Manuscript accepted on :27-10-2022
Published online on: 15-05-2023
Plagiarism Check: Yes
Reviewed by: Dr. Zainab Haitham
Second Review by: Dr Supratim Datta
Final Approval by: Dr. Patorn Promchai
Ravindra Kumar Ganjikunta1 , Rudhra Prabhakar Kadali2*
, Rudhra Prabhakar Kadali2* , Tarun Arora2
, Tarun Arora2 and Pallavi Chalivendra1
and Pallavi Chalivendra1
1Department of Pharmacology, Sri Padmavati Medical College for women, SVIMS, Tirupati-517507, India
2Department of Pharmacology, Lady Hardinge Medical College, New Delhi-110001, India.
Corresponding Author E-mail: drrudhraprabhakar@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2691
Abstract
Adverse drug reactions (ADR) can be manifested in different forms, among these cutaneous adverse drug reactions (CADRs) are the commonest. They have been steadily gaining importance and constitute a major proportion of all ADRs. As available data on CADRs is very less, more research is required to have reliable data, hence the current study was undertaken. This prospective study was carried out to evaluate the age and gender distribution, presenting complaints, spectrum of CADRs, causative drugs, causality, severity, and outcomes in patients with or suspected CADRs attending the department of Dermatology of Konaseema Institute of Medical Sciences & Research Foundation Hospital between January 2014 and June 2015. In cooperation with the Dermatologist, patients’ reactions were analyzed based on morphology, and laboratory investigations. Causality was assessed as per the World Health Organization- Uppsala Monitoring Centre (WHO-UMC)causality assessment scale. Modified Hartwig and Siegel Scale was used for the severity assessment of reactions. CADRs occurred most commonly in the 31-40 years age group (32%) with no sign of the difference in both sexes. The most common complaint of CADRs by the patients was skin rash (42%) anddiagnoses were Erythematous drug eruption (ERDE) and Fixed drug eruption (FDE) (28%). The commonest causative drug categories were antimicrobials (52%) and Non-Steroidal Anti-inflammatory Drugs (NSAIDs) (24%).Among antimicrobials, ciprofloxacin, and in NSAIDs, diclofenac were the commonest causative drugs. In causality, majority of the cases were under possible category(42%). Most of the reactions were mild (46%), and moderate (46%) in severity. The majority of the cases showed good recovery without any mortality or disability. The limitations of this study were the relatively small sample size, inability to confirm the particular causative drug in majority of the patients. Future research should focus on the genetic factors concerning to CADRs and molecular-level evaluation should be done for a better understanding of the pathophysiology of various ADRs.
Keywords
Antimicrobials; Antiepileptics; CADRs; Drug category; NSAIDs
Download this article as:| Copy the following to cite this article: Ganjikunta R. K, Kadali R. P, Arora T, Chalivendra P. Evaluation of Cutaneous Adverse Drug Reactions Reported in a Teaching Hospital of Coastal Andhra. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Ganjikunta R. K, Kadali R. P, Arora T, Chalivendra P. Evaluation of Cutaneous Adverse Drug Reactions Reported in a Teaching Hospital of Coastal Andhra. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/3nYFlRW |
Introduction
An Adverse drug reaction (ADR) is defined by World Health Organization (WHO)as “a response to a medicine which is noxious and unintended, and which occurs at doses normally used in man”1. ADRs are implicated in significant morbidity and mortality. In India, 400,000 deaths were due to ADRs per annum, and out of all visits to the medical emergency department, 6 percent are drug-related2. ADRs constitute a major clinical problem in terms of human suffering and increased healthcare expenses. Drugs are always connected with the risk of ADRs.
ADR can be manifested in different forms, among these cutaneous adverse drug reactions (CADRs) are the most common. They have been steadily gaining importance and constitute a major proportion of all ADRs. The CADRs range from rash to toxic epidermal necrolysis (TEN). CADRs are impacted by multiple elements like comorbidities, immune status, genomics, history of allergies, age, and sex.
The types of CADRs and the causative drugs are continuously changing over time, as new medications are being introduced into the market. The pattern of CADRs is also changing due to alterations in the drug of choice, drug interactions due to polypharmacy, and a rising trend in the public to self-medicate. CADR monitoring is an important aspect of ADR monitoring programs, not only for the collection of data but also for identifying and preventing risk factors. Epidemiological studies are deficient and underreporting of ADR is also a major problem in India. So available data on CADRs is very less, more research is required to have reliable data, hence the current study was undertaken.
In this study, age and gender distribution, common presenting complaints (symptoms), common diagnoses, causative drug category, severity of reactions, causality assessment, and outcome were evaluated.
Methods
This prospective study was carried out to evaluate the demographic distribution of patients, spectrum of CADRs, different causative drug categories, outcomes of CADRs, common presenting complaints, severity of reactions, and their causality assessment.
Study subjects
Patients of all age groups and both sexes with or suspected CADRs attending the Dermatology department (including both outpatients and inpatients) of Konaseema Institute of Medical Sciences & Research Foundation Hospital were included in the study. Before beginning the research, the Institutional Ethics Committee approval was obtained. The study was carried out prospectively for 18 months between January 2014 and June 2015.
Sampling
50 consecutive patients who visited the Dermatology department (both outpatients and inpatients) of Konaseema Institute of Medical Sciences & Research Foundation Hospital with or suspected CADRs were included in the study.
Study procedure
Before involving the patients in the study written informed consent was obtained. Patients were evaluated for the pattern and severity of the reactions. A detailed history including the present, and past medical history, and history of previous drug reactions was noted. In cooperation with the Dermatologist, patients’ reactions were analyzed based on morphology, clinical criteria, and laboratory investigations. Due to ethical concerns, a rechallenge test was not performed. Causality was assessed as per the WHO-UMC causality assessment scale. To determine the causality highest suspicious drugs were first discontinued. Modified Hartwig and Siegel Scale was used for the severity assessment of reactions.
Statistical analysis
Variables were analyzed with descriptive statistics such as mean and standard deviation (SD) by using Microsoft Excel2013. The results were represented in the form of percentages with tables and figures.
Results
Incidence
In this prospective spontaneous ADR monitoring study, a total of 50 (0.11%) CADRs were recorded from a total of 43,842 patients visiting the dermatology department of Konaseema Institute of Medical Sciences & Research Foundation Hospital from January 2014 to June 2015.
Age and gender distribution
The mean age with standard deviation was 34.76 ± 15.66 years shown in Figure 1, the oldest being 65 years and the youngest being 2years. Majority of the subjects belonged to the 31-40 age group (32%). There is no significant difference in the incidence of CADRs between males (24) and females (26).
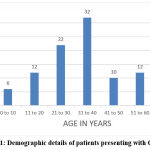 |
Figure 1: Demographic details of patients presenting with CADRs. |
Common presenting complaints (symptoms)
The Data regarding various common presenting complaints were tabulated in Table 1.In this study majority of the patient’s complaint was skin rash/Eruption, followed by skin discoloration. Only a few patients presented with erythema and pruritus.
Table 1: Clinical presentation of CADRs with their frequency
| Common presenting complaints | Frequency n (%) |
| Edema | 2(4) |
| Erythema | 1(2) |
| Skin discoloration | 12(24) |
| Pruritus | 1(2) |
| Pustules | 3(6) |
| Rash/Eruption | 21(42) |
| Vesicle | 6(12) |
| Bulla | 4(8) |
Clinical diagnoses of reactions
The proportions of various CADRs were shown in Table 2. Among all the reported CADRs ERDE and FDE were the most common variations. Only one case of each was reported in Hyperpigmentation, DRESS (Drug reaction with eosinophilia and systemic symptoms),TEN, stria, lichenoid eruption,and photosensitivity types of CADRs. Commonest CADRs ERDE and FDE occurred mainly due to antimicrobials (fluoroquinolones) and analgesics (diclofenac). Two cases of SJS, one case of TEN, and one case of DRESS were caused by Phenytoin, Lamotrigine, Nimesulide, and Phenytoin respectively.
Table 2: Showing distribution of various CADRs
| Diagnosis | Frequency n (%) |
| EMF(Erythema multiforme) major | 4(8) |
| Angioedema | 2(4) |
| ERDE(Erythematous drug eruptions) | 14(28) |
| SJS(Stevens-Johnson syndrome) | 4(8) |
| FDE(Fixed drug eruptions) | 14(28) |
| Hyperpigmentation | 1(2) |
| Urticaria | 3(6) |
| DRESS (Drug reaction with eosinophilia and systemic symptoms) | 1(2) |
| TEN(Toxic Epidermal Necrolysis) | 1(2) |
| Stria | 1(2) |
| Acneiform drug eruption | 3(6) |
| Lichenoid eruption | 1(2) |
| Photosensitivity | 1(2) |
Common causative drug categories:
Drug categories causing CADRs were shown in Table 3. According to that, the commonest causative drug category was antimicrobials 52% followed by NSAIDs 24%, and antiepileptics 8%.
In fluoroquinolones, ciprofloxacin (14%), levofloxacin (2%) and ofloxacin (2%) were predominant. Among penicillins amoxicillin (10%) constitute the bulk of CADRs. Among Anti-Tuberculosis Therapy (ATT), rifampicin (4%) and isoniazid (4%) were involved in CADRs. In cephalosporins, only cefixime was involved. Other antimicrobials involved in CADRs were antiamoebic (metronidazole 4%), Anti-Retroviral Therapy (ART) (nevirapine 4%), tetracyclines (doxycycline 2%), antimalarial (artesunate 2%).
In others group category CADRs were due to KCl, Doxylamine, and Theophylline.
Table 3: Showing distribution of various drug categories causing CADRs
| Drug category | Frequency n (%) |
| Antimicrobials | 26(52) |
| Analgesics/NSAIDs | 12(24) |
| Anticancer | 2(4) |
| Antiepileptics | 4(8) |
| Corticosteroids | 3(6) |
| Others | 3(6) |
Severity of reactions
The severity of reactions was graded as mild, moderate, and severe using Modified Hartwig and Siegel Scale as shown in Table 4.Severe cases of CADRs were very less in this study, while mild & moderate CADRs were more & equal innumber. Therewerenoreactions with the severity of 6 and 7 on the Modified Hartwig and Siegel Scale.
Table 4: Showing the association of drug categories with the severity of CADRs
| Drugcategory | Severity ofreactionsn (%) | ||
| Mild | Moderate | Severe | |
| Antimicrobials | 13(26) | 13(26) | – |
| Analgesics/NSAIDs | 6(12) | 5(10) | 1(2) |
| Anticancer | – | 1(2) | 1(2) |
| Antiepileptics | – | 2(4) | 2(4) |
| Corticosteroids | 1(2) | 2(4) | – |
| Others | 3(6) | – | – |
| Total | 23(46) | 23(46) | 4(8) |
Causality assessment
Causality of the CADRs with regard to suspected drugs was assessed by using WHO- UMC causality assessment criteria and shown in Table 5.Majority of the cases causality was assessed under the possible category. Only a few cases fall under certain & conditional categories.
Table 5: Showing the association of drug categories with Causality assessment.
|
Drug category |
Causality n (%) | ||||
| Certain | Probable | Possible | Unlikely | Conditional | |
| Antimicrobials | 1(2) | 7(14) | 14(28) | 4(8) | – |
| Analgesics/NSAIDs | – | 3(6) | 3(6) | 4(8) | 2(4) |
| Anticancer | 1(2) | 1(2) | – | – | – |
| Antiepileptics | – | 1(2) | 2(4) | 1(2) | – |
| Corticosteroids | – | 1(2) | 1(2) | 1(2) | – |
| Others | – | 1(2) | 1(2) | 1(2) | – |
| Total | 2(4) | 14(28) | 21(42) | 11(22) | 2(4) |
Outcome assessment of CADRs with severity
Different outcomes of CADRs were shown in Table 6.Majority of the cases (26%) fall under the category of “reaction persisted during observation but showed improvement”. Also, reaction resolved without sequelae and resolved with sequelae categories were equal in percentage (22%). The cases that come under the reactions persisted during observation without improvement category were12%. However, 18% of cases were not available for follow-up, hence categorized under unknown outcome conservatively. Fortunately, there were no deaths or progression of lesions during this study.
Table 6: Showing outcome of CADRs with various severity levels
| Outcome | Severity of reactions n (%) | |||
|
Mild |
Moderate |
Severe |
Total |
|
| Resolved without sequelae | 5(10) | 6(12) | – | 11(22) |
| Resolved with sequelae | 5(10) | 6(12) | – | 11(22) |
| Reaction persisted during observation but showed improvement | 6(12) | 5(10) | 2(4) | 13(26) |
| Reaction persisted during observation withoutimprovement | 1(2) | 5(10) | – | 6(12) |
| Unknown | 6(12) | 1(2) | 2(4) | 9(18) |
Discussion
The present study was carried out to evaluate the age and gender distribution, common presenting complaints, common diagnoses, causative drug categories, severity of reactions, causality assessment and outcome after the intervention of CADRs in 50 subjects. The mean age with standard deviation was 34.76 ± 15.66 years and majority of the subjects belonged to the 31-40 age group (32%).Which is in accordance with the study conducted at Vijaypura, where the mean age was 35.71±19.87 years, and the maximum number of CADRs were observed between the ages of 21-40 years3. Similar results were seen in studies conducted by Sharma V. K et al4 and Pudukadan D et al5. In contrast to the present results, a study conducted at Coimbatore showed, patients in the age group 41-60 years experienced a maximum of CADRs and the mean age was 49.26 years6. There were mild differences in the results of different studies, which may be due to different geographical variations. Normally pediatric and geriatric age groups were more prone to CADRs, due to low immunity and consuming more medications. However, in this study extremes of age group patients were less, compared to adults.
Skin rash/Eruption was a common symptom in our patients. Similar results were found in a study in Kerala, with a rash as a common complaint (33.06%)7. However, in another study in Korea, itching was the common presenting complaint (61.0%)8. These variations in presenting complaints may be attributed to genetic and environmental factors.
In this study, it was observed that the presentation of CADRs was varying from mild erythematous drug eruptions to life-threatening Toxic epidermal necrolysis. Among these, ERDE and FDE were commonest, which were similar to studies conducted at Punjab9, and Nagpur10. In contrast to the present results, a study done at Vijayapura3 and Bengaluru11 showed that FDE was the second least common. ERDE and FDE were most commonly caused by fluoroquinolones, and diclofenac in our study. Two cases of SJS, one case of TEN, and one case of DRESS were caused by Phenytoin, Lamotrigine, Nimesulide, and Phenytoin respectively. However, in a study conducted in Punjab12 cephalosporins were the most common causative drugs for ERDE. Also, in the same study, SJS was caused by ciprofloxacin. These variations could be due to different patterns of drug usage and different ethnic group characteristics.
The commonest causative drug categories in our patients were concurrent with the results of a study in Kerala, where antimicrobials were highest (47.58%) followed by NSAIDs (16.13%) and antiepileptics (13.71%)7. However, in a study conducted at Sree Balaji Medical College, though NSAIDs constitute the bulk of CADRs, the second commonest was antimicrobials, unlike NSAIDs seen in the present study13. Among antimicrobials, ciprofloxacin, and amoxicillin were the predominant drugs in our study, which is in accordance with a study conducted at Maharashtra14. Which were prescribed for indications like fever, pharyngitis, and Gastroenteritis. These differences may be due to variations in a geographical area, different disease patterns, and different types of treatment options.
The severity of reactions was graded by using the Modified Hartwig and Siegel Scale as mild, moderate, and severe. In mild and moderate cases antimicrobials were the commonest and in severe cases, antiepileptics were the commonest group to cause CADRs. Also, mild and moderate cases were equal in number and occupies a major proportion of CADRs. A Kerala study showed a similar trend, where mild, and moderate CADRs were 37.9% in each and 24.2% were in severe7. Similar results were found in a study conducted by Berihun Haile D et al15.
According to WHO- UMC causality assessment criteria, only 4% of cases were considered certain. Even though a rechallenge was not attempted due to ethical reasons, based on pre-challenge information from medical history, causality assessment was made as certain in these cases. Among all cases, 28% were assessed under probable, and a majority (42%)of the cases were assessed under possible, which is in a similar trend to Vijayapura study3, and opposite to studies done in Gujarat, where more cases of probable was followed by certain and possible categories16,17.
In the outcome assessment, “reaction resolved without sequelae and resolved with sequelae”, categories were equal in percentage. In all the cases of moderate and severe reactions, the patients were duly warned against future exposure. An alert card was given to those with serious reactions. Studies conducted at Rajahmundry18, Kerala7, Vijayapura3, and Manipal19 showed similar results in the perspective of the outcome. In the present study, the SJS, TEN, and DRESS showed good recovery, whereas in other studies4,5,20,21,22 deaths were reported because of serious organ involvement and septicemia.
Limitations
The major limitation of our study is it compiles only 50 ADRs, a rechallenge test was not performed due to ethical reasons, and some minor drug reactions encountered by clinicians have not been informed. However, a search in the active form of ADRs was done in this work which has the advantage of not depending on the quality of the records. Despite the limitations and variations in the study, this data may help clinicians to report the ADRs and to avoid irrational drug use.
Conclusion
The results of this study were slightly different from other studies. This is maybe due to geographical variations, varied drug consumption habits, and different disease patterns. In clinical practice, proper awareness of the occurrence of the reactions and special precautions while prescribing drugs, early detection, timely withdrawal of the offending drugs, and appropriate rescue measures may greatly contribute to reducing the incidence, frequency, severity, morbidity, and possible mortality. Furthermore, studies are required in this area for obtaining more data on CADRs.
Acknowledgement
I pay my highest reverence to my Professor Dr. ANAND ACHARYA, M.D., Professor and Head, Department of Pharmacology, KIMS& RF, Amalapuram.
Conflict of Interest
There is no conflict of Interest.
Funding Sources
There are no funding sources.
References
- WHO_EDM_QSM_2002.2[Internet]. Geneva: World Health Organization; 2002 [cited 2022 Oct 17]. Available from: https://apps.who.int/iris/bitstream /handle/10665/67378/WHO_EDM_QSM_2002.2.pdf?sequence=1&isAllowed=y
- Indian Medical Association [Internet]. India: Indian Medical Association;2022 [cited 2022 Jan 20]. Available from: https://www.ima-india.org/ima/left-side-bar.php?pid=210
- Anant K, Chaukimath SP, Ajit J, Leela H. A Study of Cutaneous Adverse Drug Reactions; Clinical/Morphological Pattern & Causative Agents Reported in an ADR Monitoring Centre in a Tertiary Care Hospital of North Karnataka. Biomedical and Pharmacology Journal. 2020 Sep 25;13(3):1549-54.
CrossRef - Sharma VK, Sethuraman G, Kumar B. Cutaneous adverse drug reactions: clinical pattern and causative agents–a 6-year series from Chandigarh, India. Journal of postgraduate medicine. 2001 Apr 1;47(2):95.
- Pudukadan D, Thappa DM. Adverse cutaneous drug reactions: clinical pattern and causative agents in a tertiary care center in South India. Indian Journal of Dermatology, Venereology & Leprology. 2004 Jan 1;70(1).
- Palanisamy S, Kumaran KS, Rajasekaran AI. A study on assessment, monitoring and reporting of adverse drug reactions in Indian hospital. Asian J Pharm Clin Res. 2011;4(3):112-6.
- Sharma S, Jayakumar D, Palappallil DS. Pharmacovigilance of cutaneous adverse drug reactions among patients attending dermatology department at a Tertiary Care Hospital. Indian Dermatology Online Journal. 2019 Sep;10(5):547.
CrossRef - Son YM, Lee JR, Roh JY. Causality assessment of cutaneous adverse drug reactions. Annals of dermatology. 2011 Nov 1;23(4):432-8.
CrossRef - andha R, Gupta A, Hashmi A. Cutaneous adverse drug reactions in a tertiary care teaching hospital: A North Indian perspective. International journal of Applied and Basic medical research. 2011 Jan;1(1):50.
CrossRef - Hiware S, Shrivastava M, Mishra D, Mukhi J, Puppalwar G. Evaluation of cutaneous drug reactions in patients visiting out patient departments of Indira Gandhi Government Medical College and Hospital (IGGMC and H), Nagpur. Indian journal of dermatology. 2013 Jan;58(1):18.
CrossRef - Noel MV, Sushma M, Guido S. Cutaneous adverse drug reactions in hospitalized patients in a tertiary care center. Indian journal of pharmacology. 2004 Sep 1;36(5):292.
- Jha N, Alexander E, Kanish B, Badyal DK. A study of cutaneous adverse drug reactions in a tertiary care center in Punjab. Indian dermatology online journal. 2018 Sep;9(5):299.
CrossRef - Inbaraj SD, Muniappan M, Muthiah NS, Amutha A, Rahman F. Pharmacovigilance of the cutaneous drug reactions in outpatients of dermatology department at a tertiary care hospital. Journal of clinical and diagnostic research: JCDR. 2012 Dec;6(10):1688.
- 14.Agrawal A, Ghate S, Gupta AK, Dhurat R. Clinical spectrum of cutaneous adverse drug reactions. Indian Journal of Drugs in Dermatology. 2018 Jul 1;4(2):61.
CrossRef - Haile DB, Ayen WY, Tiwari P. Prevalence and assessment of factors contributing to adverse drug reactions in wards of a tertiary care hospital, India. Ethiopian journal of health sciences. 2013 Mar 12;23(1):39-48.
- Acharya T, Mehta D, Shah H, Dave J. Pharmacovigilance study of adverse cutaneous drug reactions in a tertiary care hospital. National Journal of Physiology, Pharmacy and Pharmacology. 1970 Jan 1;3(1):75.
CrossRef - Patel TK, Thakkar SH, Sharma DC. Cutaneous adverse drug reactions in Indian population: A systematic review. Indian dermatology online journal. 2014 Dec;5(Suppl 2): S76.
CrossRef - Vijayakumar TM, Dhanaraju MD. Description of adverse drug reactions in a multi-speciality teaching hospital. Int J Integr Med. 2013;1(26):1-6.
CrossRef - Ghosh S, Acharya LD, Rao PG. Study and evaluation of the various cutaneous adverse drug reactions in Kasturba hospital, Manipal. Indian journal of pharmaceutical sciences. 2006;68(2).
CrossRef - Jhaj R, Uppal R, Malhotra S, Bhargava VK. Cutaneous adverse reactions in in-patients in a tertiary care hospital. Indian Journal of Dermatology, Venereology and Leprology. 1999 Jan 1;65(1):14-17.
- 21.Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. American journal of hospital pharmacy. 1992 Sep 1;49(9):2229-32.
CrossRef - Modi A, Desai M, Shah S, Shah B. Analysis of cutaneous adverse drug reactions reported at the regional ADR monitoring center. Indian Journal of Dermatology. 2019 May;64(3):250
CrossRef







