Manuscript accepted on :09-01-2023
Published online on: 13-04-2023
Plagiarism Check: Yes
Reviewed by: Dr. Abeer Gatea
Second Review by: Dr. Masumeh Saeedi
Final Approval by: Dr. Ayush Dogra
C. S. Brethis 1 , R Hemalathaa 2*
, R Hemalathaa 2* , Ramyaa Rajendiran 3
, Ramyaa Rajendiran 3 , Sumitha A1
, Sumitha A1 , Navneeth P1
, Navneeth P1 and Amalnath A1
and Amalnath A1
1Department of Pharmacology, ACS Medical College and Hospital, Chennai, India.
2Department of Biochemistry, Saveetha Medical College, Chennai, India.
3Department of Pediatrics, Tagore Medical College and Hospital, Chennai, India.
Corresponding Author E-mail: hemalathaaramar@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2689
Abstract
Introduction: Offspring of gestational diabetes mellitus (GDM) mothers are at high risk of developing insulin resistance, type 2 diabetes mellitus (T2 DM), and cardiovascular complications later in life. So, screening maternal blood glucose during pregnancy and identifying high-risk infants immediately after birth is necessary to prevent the potential long-term implications. Aim: To correlate the maternal fasting and post-prandial blood glucose withfetal insulin level. Materials and methods:A case-control study, was conducted at Chettinad Hospital and Research Institute, India, between May 2019 to May 2020. A 75-gram OGTT was performed among pregnant women between 24 to 28 weeks of pregnancy for diagnosing GDM according to American Diabetes Association (ADA) guidelines. 94 GDM mothers and Non-GDM mothers and theirnew-bornswere taken up for this study. 2.5ml of maternal venous blood was collected in a vacutainer containing sodium fluoride and EDTA as an anticoagulant for FBS and PPBS estimation. Some mothers on induction of labor were posted for emergency LSCS (for failed induction and non - progression of labor) and some had normal vaginal deliveries. Plasma FBS and PPBS estimation in the mother’s blood sample was assayed by the Hexokinase method in Siemen'sDimension RxLMachine immediately after centrifugation. 2.5ml of umbilical cord blood was collected in a vacutainer without an anticoagulant after the 2nd stage of labor. 0.5 ml of cord blood serum was separated and stored at -80°C in an eppendorf for later estimation of insulin by CLIA method in Beckman Coulter – Access 2 Immunoassay System. Independent students’ t-tests and Pearson’s correlation were used as statistical methods. p-value <0.05 is considered significant. Results: There is a positive correlation and significant difference between maternal FBS, PPBS, and fetal insulin levels in the GDM group (p-value 0.008, r-value 0.272 and p-value 0.005, r-value 0.286) compared to the Non-GDM group (p-value -0.087, r-value 0.243 and p-value 0.018, r-value 0.212). Conclusion: Significant hyperinsulinemia was noted in the offspring of the GDM group compared to the NON-GDM group.Those hyper-insulinemic babies are at very high risk of developing obesity, metabolic syndrome, and type 2 DM in the future and possess a threat to society.
Keywords
APGAR score; Fetal cord insulin; FBS; Gestational Diabetes mellitus; PPBS; Placenta weight
Download this article as:| Copy the following to cite this article: Brethis C. S, Hemalathaa R, Rajendiran R, Sumitha A, Navneeth P, Amalnath A. Comparison Between Maternal Blood Glucose and Fetal Cord Insulin Level Among Gestational Diabetes Mellitus Women. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Brethis C. S, Hemalathaa R, Rajendiran R, Sumitha A, Navneeth P, Amalnath A. Comparison Between Maternal Blood Glucose and Fetal Cord Insulin Level Among Gestational Diabetes Mellitus Women. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/43x3wXN |
Introduction
The physiological alterations that happen during pregnancy act as a “stress test” naturally and this pregnancy period is considered a ‘window period’ for future maternal health. Most of the women seeking medical care during pregnancy utilize this opportunity for preventive healthcare guidance 1. There is growing evidence in recent years that the maternal nutritional status and their intrauterine environment have an impact on their offspring’s health throughout their lifespan after their birth 2. The emerging field of the developmental origins of health and disease (DOHAD) states that the intrauterine and early neonatal periods have apermanent programming effect on the body’s metabolism and health later in life 3. The most common physiological alteration during pregnancy is gestational diabetes mellitus (GDM) 4. GDM is defined as the varying degree of glucose intolerance that is diagnosed first during pregnancy 5. Though the underlying pathophysiology of GDM is not fully understood, it has been thought that the hormonal imbalances in the mother could affect the pancreas and lead to β-cell dysfunction and insulin sensitivity in their offspring6. In a random survey prevalence of GDM is known to be 3.8 to 21% in India and 16.2% in Chennai urban population 7. The occurrence of GDM in pregnant women and its complications in the new-born can be overcome by proper management of blood sugar levels during the perinatal, and postnatal periods3.
The degree of maternal insulin resistance established during pregnancy is associated with the degree of glucose flux from the mother to the fetus. During pregnancy 30% of hepatic glucose production increases 8 as fetal development depends on the maternal metabolic profile throughout the whole pregnancy period, illustrated by the fact that obese women or women who develop gestational diabetes have larger fetuses already by 20 weeks of gestation 9. Maternal hyperglycemia leads to fetal hyperglycemia and hyperinsulinemia, which causes fetal macrosomia as one of the most common and serious complications of maternal GDM10. This may be a predisposing factor for newborns of GDM mothers to develop metabolic syndrome and T2DM in future 11. This vicious cycle would increase the incidence and prevalence of GDM 12. Failure to treat the elevated blood sugar levels before and during pregnancy might end up in complications like preterm delivery, caesarean section, big babies, serious complications in new-borns, and raised bilirubin levels13. Many studies have indicated the association of GDM with macrosomia and other birth defects14.
Based on this hypothesis, maternal endocrine parameters can cause obesity, metabolic syndrome, and T2DM in GDM mother’s offspring in the future. So, assessment of fetal insulin at birth will be a very sensitive parameter and will reveal the maternal hyperglycemic insult in utero and create an awareness of the risks of GDM in future. However, there is a dearth of such case-control studies to demonstrate the association of maternal parameters affecting the fetus in utero. So, this study was planned to correlate the fasting and postprandial blood glucose of GDM and NON-GDM mother with their fetal insulin levels and to identify the high-risk offspring as early as possible in order to create awareness of the risks of GDM in the fetus.
Aim and Objective
Primary
To correlate the fasting and postprandial blood glucose of GDM and NON-GDM mothers with their fetal insulin levels.
Secondary
To identify the high-risk babies as early as possible.
To create awareness of the risk ofGDM in fetuses.
Methods
Study design and population
This is a single-center case-control study conducted at Chettinad Hospital and Research Institute, India, between May 2019 to May 2020 after obtaining clearance from the Institutional Human Ethics Committee.
Inclusion criteria
Pregnant women between 18- 45years,37 – 40 weeks of gestation, height between 157-161 cms, pregnancy weight during 1st trimester between 58 -65 kgs, pregnancy BMI during 1st trimester between 20 – 25kg/m2GDM confirmed between 24-28 weeks (OGTT) and GDM on dietary management with the meal plan, pregnancy weight at term between 75-85 kgs, pregnancy BMI at term 25-30 kg/m2were included in the study.
Exclusion criteria
Previously known cases of Diabetes Mellitus(DM),GDM women on insulin and insulin secretagogues, major medical or surgical history in the past, anomalous fetus in present pregnancy, pregnancy-induced hypertension – PIH /pre-eclampsia/eclampsia, blood transfusion in present pregnancy, pregnant women on corticosteroids and antenatal corticosteroids given in last 3 days were excluded from the study.
Sample Size
The size was calculated using the following formula,
σ2(zβ– za/2)2/d2
sample size(n) = 188, standard deviation (σ2)= 46, level of statistical significance (za) =1.96, desired power (zβ), desired power = 0.84, difference in mean (d²) = 10. 94 GDM women and their newborns and 94 Non-GDM women and their newborns were taken up for this study.
Data Collection
A 75-gram OGTT was performed after 8 hours of overnight fasting in a pregnant woman without previous history of DM between 24 to 28 weeks and the plasma glucose is measured at fasting, 1st and 2nd hour. Informed consent was obtained from the antenatal women who were admitted for induction of labor and for elective LSCS. 2.5ml of maternal venous blood was collected in a vacutainer containing sodium fluoride and EDTA as an anticoagulant for FBS and PPBS estimation. Some patients on induction of labor were posted for emergency LSCS (for failed induction and non – progression of labor) and some had normal vaginal deliveries. Plasma FBS and PPBS estimation in the mother’s blood sample was done by the Hexokinase method in Siemen’s Dimension RxL Machine immediately after centrifugation. 2.5ml of umbilical cord blood was collected in a vacutainer without an anticoagulant after the 2nd stage of labor. Cord blood was centrifuged immediately and 0.5 ml of serum was separated and stored at -80°C in an Eppendorf for later estimation of insulin. Estimation of serum insulin was done by CLIA method in Beckman Coulter – Access 2 Immunoassay System.
Definition of GDM and Fetal hyperinsulinemia
The diagnosis of GDM between 24-28 weeks of pregnancy was concluded when any one of the following glucose values are met or exceeded according to American Diabetes Association (ADA):Fasting blood glucose: 95 mg per dL, 1st hr: 180 mg per dL, 2nd hr 153 mg per dl. Plasma FBS >95 mg/dL and PPBS >140 mg/dL 15 were considered high values before labor. The normal umbilical cord insulin level is <13 µIU/L 16 and levels> 13 µIU/L is considered fetal hyperinsulinemia.
Statistical Results and Analysis
Characteristics of study participants
The study included 94 GDM mothers and their new-borns (Group 1) and 94 Non-GDM mothers and their offspring (Group 2). The comparison of demographic variables of GDM and Non-GDM mothers and their new-bornsis shown in Table 1. Both the groups did not differ significantly in gestational age, maternal height, pregnancy weight during 1st trimester, pregnancy weight at term, and placental weight with a p-value >0.05. There is a significant difference between maternal age ( 25.61 + 4.06 vs 23.98 + 3.37, p-value 0.03), maternal FBS and PPBS (91.52 ± 7.99 vs 84.00 ± 8.24, p < 0.001), maternal BMI during 1st trimester (24.21 ± 2.83 vs 20.92 ± 2.03, p-value < 0.001), maternal BMI at term (29.66 ± 2.38 vs 24.62 ± 2.10, p-value < 0.001), fetal umbilical cord insulin (24.97 ± 19.36 vs 5.25 ± 6.50, p < 0.001), baby weight (3.35 ± 36.58 vs 2.96 ± 0.27, p < 0.001), 1 minute APGAR score (7.69 ± 0.58 vs 7.91 ± 0.28, p < 0.001) and 5 minutes APGAR score (8.80 ± 0.43 vs 8.91 ± 0.28, p < 0.028) between the 2 groups.
Table 1: Comparison of Demographic Variables Between GDM and Non-GDM mothers and Their New-borns by Independent student’s t-test
| S.NO | CATEGORY | GDM | NON – GDM | p VALUE
|
| 1. | Maternal age(years) | 24.61 ± 4.06 | 23.98 ± 3.37
|
0.003
|
| 2 | Gestational Age (weeks) | 37.84 + 3.76 | 42.39 + 37.29 | 0.241 |
| 3. | Maternal height (cms) | 159.83 ± 2.39 | 158.72 ± 2.27
|
1.000
|
| 4. | Pregnancy weight (kgs) during 1st trimester | 64.36 ± 5.39 | 64.36 ± 5.34 | 1.000
|
| 5. | Pregnancy BMI (kg/m2) during 1st trimester | 24.21 ± 2.83 | 20.92 ± 2.03 | < 0.001
|
| 6. | Pregnancy weight at term (kgs) | 76.44 ± 5.35 | 85.67 ± 4.36 | 1.000 |
| 7. | Pregnancy BMI at term (kg/m2) | 29.66 ± 2.38 | 24.62 ± 2.10 | < 0.001 |
| 8. | Maternal FBS before delivery (mg/dL) | 91.52 ± 7.99 | 84.00 ± 8.24 | < 0.001 |
| 9. | Maternal PPBS before delivery (mg/dL) | 148.62 ± 13.72 | 122.90 ± 6.26 | < 0.001 |
| 10. | Fetal insulin (µIU/L) | 24.97 ± 19.36 | 5.25 ± 6.50 | < 0.001 |
| 11. | Baby Weight (kgs) | 3.35 ± 0.33 | 2.96 ± 0.27 | < 0.001 |
| 12. | Placenta weight (grams) | 497.68 ± 4.27 | 489.72 ± 3.97 | 1.000 |
| 13. | APGAR score in 1 Minute | 7.69 ± 0.58 | 7.91 ± 0.28 | < 0.001 |
| 14. | APGAR score in 5 Minutes | 8.80 ± 0.43 | 8.91 ± 0.28 | 0.028 |
Abbreviations: BMI – Body Mass Index; FBS – Fasting Blood Glucose; PPBS – Postprandial Blood Glucose; APGAR – Appearance, Pulse, Grimace, Activity, and Respiration.
Continuous variables are described using mean ± standard deviation
p-value < 0.05 is considered significant.
Correlation Between Maternal Blood Glucose and Fetal Insulin Levels in Both the GDM and Non-GDM Groups
Maternal FBS and PPBS in the GDM group showed a positive correlation with fetalumbilical cord insulin levels with r-valueof 0.272 (p-value 0.008) and r-valueof 0.286 (p-value 0.005).
Table 2: Correlation Between Maternal Blood Glucose andFetal Insulin in GDM Women by Pearson’s Correlation
| FETAL INSULIN (µIU/L) | ||
| Maternal parameter | r value | p-value |
| FBS (mg/dL) | 0.272 | 0.008 |
| PPBS (mg/dL) | 0.286 | 0.005 |
p-value < 0.05 is considered significant
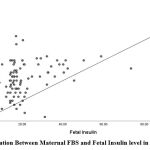 |
Figure 1: Correlation Between Maternal FBS and Fetal Insulin level in the GDM group |
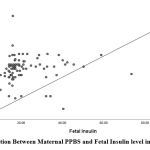 |
Figure 2: Correlation Between Maternal PPBS and Fetal Insulin level in the GDM group |
Maternal FBS and PPBS in the Non-GDM group showed a positive correlation with fetalumbilical cord Insulin levels with r-value of -0.087 (p-value 0.243) and r-valueof 0.212 (p-value 0.018).
Table 3: Correlation Between Maternal Blood Glucose and Fetal Insulin in Non-GDM Women by Pearson’s Correlation
| FETAL INSULIN (µIU/L) | ||
| Maternal parameter | r-value | p-value |
| FBS (mg/dL) | -0.087
|
0.243 |
| PPBS (mg/dL) | 0.212 | 0.018
|
p-value < 0.05 is considered significant
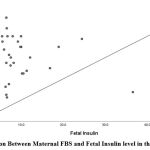 |
Figure 3: Correlation Between Maternal FBS and Fetal Insulin level in the Non-GDM Group |
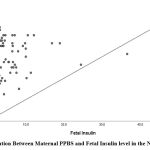 |
Figure 4: Correlation Between Maternal PPBS and Fetal Insulin level in the Non-GDM Group |
Discussion
Insulin resistance and deficiency in adults is highly related to the incidence of GDM17. Several studies on GDM have reported on the metabolic abnormalities of GDM mothers. Quite a few focused on maternal-fetal metabolic mechanisms in pregnant women with GDM 18. Our study tried to determine the effect of GDM on fetal insulin resistance.We found that Maternal FBS and PPBS in the Non-GDM group showed a positive correlation with fetal umbilical cord Insulin levels.
The mechanism that gives an explanation for the hyper insulin levels in the fetuses of GDM mothers is the hyperactivity in the fetal β-cell mass since maternal blood glucose is a critical stimulator of fetal β-cell hyperplasia 19. The pancreatic β – cell hyperplasia cited inside the pancreas of a newborn child from a GDM mother demonstrated that during the early fetal period, the proliferative potential of the β -cell is very excessive 20. Neonatal hyperinsulinemia in association with neonatal hypoglycemia is a marker of the above-mentioned mechanism. This is an energetic process during the early neonatal period and additionally during adolescence, puberty and childhood. At around two decades of life, when the β-cells of the fetus arrive at the post-mitotic stage their ability for replication is almost impossible and leads to T2DM. In order to break the such vicious cycle, it is wise to improve maternal glycemic control during pregnancy itself21. A study conducted by Wang et al., and Arshad et al., explains the anabolic effect of insulin is thought to contribute to increasingfetal adipocyte mass and placental tissues in utero that are liable to change with maternal metabolic parameters. There is fetal β–cellhypertrophy and hyperplasiawith excessive circulating insulin levels in the serum which upregulates the expression of many genes, leptin, and inflammatory cytokines levels in the placenta leading to fetal macrosomia and placental volume which compensates for the high demands of the growing fetus in utero to an extent. Thereafter, fetal hypoxia ensues leading to unexplained maternal and fetal adverse outcomes 22,23. Dabelea et al., and Desoye et al., states that intrauterine exposure to high maternal blood glucose is associated with fetal hyperinsulinemia, obesity, and T2DM in the offspring in the future. Maternal hyperinsulinemia through its own effect on lowering maternal hyperglycemia, will increase the glucose passage across the placenta and therefore the influx of glucose towards the fetus. The apparent documentation is that maternal glycemic control should be optimized very early during pregnancy to avoid fetal hyperinsulinemia 24,25.
Conclusion
In summary, GDM and fetal hyperinsulinemia are associated with the long-term development of obesity, and diabetes mellitus in the offspring, involving deleterious mechanisms of intrauterine programming.Our results suggest a positive correlation between maternal plasma blood glucose and fetal insulin levels with clear statistical evidence of metabolic programming during pregnancy which would help to identify high-risk babies early and to create awareness about GDM and its complications.
Strengths of the study
It is a case-control study.
Maternal and fetal parameters were correlated.
Timed collection and storage of cord blood samples.
Fetal cord blood insulin was assayed which is more sensitive than venous blood.
Fetal cord insulin was assayed by CLIA method.
Limitations
HOMA-IR using C-peptide for both mothers and new-born was not evaluated.
The present study is done in asingle centre.
Conflict Of Interest
There are no conflict of Interest.
References
- Wang X, Yang T, Miao J, Liu H, Wu K, Guo J, Chen J, Li T. Correlation Between Maternal and Fetal Insulin Resistance in Pregnant Women with Gestational Diabetes Mellitus. Clinical Laboratory. 2018 Jun 1;64(6):945-53.
CrossRef - Das S, Behera MK, Misra S, Baliarsihna AK. Β-cell function and insulin resistance in pregnancy and their relation to fetal development. Metabolic Syndrome and Related Disorders. 2010 Feb 1;8(1):25-32.
CrossRef - Alejandro EU, Mamerto TP, Chung G, Villavieja A, Gaus NL, Morgan E, Pineda-Cortel MR. Gestational diabetes mellitus: a harbinger of the vicious cycle of diabetes. International journal of molecular sciences. 2020 Jul 15;21(14):5003.
CrossRef - Kamana KC, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Annals of Nutrition and Metabolism. 2015;66(Suppl. 2):14-20.
CrossRef - Burtis CA, Ashwood ER, Bruns DE. Tietz textbook of clinical chemistry and molecular diagnostics-e-book. Elsevier Health Sciences; 6th edition ,2012 Oct 14. p 1416.
- Bianco ME, Josefson JL. Hyperglycaemia during pregnancy and long-term offspring outcomes. Current diabetes reports. 2019 Dec;19(12):1-8.
CrossRef - Reddy KM, Balmuri S, Jagarlamudi A, Betha K. Prevalence of gestational diabetes mellitus and perinatal outcome: a rural tertiary teaching hospital based study. International Journal of Reproduction, Contraception, Obstetrics and Gynaecology. 2017 Aug;6(8):3594-8.
CrossRef - Swetha B. Influence of maternal HbA1C on fetal insulin levels. International Journal of Contemporary Paediatrics. 2017 Mar;4(2):604.
CrossRef - Lima RA, Desoye G, Simmons D, Devlieger R, Galjaard S, Corcoy R, Adelantado JM, Dunne F, Harreiter J, Kautzky‐Willer A, Damm P. The importance of maternal insulin resistance throughout pregnancy on neonatal adiposity. Paediatric and perinatal epidemiology. 2021 Jan;35(1):83-91.
CrossRef - Kampmann U, Knorr S, Fuglsang J, Ovesen P. Determinants of maternal insulin resistance during pregnancy: an updated overview. Journal of diabetes research. 2019 Nov 19;2019.
CrossRef - Kaaja R, Rönnemaa T. Gestational diabetes: pathogenesis and consequences to mother and offspring. The review of diabetic studies: RDS. 2008;5(4):194.
CrossRef - Seshiah V, Balaji V, Balaji MS, Sanjeevi CB, Green A. Gestational diabetes mellitus in India. Japi. 2004 Sep 21;52(9):707-11.
- Wu Y, Liu B, Sun Y, Du Y, Santillan MK, Santillan DA, Snetselaar LG, Bao W. Association of maternal prepregnancy diabetes and gestational diabetes mellitus with congenital anomalies of the newborn. Diabetes Care. 2020 Dec 1;43(12):2983-90.
CrossRef - Ye W, Luo C, Huang J, Li C, Liu Z, Liu F. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. bmj. 2022 May 25;377.
CrossRef - American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes care. 2018 Jan 1;41(Supplement 1):S21.
CrossRef - Simental-Mendía LE, Castañeda-Chacón A, Rodríguez-Morán M, Guerrero-Romero F. Birth-weight, insulin levels, and HOMA-IR in newborns at term. BMC pediatrics. 2012 Dec;12(1):1-5.
CrossRef - Kua KL, Hu S, Wang C, Yao J, Dang D, Sawatzke AB, Segar JL, Wang K, Norris AW. Fetalhyperglycemia acutely induces persistent insulin resistance in skeletal muscle. Journal of Endocrinology. 2019 Jul 1;242(1):M1-5.
CrossRef - Luo ZC, Delvin E, Fraser WD, Audibert F, Deal CI, Julien P, Girard I, Shear R, Levy E, Nuyt AM. Maternal glucose tolerance in pregnancy affects fetal insulin sensitivity. Diabetes care. 2010 Sep 1;33(9):2055-61.
CrossRef - Sonagra AD, Biradar SM, Dattatreya K, DS JM. Normal pregnancy-a state of insulin resistance. Journal of clinical and diagnostic research: JCDR. 2014 Nov;8(11):CC01.
CrossRef - Tumminia A, Scalisi NM, Milluzzo A, Ettore G, Vigneri R, Sciacca L. Maternal diabetes impairs insulin and IGF-1 receptor expression and Signaling in human placenta. Frontiers in Endocrinology. 2021 Mar 10;12:621680.
CrossRef - Pascu M, Marin JA, Guja C, Ionescu-Tirgoviste co. Proinsulin level in women with gestational diabetes according to body mass index. Proc. Rom. Acad., Series B, 2011:2 p117–123.
- Wang Q, Huang R, Yu B, Cao F, Wang H, Zhang M, Wang X, Zhang B, Zhou H, Zhu Z. Higher fetal insulin resistance in Chinese pregnant women with gestational diabetes mellitus and correlation with maternal insulin resistance. PloS one. 2013;8(4).
CrossRef - Catalano PM, Presley L, Minium J, Hauguel-de Mouzon S. Fetuses of obese mothers develop insulin resistance in utero. Diabetes care. 2009 Jun 1;32(6):1076-80.
CrossRef - Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000 Dec 1;49(12):2208-11.
CrossRef - Desoye G, Nolan CJ. The fetal glucose steal: an underappreciated phenomenon in diabetic pregnancy. Diabetologia. 2016 Jun 1;59(6):1089-94.
CrossRef







