Manuscript accepted on :20-02-2023
Published online on: 18-05-2023
Plagiarism Check: Yes
Reviewed by: Dr. Dasari, Rajaneekar
Second Review by: Dr. Gavat Cristian
Final Approval by: Dr. Ian James Martin
Regina Tedjasulaksana1* and Ni Ketut Ratmini2
1Midwifery Department, Polytechnic of Health Denpasar, Bali, Indonesia
2Dental Hygienist Department, Polytechnic of Health Denpasar, Bali, Indonesia
Corresponding Author E-mail: reginatedjasulaksana@yahoo.co.id
DOI : https://dx.doi.org/10.13005/bpj/2675
Abstract
Kirinyuh leaves (Chromolaena odorata L.) are natural medicinal plants for wound healing. WHO (World Health Organization) recommends and concern about the safety of plant medicines. Due to limited toxicity study of Chromolaena odorata, this study aimed to determine the acute toxicity of the ethanolic extract of C. odorata in terms of LD50 and its effect on liver and kidney function of male white rats. The observed toxicity parameters were LD50 and delayed toxic effects for 14 days, including toxic symptoms, body weight, AST, ALT and creatinine test, liver and kidney histopathology. The study used 24 white male rats divided into 4 groups, namely the control group NaCMC 0.5%, the treatment group of C. odorata leaf ethanol extract 5 g/kg bw, 10 g/kg bw and 15g/kg bw with a single dose. The results showed that no white rats died during the 14 days of observation. Toxic symptoms that appear only white rats lack of appetite. Other parameter was body weight between days 1, 3, 7, 10 and 14 for each treatment group was significantly different and tended to decrease. The AST, ALT and creatinine values were not significantly different for all treatments. Histopathology of the liver and kidneys showed no abnormalities. Based on these results, the LD50 of kirinyuh leaf extract was 15 g/kg bw and delayed toxic effects in the form of lack of appetite which caused weight loss in all treatment groups.
Keywords
Chromolaena odorata; Delayed Toxic Effect; LD50; white rats
Download this article as:| Copy the following to cite this article: Tedjasulaksana R, Ratmini N. K. Toxicity Effects of Kirinyuh Leaf Ethanol Extract (Chromolaena Odorata L.) on White Rats (Rattus Novergicus). Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Tedjasulaksana R, Ratmini N. K. Toxicity Effects of Kirinyuh Leaf Ethanol Extract (Chromolaena Odorata L.) on White Rats (Rattus Novergicus). Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/3BConfj |
Introduction
Kirinyuh leaves (Chromolaena odorata L.) are natural medicinal plants for wound healing, cancer, diabetes, hepatotoxicity, inflammation, antimicrobials, and antioxidants1. Kirinyuh leaf (C. odorata L.) extract contains compounds such as flavonoids, tannins, antioxidants and saponins that can help the wound healing process2,3. The medicinal-activity plants should have low toxicity because of their long-term use in humans4. However, the toxicity study of Chromolaena odorata still limited, and WHO (World Health Organization) recommends and is concerned about the safety of plant medicines.
Acute toxicity test is a test on experimental animals to detect toxic effects after administration of a substance in a single dose or doses and observed for 14 days to obtain an LD50 value. LD50 is a quantitative benchmark that states the dose of a test substance that causes 50% death in experimental animals as an initial step for chemical and pharmacological toxicity screening of a test substance5,6,7,8.
Observations on experimental animals are carried out within 24 hours after giving the test material to the emergence of symptoms of poisoning such as convulsions, diarrhea, vomiting, shortness of breath and others, number of deaths, body weight and changes in the function of vital organs of the experimental animals. The living experimental animals were observed for a maximum of 14 days and then sacrificed for liver and kidney histopathological tests and serological tests in AST (aspartate aminotransferase), ALT (alanine aminotransferase), and creatinine9. This study aimed to determine the acute toxicity of the ethanolic extract of C. odorata in terms of LD50 and its effect on liver and kidney function of male white rats.
Material and Method
Plant material
C. odorata leaves test material was taken from Tabanan (Bali) to make ethanol extract of C.odorata leaves and fractionated with ethyl acetate. C. odorata leaves from Tabanan, Bali in Indonesia, show high content of tannins, flavonoids and antioxidants, which are needed in the wound healing process4.
C. odorata leaves are cleaned with water, then left until no water is attached. C odorata leaves finely chopped and dried at room temperature to become simplicia. 500 g of C. odorata simplicia was macerated with 2000 ml of ethanol for 72 hours, then filtered using Whatman No.1 filter paper. The filtrate was evaporated using a vacuum rotary evaporator at a temperature of 400C to obtain a crude extract of C. odorata leaves.
Animal preparation
Prepare samples or experimental animals, namely 24 adult male white rats, healthy and mature, 3 months old and weighing 150-180 grams with weight variations not exceeding 20% of the average body weight. Rats were adapted in cages and fed for one week. Rats fasted for 14 hours. Experimental Groups24 experimental animals were divided into one control group, namely NaCMC (sodium carboxy methyl cellulose) 0.5% and three treatment groups, namely ethanol extract of C. odorata leaves, at a dose of 5 g/kg body weight (bw), 10 g/kg bw, 15 g/kg bw.Determination of LD50 was conducted by observing the number of rat deaths for 14 days after administration of C. odorata leaf extract. For 14 days, toxic symptoms such as body weight, hair condition, salivation, appetite, respiration, vomiting, diarrhea, tremors and coma were also observed. The body weight of the white rats was observed on the 3rd, 7th, 10th and 14th days. Rats still alive after 14 days were anaesthetized with ketamine for liver and kidney harvesting for microscopic histopathological examination of the liver and kidneys and blood from the orbit for AST, ALT and creatinine examination.
Statistical analysis
The data were statistically tested with One Way Anova and Repeated Measures Anova in SPSS statistic. Statistical test results are significant if p <0.05.
Results and Discussion
Observations for signs of toxicity were carried out every 30 minutes after administration of the extract for four hours and then observed five times for 14 days. The results of the observation of the LD50 value in rats that had been given ethanol extract of C. odorata leaves showed that there was no mortality rate at the three levels of doses that had been given. In this study, an acute toxicity test was carried out where no animals died up to a dose of 15 g/kg bw. These results indicate that the ethanol extract of C. odorata leaves is practically non-toxic because it has an LD50 15 g/kg bw. This classification category is based on the toxicity of the test preparations for traditional medicines and foodstuffs, according to Hodge and Sterner 19959. The LD50 value obtained from the acute toxicity test results of the ethanol extract of C. odorata leaves in mice was 14.1416 g/kg bw or 28.82% of the extract and was included in the practically non-toxic category10. The smaller the LD50 value, the more toxic the compound is; the higher the LD50 value, the lower the toxicity9.
Table 1: LD50 of ethanol extract of C. odorata leaves in male white rats
| Dosage of test preparation | Number of rats | Number of deaths |
| NaCMC 0,5 % | 6 | 0 |
| 5 gr /kg bb | 6 | 0 |
| 10 gr / kg bb | 6 | 0 |
| 15 gr / kg bb | 6 | 0 |
Observations were taken on the first, third, seventh, tenth and fourteenth days. Symptoms of poisoning, such as seizures, diarrhea, vomiting and shortness of breath, were not found in male white rats as experimental animals during the study. White rats appeared to have decreased appetite in both the control and treatment groups (Table 2). This may be due to the stress factor such as treatment or the number of test materials given to the control and treatment groups. Rats feel unfamiliar with the intake of NaCMC, and the ethanol extract of C. odorata leaves is also not a daily food for rats. Acute stress in rats can cause appetite suppression. Stress causes the body to release stress hormones. This sudden surge of stress hormones has several physical effects, such as appetite suppression due to the release of corticotropin hormone (CRF), which affects the digestive system11-15
Table 2 : Behavior of white rats on the administration of 0.5% NaCMC and ethanol extract of C. odorata leaves
| Parameter | Observation | |||||||||||||||||||||||
| Day1 | Day3 | Day7 | Day 10 | Day14 | ||||||||||||||||||||
| C | P1 | P2 | P3 | C | P1 | P2 | P3 | C | P1 | P2 | P3 | C | P1 | P2 | P3 | C | P1 | P2 | P3 | |||||
| Fur and skin | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | ||||
| Salivation | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | ||||
| Vomit | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | ||||
| Appetite | A | A | A | N | N | N | N | N | A | A | N | A | A | A | A | A | N | N | N | N | ||||
| Breathing | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | ||||
| Urination | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | N | ||||
| Diarrhea | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | ||||
| Seizures and tremors | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | ||||
| Coma | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | ||||
| Dead | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | AS | ||||
N = Normal; C = NaCMC 0.5%. ; A =. Abnormal; P1 = Ethanol extract of C. odorata 5 g/kg bw; AS =. absence
P2 = Ethanol extract of C. odorata 10 g/kg bw; P3 = Ethanol extract of C. odorata 15 g/kg bw
The results of the One Way Anova test in this study showed that the average body weight of male white rats was not significantly different (p> 0.05) between the NaCMC group, C. odorata leaf extract 5 g/kg bw, 10 g/kg bw and 15 g/kg. Weight on day 1, day 3 (p=0.967), day 7 (p=0.944), day 10 (p=0.688) and day 14 (p=0.487) as shown in table 3. This shows that the body weight of rats for the control and treatment groups did not differ on the day of observation on the 1st, 3rd, 7th, 10th and 14th days.
Table 3 : Differences in Mean Body Weight of White Rats on Days 1, 3, 7, 10 and 14 Between Treatment Groups
| Day | NaCMC 0,5 % | C. odorata
5 g /kg bw |
C. odorata
10 g / kg bw |
C. odorata
15 g / kg bw |
Sig |
| Day 1 | 165.00 ± 5,55 | 170.33 ± 14.91 | 170.67 ± 18.50 | 170.67 ± 11.06 | 0.854 |
| Day 3 | 162.33 ± 7.1 | 165.67 ± 13.88 | 163.67 ± 19.30 | 165.33 ± 7.22 | 0.967 |
| Day 7 | 160.67 ± 8.14 | 159,00 ± 14,37 | 163.50 ± 18.10 | 161.00 ± 7.87 | 0.944 |
| Day 10 | 152.50 ± 9.09 | 150.33 ± 13.20 | 159.17 ± 21.08 | 156.33 ± 6.77 | 0.688 |
| Day 14 | 147.00 ± 1.87 | 147.50 ± 9.18 | 157.00 ± 20.34 | 154.67 ± 6.09 | 0.487 |
The results of the Repeated Measures Anova test on the body weight of white rats on the 1st, 3rd, 7th, 10th and 14th days for each group were found to be significantly different (p<0.05). Viewed from Graph 1, it can be seen that there was a decrease in body weight for each treatment and control group. So weight loss occurred in each group, and the results of the Pairwise Comparison test showed that the difference in body weight was not more than 20%. Indications of experimental animals experiencing pain or suffering are generally when their body weight has decreased by more than 20%, or body weight has decreased by more than 25% for seven days or more17. This means that the weight loss of mice in this study was not indicated by pain or suffering due to the administration of C. odorata leaf extract. Weight loss may be due to decreased appetite for white rats due to treatment given in large doses, causing discomfort to the digestive system. The occurrence of body weight loss in a day that does not reach 5% does not affect the behavior of the test animals.
Table 4 : Differences in Average Body Weight of White Rats on Days 1, 3, 7, 10 and 14. In Each Treatment Group
| Treatment | Weight | Sig | ||||
| Day 1 | Day 3 | Day 7 | Day 10 | Day 14 | ||
| NaCMC 0.5% | 165.00 ± 5.55 | 162.33 ± 7.71 | 160.67 ± 8.14 | 152.50 ± 9.09 | 147.00 ± 1.87 | 0.008 |
| Ethanol extract of C. odorata 5 g/kg bw | 170.33 ± 14.91 | 165.67 ± 13.88 | 159.00 ± 14.37 | 150.33 ± 13,20 | 147.50 ± 9.18 | 0.000 |
| Ethanol extract of C. odorata 10 g/kg bw | 170.67 ± 18.50 | 163.67 ± 19.30 | 163.50 ± 18.10 | 159.17 ± 21.08 | 157.00 ± 20.34 | 0.003 |
| Ethanol extract of C. odorata 15 g/kg bw | 170.67 ± 11.06 | 165.33 ± 7.22 | 161.00 ± 7.87 | 156.3 ± 6.77 | 154.67 ± 6.09 | 0.034 |
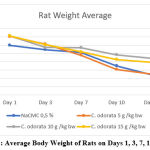 |
Graph 1: Average Body Weight of Rats on Days 1, 3, 7, 10 and 14 |
Serum levels of AST, ALT, and creatinine were not significantly different (p>0.05) between the control rat group and the group of rats given ethanol extract of C. odorata leaf 5 g/kg bw, 10 g/kg bw and 15 g/ kg bw, indicating that the ethanol extract of C. odorata leaves does not contain compounds that cause toxic effects. The liver is the largest organ and is metabolically the most complex because it is involved in the metabolism of nutrients and most drugs and toxicants18. The liver has AST and ALT enzymes, which function as biomarkers or markers of liver disorders. A very high increase in ALT enzyme levels accompanied by an increase in AST enzymes is an indicator that indicates hepatocellular changes,19. Urine is the main route of excretion of most toxicants. Creatinine is a strong indicator of kidney function, an increase of twice the normal plasma level indicates a 50% decrease in kidney function20. Histopathological showed the proportion of hydrophic degeneration is < 25%, categorized normal category (figure 1), cells appear paler than normal cells, and there are water-containing vacoules in the cytoplasm, but the proportion of damage has not reached 25% in field of view and categorized as normal.
Table 5 : Differences in Mean AST, ALT and Creatinine Between Groups of 0.5% NACMC, C. odorata leaf extract 5 g/kg bw, 10 g/kg bw and 15 g/kg bw
|
NaCMC |
C. odorata extract 5 g /kg bw | C. odorata extract 10 g/ kg bw | C. odorata extract 15 g/kg bw |
Sig |
|
| AST (u/l) | 161.33 ± 11.77 | 174.00 ± 38.55 | 160.83 ± 15.83 | 191.67 ± 34.26 | 0.214 |
| ALT (u/l) | 93.17 ± 35.71 | 92.33 ± 13.31 | 94.33 ± 10.67 | 70.50 ± 11.59 | 0.172 |
| Creatinine (mg/dl) | 0.90 ± 0.09 | 0.87 ± 0.08 | 0.87 ± 0.14 | 0.82 ± 0.12 | 0.616 |
The results of histopathological observations of liver tissue structure in the control group and the treatment of the ethanol extract of C. odorata leaves were in normal conditions. The liver is a very important organ and has various metabolic process functions, so this organ is often exposed to chemicals. These substances will undergo detoxification and inactivation to become harmless to the body21. The results of the histopathological examination of the kidney tissue structure of white rats in the control and treatment groups were normal. Kidneys are organs that have an important role in the body, this organ functions to remove metabolic waste and body toxins in the form of urine. The kidney is an organ of the body that is susceptible to the influence of chemical substances because the kidneys work in the rest of the metabolic products of the blood, so the possibility of pathological changes is very high22. However, the ethanolic extract of C. odorata leaves has the potential to protect kidney tissue from antioxidant damage that may be caused by plant bioactive agents such as flavonoids, which increase antioxidant activity and protect the kidneys against oxidative stress and damage4,23 Shown histopathological features include a glomerular shape that looks normal on all sides so that the capsular space is also clearly visible.
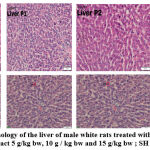 |
Figure 1: Histopathology of the liver of male white rats treated with 0.5% NaCMC, C. odorata leaf extract 5 g/kg bw, 10 g / kg bw and 15 g/kg bw ; SH : Hepatocyte cell |
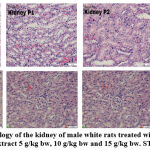 |
Figure 2 : Histopathology of the kidney of male white rats treated with 0.5% NaCMC, C. odorata leaf extract 5 g/kg bw, 10 g/kg bw and 15 g/kg bw. ST: tubular cell. |
Conclusion
This study shows that the ethanol extract of C. odorata leaves is practically non-toxic when administered acutely due to LD50 value is 15 g/kg bw. In this study, delayed toxic effects observed in white rat body weight tended to decrease for all treatment groups. Further studies are needed to evaluate long-term toxicity and improve the evidence.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper. We certify that the submission is original work and is not under review at any other publication.
Funding Source
This study was supported by the Polytechnic of Health Denpasar, Board for Development and Empowerment Human Resources of Health – The Ministry of Health Republic Indonesia, grant number HK.02.03/WD.1/910/2022.
References
- Sirinthipaporn, A, Jiraungkooskul, W. Wound Healing PropertyReview of Siam Weed, Chromolaena odorata. Pharmacogn Rev, 2017 Jan-Jun; 11(21): 35–38
CrossRef - OmokhuaG, McGawL.J, Finnie J.F, Staden, J.V. Chromolaena odorata (L.) R.M. King & H. Rob. (Asteraceae) in sub Saharan Africa: a synthesis and review of its medicinal potential. Journal of Ethnopharmacology, 2016 ; 183 : 112-122
CrossRef - Tedjasulaksana R, Nahak M.M, Ratmini, N.K. Studi Kualitatif dan Kuantitaf Fitokimia Ekstrak Air dan Ekstrak Etanol Daun Kirinyuh (Chromolaena odorata L.) yang Tumbuh di Provinsi Bali. Intisari Sains medis, 2022 ; Volume 13, Number 1 : 70-74
CrossRef - Pandhare, R.B., Santosh N Belhekar, S.N., Balakrishnan, S., Mohite, P.B. , Khanage, S.G., Deshmukh, V.K. Assessment of Acute and 28-Day Sub-Acute Oral Toxicity of a Polyherbal Formulation in Rats. Int J Pharmacogn Chinese Med 2020, 4(1): 000200.
CrossRef - Alelign T, Chalchisa D, Fekadu N, Solomon D, Sisay T, Debella A, Petros Evaluation of acute and sub-acute toxicity of selected traditional antiurolithiatic medicinal plant extracts in Wistar albino rats. Toxicol. Rep., 2020; 7: 1356–1365
CrossRef - Zhang Y, Tian R, Wu H, Li X, Li S, Bian LEvaluation of acute and sub-chronic toxicity of Lithothamnion sp. in mice and rats. Toxicol.Rep., 2020; 7: 852–858
CrossRef - Ebbo A.A, Sani D, Suleiman M.M, Ahmad A, Hassan A.Z. Acute and sub-chronic toxicity evaluation of the crude methanolic extract of Diospyros mespiliformis hochst ex a. Dc (ebenaceae) and its fractions. Toxicol. Rep., 2020; 7: 1138–1144
CrossRef - Eweka SDEO, Orhue N.E.J, Omogbai E.K.I, Amaechina F.C. Oral acute and sub-chronic toxicity assessment of aqueous leaf extract of Simarouba glauca DC (Paradise tree) Toxicol. Rep,. 2021; 8: 239–247.
CrossRef - Badan pengawas Obat dan Makanan Republik Indonesia. 2014. Pedoman Uji Toksisitas Non Klinik Secara In Vivo
- Jumain, Syahruni, Farid. 2018. Uji Toksisitas Akut dan LD50 Ekstrak Etanol Daun Kirinyuh (Euphatorium odoratum Linn) Pada Mencit (Mus musculus). http://journal.poltekkes- mks.ac.id/ojs2/index.php/mediafarmasi/article/view/82
CrossRef - Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annual review of physiol- ogy. 2005; 67:259–84.
CrossRef - Radahmadi M, Izadi MS, Rayatpour A, Ghasemi M. Comparative Study of CRH Micro injections Into PVN and CeA Nuclei on Food Intake, Ghrelin, Leptin, and Glucose Levels in Acute Stressed Rats.Basic Clin Neurosci. 2021;12(1):133-148. doi:10.32598/bcn.12.1.2346.1
CrossRef - Corwin RL, Avena NM, Boggiano M. Feeding and reward: Perspectives from three rat models of binge eating. Physiology & Behavior , 2011 ; Volume 104, Issue 1 ; 87-97
CrossRef - Klazkin R.R, Baldassaro A, Rashid S. Physiological responses to acute stress and the drive to eat: The impact of perceived life stress. Appetite, 2019 ; 133; 393-399
CrossRef - Schamer S, Stengel A. Animal models for anorexia nervosa-a systematic review. Front Hum Neurosci , 2021; 14 : 596381
CrossRef - Nurfaat D. L. Uji Toksisitas Akut Ekstrak Etanol Benalu Mangga (Dendrophthoe petandra) Terhadap Mencit Swiss Webster. Indonesian Journal of Pharmaceutical Science and Technology, 2016; 3(2), 53–65.
- Contreras-Zentella, M.L and Hernández-Muñoz, R. Is Liver Enzyme Release Really Associated with Cell Necrosis Induced by Oxidant Stress? Oxidative Medicine and Cellular Longevity Volume 2016, Article ID 3529149, 12 pages http://dx.doi.org/10.1155/2016/3529149
CrossRef - Baynes J.W, Dominiczak M.H. Medical Biochemesttry. 2014 P: 317. Fourth Philadelphia-Elsvier- Saunders.
- Huether S.E, Mc.Cance KL Understanding Pathophysiology. Chapter 28 : Structure and Function of the Renal and Urologic System. 2012.Page : 737 Fifth edition. USA : Elsevier Mosby
- Dutta S, Mishra S.P, Sahu A.K, Mishra K, Kashyap P, Sahu, B. Hepatocytes and Their Role in Metabolism. 2021 DOI: 10.5772/intechopen.99083 https://www.intechopen.com/chapters/78184
CrossRef - Radi, Z.A.Kidney Pathophysiology, Toxicology, and Drug-Induced Injury in Drug Development . International Journal of Toxicology, 2019 ;Vol. 38(3) 215-227
CrossRef - sunobun U, Inuumoru C.S, Onyeaghor C, Lawuru D, Egbo H. Nephroprotective Potential of Chromolaena odorata (L.) R.M. King & H. Rob. on Methotrexate-Induced Kidney Damage and Oxidative Stress. Journal of Research in Applied and Basic Medical Sciences, 2022; 8(3): 118-127
CrossRef







