013;7(8):1827-9.
CrossRef - Dubey R, Kumar R, Jaya, Dubey N. Evaluation of Eupatorium cannabinum Linn. oil in enhancement of shelf life of mango fruits from fungal rotting. World Journal of Microbiology and Biotechnology. 2006;23(4):467-473.
CrossRef - Louie K, Kosina S, Hu Y, Otani H, de Raad M, Kuftin A et al. Mass Spectrometry for Natural Product Discovery. Comprehensive Natural Products III. 2020:263-306.
CrossRef - England J. The Phenol Coefficient Method of Testing Disinfectants**Presented to the Pennsylvania Pharmaceutical Association, June, 1913. The Journal of the American Pharmaceutical Association.1913;2(8):955-958.
CrossRef - Pelczar J, Chan E, Krieg N. 2011. Microbiology – An Application Based Approach. New Delhi, India: Tata Mcgraw Hill Publishing Co Ltd. p. 211-215.
CrossRef - Wijaya C, Napitupulu F, Karnady V, Indariani S. A review of the bioactivity and flavor properties of the exotic spice “andaliman” (Zanthoxylum acanthopodium DC.). Food Reviews International. 2019;35(1):1-19.
CrossRef - Yu DQ, Han XJ, Shan TY, Xu R, Hu J, Cheng WX, Zha LP, Peng HS. Microscopic Characteristic and Chemical Composition Analysis of Three Medicinal Plants and Surface Frosts. Molecules. 2019;24(24):4548.
CrossRef - Veraverbeke EA, Verboven P, Van Oostveldt P, Nicolai BM. Prediction of moisture loss across the cuticle of apple (Malus sylvestris subsp. Mitis (Wallr.)) during storage: part 1. Model development and determination of diffusion coefficients. Postharvest Biol Technol. 2003;30:75–88. doi: 10.1016/S0925-5214(03)00083-8.
CrossRef - Narnoliya, L.K., Rajakani, R., Sangwan, N.S. et al. Comparative transcripts profiling of fruit mesocarp and endocarp relevant to secondary metabolism by suppression subtractive hybridization in Azadirachta indica (neem). Mol Biol Rep. 2014; 41:3147–3162.
CrossRef - He J, Liang YK. Stomata. Plant Science. 2018. DOI: 10.1002/9780470015902.a0026526
CrossRef - Kostina E, Wulff A. Growth, structure, stomatal responses and secondary metabolites of birch seedlings (Betula pendula) under elevated UV-B radiation in the field. Trees. 2001;15(8):483-491.
CrossRef - Kazusaki M, Ueda S, Takeuchi N, Ohgami Y. Validation of analytical procedures by high−performance liquid chromatography for pharmaceutical analysis. Chromatography. 2012;33(2):65-73.
CrossRef - Brookes Z, Bescos R, Belfield L, Ali K, Roberts A. Current uses of chlorhexidine for management of oral disease: a narrative review. Journal of Dentistry. 2020;103:103497.
CrossRef - Jaisinghani, R. N. Antibacterial properties of quercetin. Microbiology research. 2017;8(1): 6877
CrossRef - Baba S, Malik S. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. Journal of Taibah University for Science. 2015;9(4):449-454.
CrossRef - Yuan G, Guan Y, Yi H, Lai S, Sun Y, Cao S. Antibacterial activity and mechanism of plant flavonoids to gram-positive bacteria predicted from their lipophilicities. Scientific Reports. 2021;11(1).
CrossRef - Singh S, Konwarh R, Konwar B, Karak N. Molecular docking studies on analogues of quercetin with d-alanine:d-alanine ligase of Helicobacter pylori. Medicinal Chemistry Research. 2013;22(5):2139-2150.
CrossRef - Chinnam N, Dadi P, Sabri S, Ahmad M, Kabir M, Ahmad Z. Dietary bioflavonoids inhibit Escherichia coli ATP synthase in a differential manner. International Journal of Biological Macromolecules. 2010;46(5):478-486.
CrossRef - Feng, T., Xu, Y., Cai, X. H., Du, Z. Z., & Luo, X. D. Antimicrobially active isoquinoline alkaloids from Litsea cubeba. Planta medica. 2009;75(01):76-79.
CrossRef - Pratiwi, R. H., Oktarina, E., Mangunwardoyo, W., Hidayat, I., & Saepudin, E. (2022). Antimicrobial Compound from Endophytic Pseudomonas azotoformans UICC B-91 of Neesia altissima (Malvaceae). Pharmacognosy Journal, 14(1).
CrossRef - Sharifi-Rad, J., Kamiloglu, S., Yeskaliyeva, B., Beyatli, A., Alfred, M. A., Salehi, B., … & Martorell, M. (2020). Pharmacological activities of psoralidin: a comprehensive review of the molecular mechanisms of action. Frontiers in Pharmacology, 11, 571459.
CrossRef







