Manuscript accepted on :24-06-2023
Published online on: 05-07-2023
Plagiarism Check: Yes
Reviewed by: Dr. Omage, Joel and Dr. Feng Li
Second Review by: Dr. Hind Shakir
Final Approval by: Dr. Patorn Piromchai
Oral Medicine and Orofacial Pain, College of Dentistry, Prince Sattam Bin Abdulaziz University, Al Kharj, Riyadh, Saudi Arabia
Corresponding Author E-mail: a.binnabhan@psau.edu.sa
DOI : https://dx.doi.org/10.13005/bpj/2666
Abstract
Background: It is a widely held belief that if the trigeminal nerve is damaged, the victim would experience agonising and unrelenting external pain. A lesion to the trigeminal nerve may have a wide-reaching effect, such as on one side of the face in particular, or it might have a more localised effect, such as on some or all of your gums. The risk of damage increases the likelihood that it will be difficult to speak and swallow. This nerve provides sensation to a part of your face that may be constantly aching or tingling for some people. However, the trigeminal nerve injury-related persistent orofacial pain might be brought on by a wide variety of unknown triggers. Aim: In this study investigate the clinical manifestations of chronic orofacial pain brought on by a damage to the trigeminal nerve, as well as the diagnostic and therapeutic approaches available to treat this condition. Methodology Through the use of search phrases such as "Trigeminal nerve injury," "Trigeminal ganglion," "Trigeminal spinal subnucleus caudalis," "Craniofacial pain," "Oral prognosis," and "treatment," the computerised databases for the last twenty years have been investigated. There are now two hundred objects in total that have been accumulated. There have been around fifty of them that are pertinent to the discussion that is going on in this work. Majority of the patients fair enough with the pharmacology treatment/drugs like the carbamazepine & oxcarbazepine which forms the first line treatment options followed by lamotrigine & baclofen encompassing the second line of drugs along with adjuvant drug support of topiramate, levetiracetam, gabapentin, pregabalin. As the field of science has explored &advanced for the latest treatment options include microvascular decompression, gamma knife radiosurgery, percutaneous rhizotomies variable based on the evidences & guidelines 54 Conclusion: New diagnostic criteria and treatment alternatives have become available for people who suffer from trigeminal neuropathy and orofacial neuropathic pain as a result of recent developments in fundamental animal research that have led to their development. Despite the results, more research needs to investigate a greater variety of distinct non-neuronal cell feature approaches.
Keywords
Craniofacial Pain; Trigeminal Ganglion; Oral Diagnosis Treatment; Trigeminal Nerve Injury; Trigeminal Spinal Subnucleus Caudalis
Download this article as:| Copy the following to cite this article: Nabhan A. B. Pathophysiology, Clinical Implications and Management of Orofacial Neuropathic Pain- with Special Attention to Trigeminal Neuralgia: A Narrative Review. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Nabhan A. B. Pathophysiology, Clinical Implications and Management of Orofacial Neuropathic Pain- with Special Attention to Trigeminal Neuralgia: A Narrative Review. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/442Z5DP |
Introduction
When a peripheral nerve is destroyed, injured nerve fibres produce high-frequency injury discharges. These high-frequency unfavourable discharges in primary afferent neurons increase the amount of nociceptive neurons in the spinal dorsal horn, which are then sent to the important frightened machine through dorsal root ganglion neurons 1. Chronic, excruciating pain in the orofacial region is caused by negative discharges from trigeminal ganglion (TG) neurons in the trigeminal system being received by the upper cervical spinal cord (C1-C2) and the trigeminal spinal subnucleus caudalis (Vc) 2. At the same time as nociceptive TG, Vc, and C1-C2 neurons are becoming hyperactive, non-neuronal glial cells and macrophages are becoming activated and accumulating in these areas. Increased macrophage awareness and activation of non-neuronal glial cells are assumed to be supplied by neuron-non-neuronal cellular interaction, which also complements the activity of neuronal cells 3. . It is known fact that activated satellite tv for pheochromocytoma cells (PC cells), microglial cells and accumulating macrophages in the Vc and C1-C2 regions release a variety of cytokines, neurotrophic factors, and tumour necrosis factors 4, 5. These interactions between neurons and non-neuronal cells thought to enhance the spread of neuronal activity throughout the TG, ventral C Vc, and C1-C2 regions 6.
In addition, the interaction between astrocytes and microglia within the C1-C2 and Vc areas is thought to promote the spread of the excitability of nociceptive neurons 7. Neurons in the brain stem convey additional negative data to locations higher up in the central nervous system 8,9. Recent investigations have shown that injury to the trigeminal nerve alters the manner in which these ascending routes work. In this investigation, we investigate fresh data from animal studies on the properties of materials associated to neuron-non-neuronal cellular communication. In addition, we discuss the role that ascending pain pathways may play in the interpretation of orofacial pathological pain brought on by injury to the trigeminal nerve. In addition, we provide the clinical results of patients who have trigeminal neuralgia and orofacial neuropathic pain, as well as their clinical approaches to diagnosing and treating patients who have trigeminal neuralgia.
Orofacial Neuropathic Pain Peripheral Methods
Damage to the trigeminal nerve may cause a variety of painful pain reactions, including persistent orofacial pain, allodynia, and hyperalgesia. These phrases are used to characterise the types of pain allergy reactions that might occur 5. When peripheral nerves are damaged, a cellular response may be triggered by a number of substances that are present at the location of the lesion. An alternative inside the immune cells environment first sets off the cascade of events that leads to the inflammatory response that occurs when peripheral nerves are wounded 9. Damaged neurons and Schwann cells secrete inflammatory chemicals, which causes the peripheral nerve to become hypersensitive at the same time as immune cells infiltrate the area of the lesion. This makes it possible for the peripheral nerve to become hypersensitive 10. In addition, the injured neurons show changes in their neuronal excitability, such as a lower threshold and more spontaneous firing 11. To put that into perspective, proinflammatory mediators such as tumour necrosis factor-alpha (TNF) and nerve growth factor are found in higher amounts near the locations of peripheral nerve damage 12. TNF-induced neuronal hyperexcitation is due to the upregulation of voltage-gated sodium channels’ excitatory potential via the activation of protein kinase C. According to Leo et al.’s research from 2015, the binding location for TNF is the TNF receptor, also known as TNFR, which may be present in healthy nerve terminals 13.
Due to more appropriate artemin signalling, TRPV1 is overexpressed in tongue nociceptors. This enhances heat hypersensitivity response inside the tongue via the activation of p38 mitogen-activated protein kinase 14. In the aftermath of a lesion to a peripheral nerve, it is well knowledge that a diverse population of macrophages originating from monocytes will enter the affected area. This blood-borne macrophage buildup, which takes place specifically near wounded axons, is caused by monocyte chemoattractant protein-1 (MCP-1) signalling, which determines the course of neuropathy15. It has been cautioned that damaged to peripheral nerves leads to consequences of macrophage infiltration and recruitment to the injury site, which in turn releases insulin-like boom aspect-1 (IGF-1).IGF-1which is launched by way of the macrophages on the web site of nerve injury, communicates with Transient Receptor Potential Vanilloid (TRPV2) to inspire Transient Receptor Potential Vanilloid (TRPV4) manufacturing in the TG neurons that innervate the facial skin. 16] As an end result, the pores and skin on the face turns into routinely sensitive following peripheral nerve harm, the broken axons, the encroaching macrophages, and the Schwann cells all release materials that stimulate non-injured axons. It’s miles viable that stimulation of axons that aren’t broken is what reasons the peripheral neuronal hyper-excitability this is so carefully related to neuropathic pain 17.
Trans-cellular Interaction with both Inflammatory Cells and Neurons in TG
It is well knowledge that the TG harbours primary afferent neuron somata and that lymphocytes, macrophages, and satellite glial cells, all of which communicate with one another via the use of neurotransmitters, surround the sensory neuronal soma 8. According to the findings of recent study, nerve damage may trigger mobile-to-mobile communication in TG by utilising humoral chemicals like as cytokines, neuropeptides, and fuel. It is quite interesting to look at how macrophages cluster together in the TG, which contains the somata of the wounded primary afferent neurons and the area of peripheral nerve injury 17. Cells that cause inflammation, such as macrophages, are able to enter damaged trigeminal nerves and then cause a more rapid inflammatory response. In a variety of orofacial clinical circumstances, both invading and resident macrophages experience certain morphological changes, such as thicker ramifications and a bigger soma, that speed up the release of a variety of neurotransmitters 10. Consequently, changes in morphological appearance are assumed to indicate that macrophages have been active. In addition, macrophages may be subdivided into two distinct histological types based solely on the distinct tasks that they provide in the body. During the first phases of an anti-inflammatory response, a significant amount of reliance is placed on the M1 macrophage. The M1 macrophage is a classically activated phenotype that is responsible for the production of a variety of pro anti-inflammatory mediators. The M2 macrophage is the second kind, and it is an alternatively activated phenotype of macrophage. These macrophages produce a range of proinflammatory mediators, and their features and linkages to tissue healing are discussed below 8.
The TG is able to govern the excitability of TG neurons by means of trans-cell communication between neurons and macrophages via the use of a variety of biochemical mediators 19. Following an injury, macrophages migrate into the trigeminal nerve and begin releasing TNF into the TG. According to the previous studies 5,18-21 the aetiology of orofacial neuropathic pain is a TNF signalling consequence known as a neuronal hypersensitive response in the trigeminal ganglion.
Inter-Satellite Cell Communication in Trigeminal ganglion
Recent research has shown that the primary cause of neuronal hyperactivity is morphological and functional changes in satellite cells, which are brought on by peripheral nerve damage. These changes include enlargement of the soma and shortness of the techniques. In addition, satellite cells are able to interact with one another via the use of gap junctions, which make it possible for a wide variety of chemicals to move freely across cells 22. According to research by Kaji et al. (2016), the damage of the inferior alveolar nerve in TG leads to morphological abnormalities in satellite glial cells. These abnormalities are the primary cause of increased orofacial mechanical allergy. These results suggest that Cx43 causes activation of satellite to for pc glial cells in the TG, hence increasing the trigeminal neuronal excitability and serving as a significant contribution to ectopic orofacial pain. Therefore, ectopic or extraterritorial pain hypersensitivity, which is caused by non-neuronal mobile methods inside the TG, might be the consequence of peripheral nerve injury within the orofacial area. Orofacial ectopic pain is a common condition that is often misdiagnosed as dental pain. This error in diagnosis may lead to unnecessary and irreversible dental procedures such as pulpotomies and tooth extractions. In addition, knowledge of these processes may significantly help in the management of orofacial neuropathic pain and prevent the patient from receiving an incorrect diagnosis from a medical professional who is treating orofacial neuropathic ache 18.
Mechanisms of the Brainstem and Cervical Spinal Cord
The trigeminal spinal nucleus is an intensive shape made from the caudalis, interpolaris, and oralis subnuclei. Vc shares a layered structure with the spinal cord and takes up most of the trigeminal spinal nucleus. The Vc and the C1-C2 acquire nociceptive enter from the craniofacial location thru primary afferent fibres with somata in TG. Projective neurons inside the trigeminal nerve’s branches convey nociceptive information from the craniofacial area 23.
The higher CNS areas still have the ability to get poor signals from the neurons of the brainstem. The MT, the PBN, and the posterior medial thalamic nucleus are three essential parts of the CNS that are responsible for orofacial pathological pain and receive noxious inputs from the Vc and C1-C2 regions 24 . It is believed that the orofacial noxious route that projects to ventral posterior medial (VPM) is responsible for mediating the sensory discriminative aspect of pain. On the other hand, it is considered that the MT and PBN pathways have an influence at the motivational and emotional elements of aching 24. This theory was presented in a study that was published in 2021. These ascending routes have recently undergone some practical alterations as a result of damage to the trigeminal nerve 23,25. According to the findings of an increasing number of research conducted in recent years, the glial cells of the central anxious system play an essential role in initiating an extension of alterations in neuronal interest in the Vc after injury has been caused to the trigeminal nerve. Microglia and astrocytes, two of the most important contributors to neuropathic pain, become active when there is irritation in the orofacial region or injury to the trigeminal nerve 26.
It is well accepted information that microglia become activated during the early stages of nerve damage, which may be recognised as astrocytic activation. The immune cells known as microglia, which are similar to macrophages, are responsible for the production of a number of proinflammatory cytokines. Some examples of these cytokines include interleukin (IL)-1, IL-6, and TNF. IL-1 is one of these cytokines, and it is the one that phosphorylates N-methyl-D-aspartate (NMDA) receptors in order to accomplish its goals 27,28 . Mind-derived neurotrophic factor, or BDNF for short, is an additional microglial secretory factor that plays an important role in neuropathic pain. During the time when BDNF is binding to tropomyosin-associated kinase B (TrkB), the expression of the k+-Cl- cotransporter, potassium chloride cotransporter KCC2, is decreasing.
When the Cl- concentration within the cell becomes too high, the Cl- inflow mediated by GABA receptors switches to Cl- efflux, triggering an excitatory response (Hildebrand et al., 2016). [29]. As a result, Brain-derived neurotrophic factor BDNF can have an effect on the effect of the GABAergic machine further to regulating excitatory transmission by way of lowering inhibition or maybe switching from excitation to inhibition.
Consequences showed that pharmacological inhibition of 6GABAA receptors, a subunit of GABA receptors, decreased the raised pain threshold in continual constriction damage (CCI) version mice, suggesting that GABAergic disinhibition is likely to be a sizeable contributor to neuropathic ache 26. Through the downstream signaling associate of TrkB, Fyn kinase, microglial-secreted BDNF can phosphorylate GluN2B 29 Furthermore, it promotes glutamate release with the aid of activating NMDA receptors in number one afferent neurons’ presynaptic terminals 30. more recently, it’s been proven that neuropathic pain reasons lengthy-term potentiation in the spinal cord due to the fact BDNF, that’s produced with the aid of microglia, will increase the number of synaptic terminals in number one sensory fibers which can be high quality for CGRP 31. The potential to regulate both excitatory and inhibitory neurotransmission is furnished by BDNF. In addition to trigeminal nerve damage, extra situations would possibly motive microglia to end up active. Fractalkine is produced on the membrane surface of neurons by using the lysosomal cysteine protease cathepsin S, that’s secreted by means of microglia. Fractalkine binds to the CX3CR1 receptor, which is best expressed in microglia, and keeps these cells in an activated country32 . The extended launch of inflammatory cytokines by way of microglia consequences in painful infection. it is widely regularly occurring that microglia have a role inside the improvement of neuropathic ache. The microglial activation inhibitor minocycline, however, has little effect on pain that is already gift. This can be resulting from the section shift in glial activation that occurs after nerve harm.
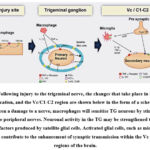 |
Figure 1: Following injury to the trigeminal nerve, the changes that take place in the TG, the wounded location, and the Vc/C1-C2 region are shown below in the form of a schematic. |
It is well knowledge that astrocytes may influence the physiological activity of neurons in the following way: the glutamine that is generated by astrocytes can be transformed into glutamate in the presynaptic terminal. Glutamate is a key component of excitatory neurotransmission. According to Allen and Eroglu (2017), adjacent astrocytes throughout the entire junction work together to coordinate their activity as neuronal assemblies 1. Carbenoxolone is a gap junction blocker, while methionine sulfoximine is a glutamine synthetase inhibitor. Both of these medications significantly reduce the nociceptive behaviour that is brought on by pulpitis and infraorbital nerve transection 33. The following are some examples of how the neurotransmitter D-serine, which is produced by astrocytes, is connected with orofacial pain: According to Zhou LJ et al.’s research from 2019, the co-agonist D-serine works on NMDA receptors to increase C-fiber-mediated long-time period potentiation 34 . Orofacial pain is caused by glial cells’ inappropriate manipulation of neuronal characteristic, which also helps to maintain its level of intensity (Figure 2).
 |
Figure 2: After damage has been done to a peripheral nerve, microglia and astrocytes are shown to become activated inside the Vc. Microglia and astrocytes may be seen in the visual cortex of both normal rats and rats with IONI in the images. |
When the primary afferent neurons of the trigeminal nerve are compromised, such as when the inferior alveolar nerve, mental nerve, or infraorbital nerve is cut, satellite glial cells inside the TG become activated. Extra activation of microglial cells and astrocytes is detected, leading to hyper-activation of Vc and C1-C2 nociceptive neurons 35. In addition to IL-1, IL-1, and IL-6, other cytokines and bio-energetic chemicals have a role in regulating microglial activity in the central nervous system. Patients who were given minocycline reported less orofacial neuropathic pain and a restoration of normal brain activity after partial infraorbital nerve ligation 35. As a result, minocycline may soon be used to treat orofacial neuropathic pain. Research into treatment options for orofacial neuropathic pain should also concentrate on creating therapies with minimal adverse effects.
Diagnosis of Orofacial Neuropathic Pain
In step with the worldwide class of Orofacial pain (ICOP), orofacial neuropathic ache is “Orofacial pain because of lesion or contamination of the cranial nerves”[36].Orofacial neuropathic pain poses particular problems in assessment to neuropathic ache inside the spinal wire. The orofacial region’s anatomical limitations and associated scientific speciality demarcations, according to the international Headache Society, are a contributing element inside the problem 36. as an instance, it may be difficult to make a proper diagnosis and determine on the exceptional direction of remedy due to the fact the symptoms of neuropathic ache within the orofacial area regularly resemble the ones of odontogenic toothache [36,37]. Many non-odontogenic toothaches are introduced on by way of the complexity of the orofacial vicinity, which includes numerous structures innervated via the trigeminal machine, head, sinus, masticatory muscle groups, temporomandibular joint, jaw, enamel, and gingiva 38. Moreover, it is difficult to degree orofacial discomfort due to the fact it’s miles subjective. To avoid pointless dental treatment, it’s far important to discover the true supply of the ache.
The mandibular nerve, the 0.33 department of the trigeminal nerve, is the web page of the most not unusual kind of TN. in the enamel, paroxysmal ache is regularly felt and can be an illustration of endodontic pain and necessitate useless endodontic remedy 2. The history of the affected person and the patient’s clarification in their symptoms are used to make the TN analysis. As a result, with a view to make a clear prognosis, a lengthy verbal exchange with the affected person is required. TN pain is characterized by using brief—up to 2 min long—acute, abrupt, shooting ache bursts that seem electric surprise-like. Whether or not consuming, brushing one’s enamel, talking, or using makeup, these may be slightly stimulated orofacially. In conventional TN, blood vessels at the brain stem’s root front region constantly squeeze the trigeminal nerve. Subsequently, to diagnose TN, investigations and in-intensity interviews are needed. A few examples encompass the evaluation of the cranial nerves, mind MRIs, and brain magnetic resonance angiography (MRA). One normal type of chronic neuropathic pain is put up-annoying trigeminal neuropathy, though a few instances can be episodic and ultimate anywhere from a couple of minutes to numerous days. The orofacial place may be impacted with the aid of third molar extractions, implants, root canal therapy, orthognathic surgical procedure, and facial fractures. The ache is generally defined as a consistent burning and/or taking pictures sensation on the injury web page or in the distal dermatome of the injured nerve. Early on, odontogenic ache is generally fallacious for intra-oral submit-disturbing trigeminal neuropathic ache 39
On account of the whole trauma history and the typical publish-worrying trigeminal neuropathic pain symptoms, a specific diagnosis is likewise made. Therefore, as a way to diagnose submit-traumatic trigeminal neuropathic ache, it’s miles critical to behavior chairside sensory trying out, such as quantitative sensory testing (QST), and to be aware about the patient’s particular scientific records 40, 41. Put up-demanding trigeminal neuropathic pain is defined as “unilateral or bilateral facial or mouth pain following and prompted through trauma to the trigeminal nerve(s), with associated signs and symptoms and/or scientific proof of trigeminal nerve dysfunction, and persisting or recurrent for greater than 3 months” 36.
Trigeminal post herpetic neuralgia is some other not unusual example of persistent neuropathic ache. It’s far a herpes zoster (HZ) sickness that developed from trigeminal neuropathic pain delivered on by the virus. This pain is generally misdiagnosed as a toothache within the early stages and outcomes in pointless dental remedy 42. No matter the lesions healed after some months, the infection by HZ reasons a intense trigeminal neuropathy. Typically, 10 to fifteen percent of HZ patients will expand trigeminal post herpetic neuralgia. More than 50% of those over 60 go through with trigeminal post herpetic neuralgia. Its scientific capabilities include allodynia, hyperalgesia to mechanical and thermal stimuli, and pain that is scorching, capturing, or electric shock-like those functions are also present in post-worrying trigeminal neuropathic pain. As a result, to make the diagnosis of trigeminal post herpetic neuralgia, a whole affected person records and sensory evaluation are wished. Notwithstanding the rarity of these latter neuropathic lawsuits, a thorough analysis method is wanted36.
Neuropathic pain outcomes in ectopic or extraterritorial pain while nerves within the spinal area and orofacial place are damaged. But, because dental pain is frequently confused for the referred pain inside the orofacial location, useless and irreversible dental operations like pulpotomies or teeth extractions are carried out. Even though the neuronal convergence and sensitization hypotheses had been used to give an explanation for referred pain, it’s miles hard to define all the events which might be induced by way of referred pain43. As already indicated, the mechanism of referred ache has lately been attributed to a diffusion of molecular and mobile changes in peripheral, imperative, neuronal, and non-neuronal cells. Medicinal experiments the use of those cells’ inhibitors and modulators have produced a few encouraging outcomes, even though the tiers of efficacy are still inconsistent 30. As these medicines broaden, the management of orofacial neuropathic ache and warding off beside the point remedy may also end up much less complicated for the practitioner.
Clinical implication of trigeminal neuralgia:
The clinical aspect includes spontaneously pain experienced along the upper jaw or lower jaw assuming that it may due to dental abscess or cyst & going for the endodontic treatment or extraction. But when the pain persist even after the dental treatment then they realized it is due to some other reason. The character of pain is intensely sharp, stabbing sporadic burning or shock like pain around the eyes lips nose jaw forehead & scalp. This pain runs in cyclic manner with frequent attacks occurring by weeks, months or even years. The pattern od pain typically begins with sensation electrical shock that culminates in an excruciating stabbing pain within 30 seconds accompanied facial twitch focusing at on point r spreading type affecting one face unilaterally. The triggering factor include: touching the skin, washing, shaving, brushing, smiling, talking, encountering a breeze of air,
Differential diagnosis: there are few lesion which can show overlapping features similar to trigeminal neuralgia that includes cluster headaches, giant cell arteritis, dental pain, post herpetic neuralgia, sinus pain, ear infection, temporomandibular joint syndrome, glossopharyngeal neuralgia, eagle syndrome.
Clinical Management of Pain in the Orofacial Region
In comparison to other neuropathic pain syndromes, such as post-herpetic neuralgia, severe diabetic neuropathy, and painful spinal stressful neuropathy, which have a pharmaceutical response charge of 20–forty%, Painful traumatic trigeminal neuropathy (PTTN) is stated to have a low reaction rate of just 11%. However, PPTTN, trigeminal neuralgia, and PHTN are the three orofacial neuropathic pain conditions that are most often seen. PPTTN is caused by injury to the trigeminal nerve, which may occur as a result of treatments such as pulp extirpation, apicectomy, the extraction of teeth, or general endodontic therapy. According to estimates provided PPTTN develops in between 3 and 5% of all treatments of this kind 39. According to Okada-Ogawa et al. (2015), PPTTN typically results in a searing pain that is localised to one side of the body and lasts for an extended period of time close to or on the location of the injury. This pain is frequently followed by an acute, stabbing sensation26. Loss of sensory perception is another possibility. After a period of time, it becomes clear that the central nervous system is also involved, despite the fact that at first it may seem that only the peripheral nervous system is impacted. It is possible to be preferred to manage topical and/or systemic treatment to the peripheral, vital, or both terrified structures in order to control this problem. This is something that may be done.
Systemic Pharmacotherapy
Professional associations from all around the world, such as those for your area, have posted scientific coaching recommendations on the usage of pharmaceuticals to treat neuropathic ache. The maximum often recommended centrally focused analgesics include gabapentin, pregabalin, venlafaxine, tricyclic antidepressants (TCAs; e.g., amitriptyline and nortriptyline), and serotonin-norepinephrine reuptake inhibitors (SNRIs; e.g., duloxetine and venlafaxine)46. The primary PPTTN drugs utilized were the latter an aggregate of gabapentin or pregabalin plus duloxetine or amitriptyline must be used as an alternate remedy for PPTTN. If the primary method fails, opioids and opioid cocktail combos is probably a possible backup. Pregabalin and gabapentin are each anticonvulsants. These two tablets block the discharge of excitatory neurotransmitters like glutamate and SP by cooperating with the voltage-gated calcium channel’s subunit 2. Pregabalin and gabapentin are clinically effective for treating diabetic neuropathy, in addition to chronic ache and numbness related to PHTN 47. In massive scientific research related to PHTN patients, pregabalin and gabapentin proven considerable efficacy on PHTN ache; this analgesic impact is equivalent to that observed in studies the usage of antidepressants.
Mirogabalin, a these days advanced gabapentinoid, is a strong and particular 2 ligand. It’s been permitted in Japan for the treatment of painful diabetic peripheral neuropathy, PHTN, in addition to other peripheral neuropathic symptoms 48,49 . The nociceptive afferent input to the medullary dorsal horn can be immediately decreased or removed due to mirogabalin’s results on ectopic afferent pastime. In keeping with a latest have a look at, PPTTN sufferers’ ache-inhibitory machine gradually became less attentive to medicine 50. As an end result, patients with PPTTN ought to be controlled with a focus on insufficient inhibitory pain law. It’s far believed that TCAs and SNRIs prevent norepinephrine and serotonin from reuptaking 50
Carbamazepine and oxcarbazepine are often recommended as the first-line treatment for trigeminal neuralgia; however, a comprehensive study found that ninety percent of people who suffer from trigeminal neuralgia report much reduced discomfort when using these medications. According to Moore et al. (2019), the secondary treatment for classic trigeminal neuralgia includes the medications baclofen, lamotrigine, pregabalin, and gabapentin 51. In modern day treatment, it is recommended to make use of intravenous fosphenytoin, topical or injectable sumatriptan, or intravenous lidocaine in order to boost the efficacy of oral anticonvulsants and put a halt to an acute episode 51. In order to reduce the risk of developing orofacial neuropathic pain, a preoperative treatment known as pre-emptive analgesia may be administered. This treatment, in general, aims to reduce the initial damage-induced afferent volley and significant sensitization while also lowering the formation of anti-inflammatory mediators. This is accomplished through the utilisation of local anaesthetic blocks during invasive dentistry or oral surgical operation procedures 52. The relative timing of anaesthetic treatments is a key consideration in preemptive or preventative analgesia, which is also known as preventive analgesia. It lessens the side effects of peripheral nociceptive transduction brought on by painful stimuli before, during, and/or after surgical procedures 53. Clinicians may want to consider making use of local anaesthetic for invasive dental operations in addition to making use of preventative analgesics and/or anti-inflammatory medications in order to minimise the amount of postoperative pain experienced by patients in the patient setting.
Majority of the patients fair enough with the pharmacology treatment/drugs like the carbamazepine & oxcarbazepine which forms the first line treatment options followed by lamotrigine & baclofen encompassing the second line of drugs along with adjuvant drug support of topiramate, levetiracetam, gabapentin, pregabalin. As the field of science has explored &advanced for the latest treatment options include microvascular decompression, gamma knife radiosurgery, percutaneous rhizotomies variable based on the evidences & guidelines 54.
Conclusion
As a consequence of injury to the trigeminal nerve, astrocytes, microglial cells, and astroglial cells become activated, and macrophages accumulate. The compounds that may be created by employing these non-neuronal cells and then released play a part in enhancing the unpleasant neuronal attention that is being experience. The findings of this study may already be used as a valuable resource in the diagnosis and treatment of individuals with orofacial neuropathic pain; however, more research will need to delve at more specific non-neuronal cellular characteristic processes in order to fully understand these conditions.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Allen NJ, Eroglu C. Cell Biology of Astrocyte-Synapse Interactions. Neuron. 2017 Nov 1; 96(3):697-708. Doi: 10.1016/j.neuron.2017.09.056. PMID: 29096081; PMCID: PMC5687890.
CrossRef - Antonaci, F., Arceri, S., Rakusa, M. et al. Pitfals in recognition and management of trigeminal neuralgia. J Headache Pain 21, 82 (2020). https://doi.org/10.1186/s10194-020-01149-8
CrossRef - Asano S, Hayashi Y, Iwata K, Okada-Ogawa A, Hitomi S, Shibuta I, Imamura Y, Shinoda M. Microglia-Astrocyte Communication via C1q Contributes to Orofacial Neuropathic Pain Associated with Infraorbital Nerve Injury. Int J Mol Sci. 2020 Sep 17; 21(18):6834. Doi: 10.3390/ijms21186834. PMID: 32957694; PMCID: PMC7560139.
CrossRef - Baad-Hansen L, Benoliel R. Neuropathic orofacial pain: Facts and fiction. Cephalalgia. 2017 Jun; 37(7):670-679. Doi: 10.1177/0333102417706310. Epub 2017 Apr 12. PMID: 28403646.
CrossRef - Batbold D, Shinoda M, Honda K, Furukawa A, Koizumi M, Akasaka R, Yamaguchi S, Iwata K. Macrophages in trigeminal ganglion contribute to ectopic mechanical hypersensitivity following inferior alveolar nerve injury in rats. J Neuroinflammation. 2017 Dec 16;14(1):249. doi: 10.1186/s12974-017-1022-3. PMID: 29246259; PMCID: PMC5732495.
CrossRef - Bates, D., Schultheis, B. C., Hanes, M. C., Jolly, S. M., Chakravarthy, K. V., Deer, T. R., et al. A comprehensive algorithm for management of neuropathic pain. Pain Med. (2019). 20(Suppl. 1), S2–S12. Doi: 10.1093/pm/pnz075.
CrossRef - Bayat A, Burbelo PD, Browne SK, Quinlivan M, Martinez B, Holland SM, Buvanendran A, Kroin JS, Mannes AJ, Breuer J, Cohen JI, Iadarola MJ. Anti-cytokine autoantibodies in post herpetic neuralgia. J Transl Med. 2015 Oct 20; 13:333. Doi: 10.1186/s12967-015-0695-6. PMID: 26482341; PMCID: PMC4617715.
CrossRef - Chen MJ, Kress B, Han X, Moll K, Peng W, Ji RR, Nedergaard M. Astrocytic CX43 hemichannels and gap junctions play a crucial role in development of chronic neuropathic pain following spinal cord injury. Glia. 2012 Nov; 60(11):1660-70. Doi: 10.1002/glia.22384. Epub 2012 Aug 1. PMID: 22951907; PMCID: PMC3604747.
CrossRef - Koichi Iwata, Mamoru Takeda, Seog Bae Oh, Masamichi Shinoda Neurophysiology of Orofacial Pain Contemporary Oral Medicine, 2017. ISBN : 978-3-319-28100-1
CrossRef - Shinoda M, Imamura Y, Hayashi Y, Noma N, Okada-Ogawa A, Hitomi S, Iwata K. Orofacial Neuropathic Pain-Basic Research and Their Clinical Relevancies. Front Mol Neurosci. 2021 Jul 6; 14:691396. Doi: 10.3389/fnmol.2021.691396. PMID: 34295221; PMCID: PMC8291146.
CrossRef - Peng Q, Wang Y, Li M, Yuan D, Xu M, Li C, Gong Z, Jiao R, Liu L. cGMP-Dependent Protein Kinase Encoded by foraging Regulates Motor Axon Guidance in Drosophila by Suppressing Lola Function. J Neurosci. 2016 Apr 20;36(16):4635-46. doi: 10.1523/JNEUROSCI.3726-15.2016. PMID: 27098704; PMCID: PMC6601827.
CrossRef - Chu LW, Cheng KI, Chen JY, Cheng YC, Chang YC, Yeh JL, Hsu JH, Dai ZK, Wu BN. Loganin prevents chronic constriction injury-provoked neuropathic pain by reducing TNF-α/IL-1β-mediated NF-κB activation and Schwann cell demyelination. Phytomedicine. 2020 Feb; 67:153166. Doi: 10.1016/j.phymed.2019.153166. Epub 2019 Dec 30. PMID: 31955133.
CrossRef - Andrea Leo, Giacomo Handjaras, Matteo Bianchi, Hamal Marino, Marco Gabiccini, Andrea Guidi, Enzo Pasquale Scilingo, Pietro Pietrini, Antonio Bicchi, Marco Santello, Emiliano Ricciardi (2016) A synergy-based hand control is encoded in human motor cortical areaseLife 5:e13420. https://doi.org/10.7554/eLife.13420
CrossRef - Shinoda K, Luijten IH, Hasegawa Y, Hong H, Sonne SB, Kim M, Xue R, Chondronikola M, Cypess AM, Tseng YH, Nedergaard J, Sidossis LS, Kajimura S. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat Med. 2015 Apr;21(4):389-94. doi: 10.1038/nm.3819. Epub 2015 Mar 16. PMID: 25774848; PMCID: PMC4427356.
CrossRef - Laura Minnema, Ankita Gupta, Santosh K. Mishra, B. Duncan X. Lascelles. Investigating the role of artemin & its cognitive receptor, GFR3, in osteoarthritis pain. Front. Neurosci, 27 January 2022 sec neuropharmacology vol 16-2022. https://doi.org/10.3389/fnins.2022.738976
CrossRef - Bailes J, Soloviev M. Insulin-Like Growth Factor-1 (IGF-1) and Its Monitoring in Medical Diagnostic and in Sports. Biomolecules. 2021 Feb 4; 11(2):217. Doi: 10.3390/biom11020217. PMID: 33557137; PMCID: PMC7913862.
CrossRef - Shinoda N, Hanawa N, Chihara T, Koto A, Miura M. Dronc-independent basal executioner caspase activity sustains Drosophila imaginal tissue growth. Proc Natl Acad Sci U S A. 2019 Oct 8; 116(41):20539-20544. Doi: 10.1073/pnas.1904647116. Epub 2019 Sep 23. PMID: 31548372; PMCID: PMC6789915.
CrossRef - Iwata K, Sessle BJ. The Evolution of Neuroscience as a Research Field Relevant to Dentistry. J Dent Res. 2019 Dec; 98(13):1407-1417. Doi: 10.1177/0022034519875724. PMID: 31746682.
CrossRef - Profiling sensory neuron microenvironment after peripheral and central axon injury reveals key pathways for neural repair.Avraham,Feng R,Ewan EE, Rustenhoven J, Zhao G, Cavalli V. Elife, 29 Sep 2021, 10:e68457 DOI: 10.7554/elife.68457
CrossRef - K.M. Hargreaves Review Orofacial pain / PAIN 152 (2011) S25–S32. 0304-3959/$36.00 2011 International Association for the Study of Pain. Published by Elsevier B.V. doi:10.1016/j.pain.2010.12.024
CrossRef - Liu, Y., Lin, W., Liu, C. et al. Memory consolidation reconfigures neural pathways involved in the suppression of emotional memories. Nat Commun 7, 13375 (2016). https://doi.org/10.1038/ncomms13375
CrossRef - Hansson, E., Skiöldebrand, E. Coupled cell networks are target cells of inflammation, which can spread between different body organs and develop into systemic chronic inflammation. J Inflamm 12, 44 (2015). https://doi.org/10.1186/s12950-015-0091-2
CrossRef - Möckel L, Gerhard A, Mohr M, Armbrust CI, Möckel C. Prevalence of pain, analgesic self-medication and mental health in German pre-hospital emergency medical service personnel: a nationwide survey pilot-study. Int Arch Occup Environ Health. 2021 Nov; 94(8):1975-1982. doi: 10.1007/s00420-021-01730-x. Epub 2021 Jun 7. PMID: 34097107; PMCID: PMC8180540.
CrossRef - Liang H, Hu H, Shan D, et al. CGRP Modulates Orofacial Pain through Mediating Neuron-Glia Crosstalk. Journal of Dental Research. 2021; 100(1):98-105. doi:10.1177/0022034520950296
CrossRef - Dubner R, Ren K. Brainstem mechanisms of persistent pain following injury. J Orofac Pain. 2004 Fall; 18(4):299-305. PMID: 15636012.
- Akiko OkadaOgawa a b, Yuka Nakaya a, Yoshiki Imamura a b, Masayuki Kobayashi c, Masamichi Shinoda b d, Kozue Kita b, Barry J. Sessle e, Koichi Iwata Involvement of medullary GABAergic system in extraterritorial neuropathic pain mechanisms associated with inferior alveolar nerve transection b Experimental NeurologyVolume 267, May 2015, Pages 42-52. https://doi.org/10.1016/j.expneurol. 2015.02.030
CrossRef - Guo, W., Wang, H., Watanabe, M., Shimizu, K., Zou, S., LaGraize, S. C., et al. (2007). Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J. Neurosci. 27, 6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007
CrossRef - Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008 May 14; 28(20):5189-94. Doi: 10.1523/JNEUROSCI.3338-07.2008. PMID: 18480275; PMCID: PMC2408767.
CrossRef - Hildebrand, M. E., Xu, J., Dedek, A., Li, Y., Sengar, A. S., Beggs, S., et al. (2016). Potentiation of synaptic GluN2B NMDAR currents by fyn kinase ss gated through BDNF-mediated disinhibition in spinal pain processing. Cell Rep. 17, 2753–2765. Doi: 10.1016/j.celrep.2016.11.024.
CrossRef - Zhou M, Chen N, Tian J, Zeng J, Zhang Y, Zhang X, Guo J, Sun J, Li Y, Guo A, Li Y. Suppression of GABAergic neurons through D2-like receptor secures efficient conditioning in Drosophila aversive olfactory learning. Proc Natl Acad Sci U S A. 2019 Mar 12; 116(11):5118-5125. Doi: 10.1073/pnas.1812342116. Epub 2019 Feb 22. PMID: 30796183; PMCID: PMC6421402.
CrossRef - Clark, A. K., Yip, P. K., Grist, J., Gentry, C., Staniland, A. A., Marchand, F., et al. (2007). Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc. Natl. Acad. Sci. U.S.A 104, 10655–10660. Doi: 10.1073/pnas.0610811104.
CrossRef - Tsuboi Y, Iwata K, Dostrovsky JO, Chiang CY, Sessle BJ, Hu JW. Modulation of astroglial glutamine synthetase activity affects nociceptive behavior and central sensitization of medullary dorsal horn nociceptive neurons in a rat model of chronic pulpitis. Eur J Neurosci. 2011 Jul; 34(2):292-302. Doi: 10.1111/j.1460-9568.2011.07747.x. Epub 2011 Jun 27. PMID: 21707791.
CrossRef - Zhou LJ, Peng J, Xu YN, Zeng WJ, Zhang J, Wei X, Mai CL, Lin ZJ, Liu Y, Murugan M, Eyo UB, Umpierre AD, Xin WJ, Chen T, Li M, Wang H, Richardson JR, Tan Z, Liu XG, Wu LJ. Microglia Are Indispensable for Synaptic Plasticity in the Spinal Dorsal Horn and Chronic Pain. Cell Rep. 2019 Jun 25; 27(13):3844-3859.e6. Doi: 10.1016/j.celrep. 2019.05.087. PMID: 31242418; PMCID: PMC7060767.
CrossRef - Iwata K, Sessle BJ. The Evolution of Neuroscience as a Research Field Relevant to Dentistry. J Dent Res. 2019 Dec; 98(13):1407-1417. Doi: 10.1177/0022034519875724. PMID: 31746682.
CrossRef - ICOP (2020). International classification of orofacial pain, 1st edition (ICOP). Cephalalgia 40, 129–221. doi: 10.1177/0333102419893823
CrossRef - Christoforou J. Neuropathic Orofacial Pain. Dent Clin North Am. 2018 Oct; 62(4):565-584. Doi: 10.1016/j.cden.2018.05.005. Epub 2018 Jul 31. PMID: 30189983.
CrossRef - Christoforou, J., Balasubramaniam, R. & Klasser, G.D. Neuropathic Orofacial Pain. Curr Oral Health Rep 2, 148–157 (2015). https://doi.org/10.1007/s40496-015-0052-0
CrossRef - Sara Zamiri,1Mohammad Jafar Shaterzadeh Yazdi,2Elham Maraghi,3and Ismail Ebrahimi Takamjani The Effectiveness of Classification-Specific Physical Therapy for People with Low Back pain Within Dominant Movement-Based Schemes: A Systematic Review Iran Red Crescent Med J. 2016 December; 18(12):e41959. Published online 2016 December 4. doi: 10.5812/ircmj.41959.
CrossRef - Baad-Hansen L, Benoliel R. Neuropathic orofacial pain: Facts and fiction. Cephalalgia. 2017 Jun; 37(7):670-679. Doi: 10.1177/0333102417706310. Epub 2017 Apr 12. PMID: 28403646.
CrossRef - Devine, M., Hirani, M., Durham, J., Nixdorf, D. R., and Renton, T. (2018). Identifying criteria for diagnosis of post-traumatic pain and altered sensation of the maxillary and mandibular branches of the trigeminal nerve: a systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 125, 526–540. Doi: 10.1016/j.oooo.2017.12.020.
CrossRef - Rotpenpian N, Yakkaphan P. Review of Literatures: Physiology of Orofacial Pain in Dentistry. eNeuro. 2021 Apr 27; 8(2):ENEURO.0535-20.2021. Doi: 10.1523/ENEURO.0535-20.2021. PMID: 33820801; PMCID: PMC8086974.
CrossRef - Paquin S, Lacourse E, Brendgen M, Vitaro F, Dionne G, Tremblay RE, Boivin M. Heterogeneity in the development of proactive and reactive aggression in childhood: Common and specific genetic – environmental factors. PLoS One. 2017 Dec 6; 12(12):e0188730. Doi: 10.1371/journal.pone.0188730. PMID: 29211810; PMCID: PMC5718601.
CrossRef - Markman JD, Gewandter JS, Frazer ME. Comparison of a Pain Tolerability Question with the Numeric Rating Scale for Assessment of Self-reported Chronic Pain. JAMA Netw Open. 2020; 3(4):e203155. doi:10.1001/jamanetworkopen.2020.3155
CrossRef - Chen, W., Walwyn, W., Ennes, H. S., Kim, H., McRoberts, J. A., and Marvizon, J. C. (2014). BDNF released during neuropathic pain potentiates NMDA receptors in primary afferent terminals. Eur. J. Neurosci. 39, 1439–1454. Doi: 10.1111/ejn.12516.
CrossRef - Haviv, Y., Zadik, Y., Sharav, Y., and Benoliel, R. (2014). Painful traumatic trigeminal neuropathy: an open study on the pharmacotherapeutic response to stepped treatment. J. Oral Facial Pain Headache 28, 52–60. doi: 10.11607/jot.1154
CrossRef - Derry, S., Wiffen, P. J., Moore, R. A., and Quinlan, J. (2014). Topical lidocaine for neuropathic pain in adults. Cochrane Database Syst. Rev. 2014:CD010958. Doi: 10.1002/14651858.CD010958.pub2.
CrossRef - Dieb, W., and Hafidi, A. (2013). Astrocytes are involved in trigeminal dynamic mechanical allodynia: potential role of D-serine. J. Dent. Res. 92, 808–813. doi: 10.1177/0022034513498898.
CrossRef - Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, Yen CC, Kubota Y, Kim SB, Hsu CH, Holtved E, Xynos I, Kodani M, Kitagawa Y. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicenter, randomized, open-label, phase 3 trial. Lancet Oncol. 2019 Nov; 20(11):1506-1517. Doi: 10.1016/S1470-2045(19)30626-6. Epub 2019 Sep 30. Erratum in: Lancet Oncol. 2019 Nov; 20(11):e613. PMID: 31582355.
CrossRef - Nasri-Heir C, Zagury JG, Thomas D, Ananthan S. Burning mouth syndrome: Current concepts. J Indian Prosthodont Soc. 2015 Oct-Dec; 15(4):300-7. Doi: 10.4103/0972-4052.171823. PMID: 26929531; PMCID: PMC4762357.
CrossRef - Olga Korczeniewska,Junad Khan,Eli Eliav,Rafael Benoliel. Molecular Mechanisms of Painful Traumatic Trigeminal Neuropathy – Evidence from Animal Research and Clinical Correlate June 2020 Journal of Oral Pathology and Medicine 49(6) DOI:10.1111/jop.13078
CrossRef - Moore DJ, Meints SM, Lazaridou A, Johnson D, Franceschelli O, Cornelius M, Schreiber K, Edwards RR. The Effect of Induced and Chronic Pain on Attention. J Pain. 2019 Nov; 20(11):1353-1361. Doi: 10.1016/j.jpain.2019.05.004. Epub 2019 May 9. PMID: 31077797.
CrossRef - Aldington D, Eccleston C. Evidence-Based Pain Management: Building on the Foundations of Cochrane Systematic Reviews. Am J Public Health. 2019 Jan; 109(1):46-49. Doi: 10.2105/AJPH.2018.304745. Epub 2018 Nov 29. PMID: 30495991; PMCID: PMC6301419.
CrossRef - Korczeniewska OA, James MH, Eliav T, Katzmann Rider G, Mehr JB, Affendi H, Aston-Jones G, Benoliel R. Chemogenetic inhibition of trigeminal ganglion neurons attenuates behavioural and neural pain responses in a model of trigeminal neuropathic pain. Eur J Pain. 2022 Mar; 26(3):634-647. Doi: 10.1002/ejp.1887. Epub 2021 Dec 6. PMID: 34767278; PMCID: PMC8847328.
CrossRef - Al-Quliti KW. Update on neuropathic pain treatment for trigeminal neuralgia. The pharmacological and surgical options. Neurosciences (Riyadh). 2015 Apr;20(2):107-14. doi: 10.17712/nsj.2015.2.20140501. PMID: 25864062; PMCID: PMC4727618.
CrossRef
Abbreviations
AMPA, alpha amino-3-hydroxy-5-methyl-4-isoxazole-propionate; BDNF, brain-derived neurotrophic factor; CCI, Chronic Constriction Injury; CCL2, the chemokine C-C motif ligand 2; C1-C2, the upper cervical spinal cord; Cx43, Connexin 43; HZ, herpes zoster; IASP, International Association for the Study of Pain; IGF-1, insulin-like growth factor-1; IL, interleukin; KCC2, K+-Cl– cotransporter; MCP-1, monocyte chemoattractant protein-1; MRA, magnetic resonance angiography; MRI, magnetic resonance imaging; MT, medial thalamic nuclei; Nav1.8, voltage-gated sodium channels 1.8; NMDA, N-methyl -D-aspartate; PBN, parabrachial nucleus; QST, quantitative sensory testing; sEPSCs, spontaneous excitatory postsynaptic currents; sIPSCs, spontaneous inhibitory postsynaptic currents; SNRIs, serotonin-norepinephrine reuptake inhibitors; SP, Substance P; PHTN, Post herpetic trigeminal neuralgia; PPTTN, peripheral, painful, traumatic trigeminal neuropathy; TCAs, tricyclic antidepressants; TG, trigeminal ganglion; TN, trigeminal neuralgia; TNFR, TNF receptor; TNFα, tumor necrosis factor alpha; TrkB, tropomyosin-related kinase B; Vc, trigeminal spinal subnucleus caudalis.
(Visited 240 times, 3 visits today)







