Jihan Hussein1* , Mona El Bana1
, Mona El Bana1 , Yasmin Abdel Latif1
, Yasmin Abdel Latif1 , Safaa Saleh2
, Safaa Saleh2 and Emad tolba3
and Emad tolba3
1Medical Biochemistry Department, National Research Centre, Doki, Giza, Egypt.
2Branch of Biophysics, Faculty of Science (Girls), Al Azhar University, Egypt.
3Polymers and Pigments Department, National Research Centre, 33 El-Bohouth st., Dokki, Giza, Egypt.
Corresponding Author E-mail: jihan_husein@yahoo.co
DOI : https://dx.doi.org/10.13005/bpj/2587
Abstract
Diabetes is connected with diminished wound healing, that makes patients liable to continuing difficult wounds. Metal nanomaterials as single conjugates have established to keep possible properties of wound when metal nanoparticles are coupled with other wound covering materials. This study aimed to investigate a possible role of cotton fabrics full with silver nanoparticles (AgNPs) to enhance wound healing in diabetic model induced by streptozotocin (STZ).Animals were classified into four groups including the wounded group that were equivalently covered with sterile dressing that made of cotton fabric which had been saturated with different concentrations of silver nanoparticles, and the control group that was preserved with only cotton covering without any treatment (blank group); percent of wound contraction in different studied groups was estimated. Plasma nitric oxide (NO), malodialdehyde (MDA), reduced glutathione (GSH) were measured. Serum neutrophil elastase and nuclear factor kappa b (NF-κb) were assayed by ELISA. Homocystiene (Hcy) was estimated by HPLC. Our results revealed an elevation in wound area, MDA, NF-κb, Hcy, and elastase in the wound group compared to treated groups concomitant with a decrease in plasma nitric oxide and reduced glutathione activities, while treatment with AgNPs significantly ameliorated these parameters in treated group compared to blank group. AgNPs showed high wound contraction rates according to their used concentration .In conclusion; AgNPs have gained considerable attention amongst researchers in wound healing applications, owing to their physicochemical and biological properties. AgNPs promote wound healing and effectively control the growth of microorganisms at the wound site, and this strategy plays an important role in the treatment of wounds.
Keywords
Diabetes Mellitus; HPLC; Homocystiene; Silver Nanoparticles; Wound Healing
Download this article as:| Copy the following to cite this article: Hussein J, Bana M. E, Latif Y. A, Saleh S, Tolba E. Wound Healing Activity of Cotton Fabrics Loaded with Silver Nanoparticles in Experimental Model of Diabetes. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Hussein J, Bana M. E, Latif Y. A, Saleh S, Tolba E. Wound Healing Activity of Cotton Fabrics Loaded with Silver Nanoparticles in Experimental Model of Diabetes. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3Tm1NQ6 |
Introduction
Wound healing is a forced response to various types of stimuli that affects skin or any organ. In the case of tissue or skin injury, a sequential overlapping cascade of events occurs which eventually were resulted in the restoration of normal tissue1 . Wound healing is typically divided into different phases, namely inflammation, cell proliferation, angiogenesis, collagen deposition, and re-epithelization 2,3 . Impaired wound healing can lead to difficulties in treating deep tissue infections 1 . There are many human diseases characterized by impaired wound healing and chronic skin ulcers4 . Thus, chronic wounds are a common complication in patients with diabetes that often lead to resection. Diabetes is associated with impaired wound healing, making patients susceptible to chronic non-healing wounds 5. Such wounds preceded 84% of all diabetic lower extremity amputations5-6 and once amputation occurs, patients have a 5-year mortality rate of 50%5, 7.
Chronic diabetic wounds are trapped in a persistent inflammatory state with elevated levels of pro-inflammatory cytokines and proteases together with impaired expression of growth factors 5, in addition to elevation of oxidative stress state when the production of ROS exceeds the anti-oxidant capacity in diabetic patients 8.The formation of advanced glycation end-products (AGEs) under hyperglycemia and the interaction with their receptors (RAGE) are associated with impaired wound healing in diabetic mice as well 9 . Neutrophils are the predominant cell type in the first inflammation phase and begin to shrink after 24- 36 h by apoptosis in the time of circulating monocytes enter the wound and mature into tissue macrophages that play a very important role in the wound healing. This process is mediated by the chemokines IL-8 which is released by neutrophils, then attracts the macrophages and other cells to the wound site 10. Additionally, the enhancement of tumor necrosis factor –α (TNF-α) & IL-6 production induced nerve destruction 11 .
Indeed, skin wounds are typically treated with the combination of protective barrier, antibiotics and topical growth agents to shorten inflammatory phase which is a well-known symptom for chronic wounds. Although various wound healing agents are widely used, it has recently been shown that the topical drug delivery systems using nanoantibiotics (nAbts) represents a new paradigm involving nanomaterials to fight against microbial infection compared to the traditional antibiotic agents 12.Thus, preparation of metal-based antimicrobial agents has drawn a significant attention in the past few years, as alternative and reports indicated that microbial resistance to the practiced antibiotics can be reversed by active nanometals13 . Metal-based nanoparticles including silver (AgNPs), gold (AuNPs), zinc oxide (ZnONPs), titiunm oxide (TiO2NP) and copper oxide (CuONPs) have been shown to exhibit remarkable strong and sustainable antimicrobial activity against a wide array of bacteria, fungi, algae and even virus 14 and 15. The metal-based nanoparticles involve distinct multiple bactericidal mechanisms including oxidative stress, destroying bacterial cell membrane or other cellular organelles and non-specific mechanism (i.e. disruption of cell signaling process), which (match the color) evidently underline the powerful potential of nanosize metal-based particles as effective alternative antibacterial agents to avoid bacteria antibiotic-resistance mechanism16 , 17.
Among various metal nanomaterials, silver nanoparticles (AgNPs), which are widely utilized in formulating ointments for hurts 18 ,19 . AgNPs are well known for their comprehensive range of antimicrobial activity, competently abolishing bacteria, viruses ,fungi, protozoa and 20 . In in vitro study, AgNP was used for treatment of human keratinocytes and fibroblasts, and it effectively reduced inflammatory cytokines, oxidative stress and stimulated healing 21 . Additionally, the current application of AgNP reduced the counts of neutrophils and also interleukin (IL)-6 concentration, and linked with the elevation of vascular endothelial growth factor (VEGF) , IL-10 , and TGF-β levels 22 . From these findings, we aimed to investigate a possible role of cotton fabrics loaded with silver nanoparticles in improving wound healing in experimental model of diabetes.
Materials and methods:
Materials
Hcy ( HPLC standard ) was obtained from Sigma-Aldrich Co. (St. Louis, MO, USA), low molecular weight CMC (89% degree of deacetylation), and silver nitrate (AgNO3) were bought from Sigma-Aldrich Co. (St. Louis, MO, USA). Traditional wound coverings were obtained from a local provider. Analytical grade anhydrous ethanol was purchased from Elnaser Company Egypt. All the reagents were used as received.
Animals
Forty male albino rats weighing 180±10 g were used. All rats were housed separately in sterile stainless steel cages for one week in standard conditions of temperature and light; fed standard rodent chow. Animal procedures were performed in accordance with the Ethics Committee of the National Research Centre and followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Methods
Synthesis of silver colloidal solutions using chemical reduction
Carboxymethyl Chitosan (CMC) containing Silver NPs was prepared in water-ethanol mixture. In brief, one gram of CMC powder was dissolved in 50 ml solution with continuous string for 1h. Then, about 10 mL of freshly prepared AgNO3 (0.2 M) was added drop wise into CMC aqueous solution. The color changes to dark brown after adding silver nitrate solution. The mixture solution was kept at room temperature for 30 min in dark. Then, 10 ml of anhydrous ethanol were slowly added to the CMC/AgNO3 mixture. The mixture was vigorously stirred and heated to reach 80°C (1°C/min) holding for 3 h until obtaining a homogenous colloidal solution without aggregating. The color of the solution was gradually changed from dark brown to yellowish brown indicating the formation of Ag NPs.
Cotton fabric treatment with CMC/silver colloidal solutions
In order to obtain the desired wound dressings, the traditional wound dressings were introduced in the CMC-silver nanoparticles colloidal solution, until the nanoparticles were incorporated in the wound dressing. Then, the wound dressings were removed from the solution and genially wiped over two filter paper to remove the excess of CMC/AgNPs solution. Finally, the wound dressings were soaked in ethanol solution for 3h and then collected on a glass Petri dish to dry at 60°C.
Characterization of as-prepared CMC/Ag NPs sample
Synthesized AgNPs absorption peak was observed in the UV–visible spectrophotometer (Shimadzu (UV 2500), Japan). UV–visible spectrophotometer range was from 200 nm to 900 nm. The TEM technique was employed to visualize the morphology and the size of the synthesized Ag NPs using Ultra High Resolution Transmission Electron Microscope( TEM) ,JEOL-2010). TEM grids were prepared by placing a drop of the particle solution on a carbon-coated copper grid and drying under lamp. In addition, the wound dressing treated samples were coated with gold and examined using field emission Scanning Electron Microscope SEM (Jeol JXA 840, Japan).
Study of antibacterial potential
The antibacterial activity of wound dressing samples was performed in nutrient agar medium against P. aeruginosa (Gram-negative bacteria: ATCC 433) and S. aureus: (Gram-positive bacteria: ATCC 1688), the bacteria most commonly isolated from wound infections. In details, the medium was prepared and sterilized at 120 °C for 1 h in an autoclave and then it was incubated at 50 °C (0.5 mL/50 mL of medium) with the suspension (52.2 cfu mL−1) of microorganism (McFarland barium sulfate standard) and transferred into petri dish . Both bacteria were added separately into broth solution in separate test tubes. The dry sample was added to the dishes; the resulting growth of bacteria was observed when the plates were pre-incubated for 24 h at 37 °C for antibacterial activities. The existence of inhibition zones was measured as the distance from the border of disk to the edge of the bacterial growth.
Biological studies
In vitro study
Cell viability was assessed by the mitochondrial dependent reduction of yellow MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) to purple formazan (Mosmann,1983).
Procedure
All the following procedures were done in a sterile area using a Laminar flow biosafety cabinet Class II A2 (Manufactured by: Labconco).Cells were suspended in DMEM medium, 1% antibiotic-antimycotic mixture (10,000U/ml Potassium Penicillin, 10,000µg/ml Streptomycin Sulfate and 25µg/ml Amphotericin B) and 1% L-glutamine and 5% fetal bovine serum at 37 ºC under 5% CO2 using CO2 incubator ( Sartorius stedium ,biotech).
Human skin fibroblast (HSF) cell lines were purchased from American Type Culture Collection (ATCC) and batch cultured for 10 days, then seeded at a concentration of 10×103 cells/well in fresh complete growth medium in 96-well plastic plates at 37 ºC for 24 h under 5% CO2 either alone (negative control) or with different concentrations of silver nanoparticles (500, 250, 125, 62.5, 31.25, 15.625 ug/ml). After 48 h of incubation, medium was aspirated, 20 ul MTT salt (2.5μg/ml) were added to each well and incubated for further four hours at 37ºC under 5% CO2. To stop the reaction and dissolving the formed crystals, 200μL of 10% sodium dodecyl sulphate (SDS) in 0.01M HCL was added to each well and incubated overnight at 37ºC 23.
The absorbance was then measured using a microplate multi-well reader (Bio-Rad Laboratories Inc., model 3350, Hercules, California, USA) at 595nm and a reference wavelength of 620nm.
Viability = absorbance of drug / absorbance of control x 100
Cytotoxicity = 100- viability
In vivo study
Induction of diabetes
Streptozotocin was dissolved in 50 mM sodium citrate solution (pH 4.5) containing 150 mM sodium chloride; the solution (6 mg/100 g body weight) was subcutaneously administered in rats. Fasting blood sugar was estimated after 3 days to confirm the development of diabetes mellitus. The animals were considered diabetic if fasting glucose level was ≥ 200mg/dl 24 .
Incision wound operation
Diabetic rats were anesthetized with diethyl ether (40%) and their dorsal surface was shaved with a sterile blade. The shaved area was sterilized with ethanol (70%). A single longitudinal skin incision was done on the shaved area.
Experimental design
The wounded skin animals were uniformly dressed with experimental sterile dressing made of cotton fabric which had been impregnated with different concentrations of AgNPs. The negative control group was maintained with only cotton dressing without any treatment (Blank group). This experiment was conducted for a period of 7 days. Every day, the animals were dressed with a new cotton fabric impregnated with the group-respective concentration of AgNPs. Fasting blood sugar was recorded on the initial and final day of the study.
The diabetic wounded skin animals were uniformly dressed with experimental sterile dressing made of cotton fabric which had been impregnated with different concentrations of AgNPs as follow:
Group I: Diabetic wounded rats treated with only cotton dressing (Blank group)
Group II: Diabetic wounded rats treated with the CMC-silver nanoparticles colloidal at concentration 130.676 μg /ml?
Group III: Diabetic wounded rats treated with Ag NP-based dressing at with the CMC-silver nanoparticles colloidal at concentration 65.338 μg /ml.
Group IV: Diabetic wounded rats treated with cotton dressing treatment with CMC/silver colloidal solutions at concentration 32.669 μg /ml.
Measurement of the wound area
The progressive changes in wound area (mm) were recorded on the 1st, 4th and 7th days along the experimental period. The size of the wound area was photographed and is presented in Fig1. The percentage of wound contraction was also calculated from the equation that was described previously 25 .
n : number of days
After the experimental period, blood was withdrawn from the retro-orbital venous plexus of the eye using heparinized capillary tubes and collected in two tubes; the first one contains sodium fluoride for determination of fasting blood sugar and the second tube was dry clean tube without anti-coagulant for serum separation. All tubes were centrifuged at 5000 rpm using cooling centrifuge (Laborzentrifugen, 2K15, Sigma, Germany) for 10 min. Serum was separated and stored at −20ºC until determination of biochemical parameters.
Biochemical assays
Blood glucose was measured according to the method described by Trinder 26, nitric oxide level (NO) was measured as nitrite and determined using Griess reagent, according to the method of Moshage et al., 27 . Lipid peroxidation was assayed by measuring the level of malondialdehyde using the previous method 28 in which the thiobarbituric acid reactive substances (TBARS) react with thiobarbituric acid to produce a red colored complex having peak absorbance at 532 nm. Serum neutrophil elastase and NF-κb were measured by ELISA according to the method described by the manufacturing kit.
Determination of serum Homocystiene (hcy).
Hcy estimated by high-performance liquid chromatography (HPLC) system, Agilent technologies 1100 series equipped with a quaternary pump (G131A model) according to the method described previously 29.
Sample extraction: deproteinization of all samples was achieved using trichloro acetic acid (2% TCA).
HPLC condition: 30 μl from each extracted samples were injected into HPLC; separation was accomplished on reversed phase column (C18 x 25 cm x 0.46 cm, and I.D. 5 μm). The mobile phase was consisted of 40 mmol/L sodium phosphate monobasic monohydrate, 8 mmol/L heptanesulfonic acid, and 18% (v/v) methanol; pH of the mobile phase was adjusted to 3.1 then filtered 2 times through a 0.45 μm membrane filter ( 0.45 µ m pore size, WCN type) . The mobile phase was then delivered at a flow rate of 1 ml/min at 40°C. UV detection performed at 260 nm. Serial dilutions of hcy standard were injected into HPLC. The concentrations in samples were calculated from the standard curve constructed by plotting peak areas versus the corresponding concentrations using Agilent Chem Station software for LC and LC/MC system (Agilent Technologies).
Statistical Analysis
All experiments were done in triplicate, and the results were presented as mean ± standard deviation. Statistical analyses were performed with the one-way ANOVA test, by using Sigma Stat 3.5 software (Dundas Software Ltd, Toronto; Canada). P values ≤0.05 were considered statistically significant.
Results and discussion
Characterization of the CMCs-Ag NPs and wound dressing materials
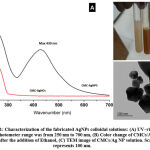
Design and development of new composite materials including metal/polymer nanocomposites have attracted much attention in medicine due to their novel physicochemical features and improved biological performances in vivo, upon comparing with bulk forms. Numerous studies have been reported on the preparation of silver nanoparticles in the presence of polymeric materials, as stabilizing agents, to provide a high colloidal stability of AgNPs during and after the synthesis process (Kumar, et al. 2019). In this study, CMCs/Silver nanoparticles colloidal solutions were synthesized using anhydrous ethanol, as chemical reducing agent. The UV-Vis spectra of synthesized AgNPs are shown in Figure 1-A. The formation of AgNPs after the reaction with AgNO3 solution was identified from the peak at around 430 nm due to the plasmon resonance formation. In addition, Figure 1-C represents the morphology and size of the prepared AgNPs confirmed by TEM analysis. The synthesis method produce nanoparticle sizes distributed at around 50-200 nm, it is important to mention that this methods tend to produce AgNPs with hexagonal-like forms.
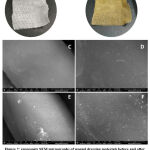
The wound dressing materials were treated with CMCs-AgNPs colloidal solutions by a simple immersion method. The surface morphology of the wound dressing sample was investigated by SEM as shown in Fig. 2.Images of raw wound dressing (Fig. 2a and b) shows smooth longitudinalfiber structure without any contaminating particles on their surfaces. While, the wound dressing treated with CMCs/Ag NPs was covered with ultrafine silver nanoparticles (Fig. 2c and d).
Antibacterial test
In the current study, the antimicrobial activity of wound dressing materials was assessed against two different pathogens: S. aureus (Gram-positive bacteria) and P. aeruginosa (Gram-negative bacteria)
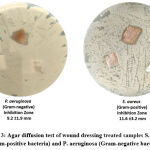 |
Figure 3: Agar diffusion test of wound dressing treated samples S. aureus (Gram-positive bacteria) and P. aeruginosa (Gram-negative bacteria). |
Cell viability
Cytotoxicity of the nanoparticles was carrying out and indicated that the highest concentration (500 ug/ml) is the only concentration appeared cytotoxicity (19.66%) while other concentrations appeared 0 toxicity; In addition, IC50 was calculated and it equal to 1306.76 ug/ml (table 1, fig.4). Accordingly we used the high dose which is tenth the IC50 equal to 130.676 μg/ml, and the other doses equal 65.338, and 32.669 ug/ml.
Table 1: Cytotoxicity of different concentration of Ag
| IC50 (µg/ml) | Cytotoxicity % | Viability
% |
Different dilution of drug 1 |
| = 1306.76 | 19.66 | 80.34 | 500 ug/ml |
| 00 | 100 | 250 ug/ml | |
| 00 | 100 | 125ug/ml | |
| 00 | 100 | 62.5 ug/ml | |
| 00 | 100 | 31.25 ug/ml |
IC50: Lethal concentration of the sample which causes the death of 50% of cells in 48 hours.
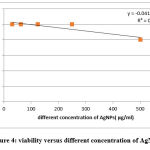 |
Figure 4: viability versus different concentration of AgNPs |
In vivo wound assay
In diabetic subjects, the wound healing process takes a long time and it is impaired rather than prevented 2.A non-healing wound is prone to produce a lot of complications which considered an important factor in delaying healing process. These complications include functional limitations, including alteration in gait and difficulty in walking; infections like cellulitis, abscess, osteomyelitis; gangrene and septicaemia and possible malignant changes in some cases 30 . Chronic wounds are at risk of developing malignant changes, known as a Marjolin’s ulcer, an aggressive form of squamous cell carcinoma31 .
In the current study the percentages of wound contraction in diabetic group that was maintained with only cotton dressing without any treatment (blank group) was only 48% (table 2, fig 4).
Thus, diabetes is a disease of altered glucose homeostasis and persistent hyperglycemia lead to advanced glycation end product (AGE) which is primarily responsible for the damage of cells and have a slow turn over. This hyperglycemia leads to elevation of reactive oxygen species (ROS) production and releasing of cytochrome C followed by activation of caspase-3and myocardial cell apoptosis 32, 33 . Partial inhibition of increased glucose levels by insulin almost completely prevents myocardial cell death; otherwise, it could be argued that there is a significant increase in apoptosis with hyperglycemia. Deregulation of apoptosis in response to hyperglycemia is generalized all over the body, leading to impaired wound healing along with the involvement of other target organs 34 .
Table 2: Wounded skin contraction in experimental rats in days1,4 and 7.
| Parameters
Groups |
Wound contraction (%) after one day | Wound contraction (%) after four days | Wound contraction (%) after seven days
|
| Group I( Blank group ) | 5 | 22 | 48 |
| Group II (treated group 1) | 7 | 39a | 78.9a |
| Group III (treated group 2) | 8 | 45a | 87.6ab |
| Group IV (treated group 3) | 8 | 44a | 93.5ab |
Pa value significant difference compared to group I.
Pb value significant difference compared to group II.
Pc value significant difference compared to group III.
Formation of AGEs under hyperglycemia elicited production of ROS by binding to its receptor (RAGE) expressed in various skin cells including keratinocytes, fibroblasts, dendrocytes, and to a lesser extent in endothelial cells and mononuclear cells 8, and induce the activation of NF-κB 35 which results in pathological gene expression and further impedes the normal activity of these cells during wound healing and impaired wound healing 36.
Uncontrolled diabetes leads to wound infections and inflammation due to high levels of pro-inflammatory cytokines such as TNF-α, NF-κB and IL- 6 (Wang et al., 2012). The elevated levels of these cytokines were linked to delay healing causing lesser migration and proliferation of fibroblasts and keratinocytes, reduced accumulation of collagen and deferred re-epithelialization 37 .
In agreement, the current study appeared that, the inflammatory biomarkers homocysteine, neutrophil elastase, and NF-κB in group I represented inflammatory chronicity whereas a lesser degree of inflammation was seen in wounds treated with AgNPs in treated groups I, II, and III (table 3).
Table 3: Inflammatory markers in different studied groups
| Parameters
Groups |
Hcys
mg/ml |
Neutrophil elastase ng/ml | NF-κB
ng/ml |
| Group I | 13.2±0.7 | 15.4±0.9 | 5.7±0.1 |
| Group II | 10.8±1.5 a | 12.6±0.3 a | 4.5±0.2 a |
| Group III | 6.0±0.5ab | 11.7±0.3 a | 3.5±0.1ab |
| Group IV | 2.5±0.4abc | 8.5±1.2abc | 2.9±0.1abc |
Pa value significant difference compared to group I.
Pb value significant difference compared to group II.
Pc value significant difference compared to group III.
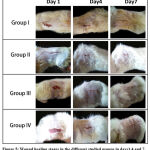 |
Figure 5: Wound healing stages in the different studied groups in days1,4 and 7. |
Table 4: Oxidant/antioxidant parameters in different studied groups
| Parameters
Groups |
NO
μmol/g |
MDA
nmole/gm |
GSH
μmol/g |
| Group I | 9.7±0.2 | 181.5±7.6 | 38.7±2.2 |
| Group II | 7.9±0.1a | 151.0±7.8 a | 53.5±1.7 |
| Group III | 6.4±0.6ab | 133.75±2.4 a | 70.2±1.1ab |
| Group IV | 5.0±0.3abc | 120.2±4.9ab | 74.9±1.4ab |
Pa value significant difference compared to group I.
Pb value significant difference compared to group II.
Pc value significant difference compared to group III.
Nanotechnology is a multidisciplinary scientific field that has drawn worldwide attention from various researchers in science and industry. Nanotechnology offers the facile synthesis of metal-based biocompatible nanomaterials that can be applied to a wide range of potential applications in medical and biological sciences 38 . In wound healing, nanoparticles have a broad range of applications and they offer a novel solution in wound care39
In the current study the percentages of wound contraction in treated groups (group II, III and IV) were significantly higher as recorded (78.9%, 87.6% and 93.5%) respectively; that is mean the high concentration of AgNPs gave the best effect and showed high wound contraction rates than the lower concentrations; thus the healing of these wound in our study appeared as a dose dependent (table 2, fig 5).
AgNPs offer a high-degree of biocompatibility and biodegradability in physiological conditions and can be considered as an effective material for wound dressings in the treatment of different wounds 39. Thus, silver nanoparticles (AgNPs) are classified as metal-based nanoparticles and have gained considerable attention amongst researchers in wound healing applications, owing to their physicochemical and biological properties. AgNPs are non-cytotoxic and safe for patients in wound care management. The unique intrinsic features of AgNPs promote wound healing and effectively control the growth of microorganisms at the wound site, and this strategy plays an important role in the treatment of both acute and chronic wounds.
In the current study there was a significant increase in antioxidant enzyme activities represented by GSH with a significant decrease in MDA and NO levels in the treated groups compared with the blank group (table 4). In addition , these results were in agreement with the work of Arora et al., 40 who investigated the effects of silver particles of 7–20 nm on primary mouse cells and observed an increase in levels of reduced GSH and superoxide dismutase (SOD). The authors hypothesized that, at lower concentrations of silver (up to 25μg/ml and 100μg/ml), there was an enhancement of cellular protection mechanisms; in their earlier work on secondary skin cell lines, this enhancement was restricted to lower doses of silver, but it is noteworthy that the non-toxic concentration range is comparable to that encountered in topical applications of silver products, providing evidence for the safety of silver nanomaterials at such doses 40.
Inflammatory biomarkers presented as homocystien, neutrophil elastase and NF-κB in group I representing inflammatory chronicity whereas a lesser degree of inflammation was seen in wounds treated with AgNPs in treated groups (table 3). In addition there is significant differences between treated groups and each other indicating a recognizable effect of the high concentration of nanoparticles in group 4 in compared to other treated groups (group 2 and 3). Thus, AgNPs are believed to decrease the time for fibroblast’s invasion into wound tissue, and also possess anti-inflammatory properties 41 .AgNPs are known as a protease inactivator that acts to decrease inflammation and also reduce the time for tissue formation 42, which is considered an important factor in wound healing among diabetic subjects.
Conclusion
Actually, metal nanomaterials, as single conjugates have proven to possess potential wound healing properties; when metal nanoparticles are coupled with other wound dressing materials, it effectively removes microbes from the wound site. The output of this study has reported that treatment with cotton fabrics loaded with silver nanoparticles AgNPs resulted in a significant improvement in wound healing in vivo through increased antioxidant activity and decreased inflammatory markers in chronic disease causing hyper proliferative wounds.
Conflict of Interest
There are no conflict of interest.
Funding Sources
There are no funding source.
References
- Salazar, V. S.; Gamer, L. W.; Rosen, V., BMP signalling in skeletal development, disease and repair. Nature Reviews Endocrinology 2016, 12 (4), 203-221.
CrossRef - Falanga, V., Wound healing and its impairment in the diabetic foot. The Lancet 2005, 366 (9498), 1736-1743.
CrossRef - Reiber, G. E.; Raugi, G. J., Preventing foot ulcers and amputations in diabetes. The Lancet 2005, 366 (9498), 1676-1677.
CrossRef - LeBert, D. C.; Huttenlocher, A. In Inflammation and wound repair, Seminars in immunology, Elsevier: 2014; pp 315-320.
CrossRef - Eming, S. A.; Martin, P.; Tomic-Canic, M., Wound repair and regeneration: mechanisms, signaling, and translation. Science translational medicine 2014, 6 (265), 265sr6-265sr6.
CrossRef - Reiber, G. E.; Vileikyte, L.; Boyko, E. d.; Del Aguila, M.; Smith, D. G.; Lavery, L. A.; Boulton, A., Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes care 1999, 22 (1), 157-162.
CrossRef - Gregg, E. W.; Williams, D. E.; Geiss, L., Changes in diabetes-related complications in the United States. The New England journal of medicine 2014, 371 (3), 286-287.
CrossRef - Vincent, A. M.; Russell, J. W.; Low, P.; Feldman, E. L., Oxidative stress in the pathogenesis of diabetic neuropathy. Endocrine reviews 2004, 25 (4), 612-628.
CrossRef - Bodiga, V. L.; Eda, S. R.; Bodiga, S., Advanced glycation end products: role in pathology of diabetic cardiomyopathy. Heart failure reviews 2014, 19 (1), 49-63.
CrossRef - Qing, C., The molecular biology in wound healing & non-healing wound. Chinese Journal of Traumatology 2017, 20 (4), 189-193.
CrossRef - Abdallah, M.; Emam, H.; Attia, E.; Hussein, J.; Mohamed, N., Estimation of serum level of interleukin-17 and interleukin-4 in leprosy, towards more understanding of leprosy immunopathogenesis. Indian Journal of Dermatology, Venereology & Leprology 2013, 79 (6).
CrossRef - Zhang, W.; Hu, E.; Wang, Y.; Miao, S.; Liu, Y.; Hu III, Y.; Liu, J.; Xu, B.; Chen, D.; Shen, Y., Emerging antibacterial strategies with application of targeting drug delivery system and combined treatment. International Journal of Nanomedicine 2021, 16, 6141.
CrossRef - Metwaly, H. H.; Fathy, S. A.; Abdel Moneim, M. M.; Emam, M. A.; Soliman, A. F.; El-Naggar, M. E.; Omara, E. A.; El-Bana, M. A., Chitosan and solid lipid nanoparticles enhance the efficiency of alpha-lipoic acid against experimental neurotoxicity. Toxicology Mechanisms and Methods 2022, 32 (4), 268-279.
CrossRef - Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A. L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A., Metal-based nanoparticles as antimicrobial agents: an overview. Nanomaterials 2020, 10 (2), 292.
CrossRef - Eivazzadeh‐Keihan, R.; Bahojb Noruzi, E.; Khanmohammadi Chenab, K.; Jafari, A.; Radinekiyan, F.; Hashemi, S. M.; Ahmadpour, F.; Behboudi, A.; Mosafer, J.; Mokhtarzadeh, A., Metal‐based nanoparticles for bone tissue engineering. Journal of Tissue Engineering and Regenerative Medicine 2020, 14 (12), 1687-1714.
CrossRef - Correa, M. G.; Martínez, F. B.; Vidal, C. P.; Streitt, C.; Escrig, J.; de Dicastillo, C. L., Antimicrobial metal-based nanoparticles: a review on their synthesis, types and antimicrobial action. Beilstein journal of nanotechnology 2020, 11 (1), 1450-1469.
CrossRef - Tortella, G.; Rubilar, O.; Fincheira, P.; Pieretti, J. C.; Duran, P.; Lourenço, I. M.; Seabra, A. B., Bactericidal and virucidal activities of biogenic metal-based nanoparticles: Advances and perspectives. Antibiotics 2021, 10 (7), 783.
CrossRef - Olczyk, P.; Mencner, Ł.; Komosinska-Vassev, K., The role of the extracellular matrix components in cutaneous wound healing. BioMed research international 2014, 2014.
CrossRef - Zhou, Y.; Chen, R.; He, T.; Xu, K.; Du, D.; Zhao, N.; Cheng, X.; Yang, J.; Shi, H.; Lin, Y., Biomedical potential of ultrafine Ag/AgCl nanoparticles coated on graphene with special reference to antimicrobial performances and burn wound healing. ACS applied materials & interfaces 2016, 8 (24), 15067-15075.
CrossRef - Cameron, P.; Gaiser, B. K.; Bhandari, B.; Bartley, P. M.; Katzer, F.; Bridle, H., Silver nanoparticles decrease the viability of Cryptosporidium parvum oocysts. Applied and environmental microbiology 2016, 82 (2), 431-437.
CrossRef - Shaheen, T. I.; El-Naggar, M. E.; Hussein, J. S.; El-Bana, M.; Emara, E.; El-Khayat, Z.; Fouda, M. M.; Ebaid, H.; Hebeish, A., Antidiabetic assessment; in vivo study of gold and core-shell silver-gold nanoparticles on streptozotocin-induced diabetic rats. Biomedicine & Pharmacotherapy 2016, 83, 865-875.
CrossRef - Tian, J.; Wong, K. K.; Ho, C. M.; Lok, C. N.; Yu, W. Y.; Che, C. M.; Chiu, J. F.; Tam, P. K., Topical delivery of silver nanoparticles promotes wound healing. ChemMedChem: Chemistry Enabling Drug Discovery 2007, 2 (1), 129-136.
CrossRef - Septisetyani, E. P.; Ningrum, R. A.; Romadhani, Y.; Wisnuwardhani, P. H.; Santoso, A., Optimization of sodium dodecyl sulphate as a formazan solvent and comparison of 3-(4,-5-dimethylthiazo-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay with wst-1 assay in mcf-7 cells. Indonesian Journal of Pharmacy 2014, 25 (4), 245.
CrossRef - Kohli, R.; Meininger, C. J.; Haynes, T. E.; Yan, W.; Self, J. T.; Wu, G., Dietary L-arginine supplementation enhances endothelial nitric oxide synthesis in streptozotocin-induced diabetic rats. The Journal of nutrition 2004, 134 (3), 600-608.
CrossRef - Werner, S.; Breeden, M.; Hübner, G.; Greenhalgh, D. G.; Longaker, M. T., Induction of keratinocyte growth factor expression is reduced and delayed during wound healing in the genetically diabetic mouse. Journal of Investigative Dermatology 1994, 103 (4), 469-473.
CrossRef - Trinder, P., Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Annals of clinical Biochemistry 1969, 6 (1), 24-27.
CrossRef - Moshage, H.; Kok, B.; Huizenga, J. R.; Jansen, P., Nitrite and nitrate determinations in plasma: a critical evaluation. Clinical chemistry 1995, 41 (6), 892-896.
CrossRef - Mattson, M. P.; Furukawa, K.; Bruce, A. J.; Mark, R. J.; Blanc, E., as Convergence Points in the Pathophysiology. Molecular Mechanisms of Dementia 1996, 103.
CrossRef - (a) Hussein, J.; El-Naggar, M.; Badawy, E.; El-Laithy, N.; El-Waseef, M.; Hassan, H.; Abdel-Latif, Y., Homocysteine and asymmetrical dimethylarginine in diabetic rats treated with docosahexaenoic acid–loaded zinc oxide nanoparticles. Applied Biochemistry and Biotechnology 2020, 191 (3), 1127-1139; (b) El-Naggar, M. E.; Hussein, J.; El-sayed, S. M.; Youssef, A. M.; El Bana, M.; Latif, Y. A.; Medhat, D., Protective effect of the functional yogurt based on Malva parviflora leaves extract nanoemulsion on acetic acid-induced ulcerative colitis in rats. Journal of Materials Research and Technology 2020, 9 (6), 14500-14508.
CrossRef - Menke, N. B.; Ward, K. R.; Witten, T. M.; Bonchev, D. G.; Diegelmann, R. F., Impaired wound healing. Clinics in dermatology 2007, 25 (1), 19-25.
CrossRef - Stadelmann, W. K.; Digenis, A. G.; Tobin, G. R., Impediments to wound healing. The American journal of surgery 1998, 176 (2), 39S-47S.
CrossRef - Baynes, J. W.; Thorpe, S. R., Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 1999, 48 (1), 1-9.
CrossRef - Cai, L.; Li, W.; Wang, G.; Guo, L.; Jiang, Y.; Kang, Y. J., Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C–mediated caspase-3 activation pathway. Diabetes 2002, 51 (6), 1938-1948.
CrossRef - Mishra, M.; Kumar, H.; Tripathi, K., Diabetic delayed wound healing and the role of silver nanoparticles. Dig J Nanomater Bios 2008, 3 (2), 49-54.
- Lohwasser, C.; Neureiter, D.; Weigle, B.; Kirchner, T.; Schuppan, D., The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor-alpha. Journal of Investigative Dermatology 2006, 126 (2), 291-299.
CrossRef - Prompers, L.; Huijberts, M.; Apelqvist, J.; Jude, E.; Piaggesi, A.; Bakker, K.; Edmonds, M.; Holstein, P.; Jirkovska, A.; Mauricio, D., Delivery of care to diabetic patients with foot ulcers in daily practice: results of the Eurodiale Study, a prospective cohort study. Diabetic medicine 2008, 25 (6), 700-707.
CrossRef - Dinh, T.; Tecilazich, F.; Kafanas, A.; Doupis, J.; Gnardellis, C.; Leal, E.; Tellechea, A.; Pradhan, L.; Lyons, T. E.; Giurini, J. M., Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes 2012, 61 (11), 2937-2947.
CrossRef - (a) Mahmoud, M. A.; El-Bana, M. A.; Morsy, S. M.; Badawy, E. A.; Farrag, A.-E.; Badawy, A. M.; Abdel-Wahhab, M. A.; El-Dosoky, M. A., Synthesis and characterization of berberine-loaded chitosan nanoparticles for the protection of urethane-induced lung cancer. International Journal of Pharmaceutics 2022, 618, 121652; (b) Hussein, J.; El-Naggar, M. E.; Latif, Y. A.; Medhat, D.; El Bana, M.; Refaat, E.; Morsy, S., Solvent-free and one-pot synthesis of silver and zinc oxide nanoparticles: activity toward cell membrane component and insulin signaling pathway in experimental diabetes. Colloids and Surfaces B: Biointerfaces 2018, 170, 76-84.
CrossRef - Kumar, S. S. D.; Rajendran, N. K.; Houreld, N. N.; Abrahamse, H., Recent advances on silver nanoparticle and biopolymer-based biomaterials for wound healing applications. International journal of biological macromolecules 2018, 115, 165-175.
CrossRef - Arora, S.; Jain, J.; Rajwade, J.; Paknikar, K., Interactions of silver nanoparticles with primary mouse fibroblasts and liver cells. Toxicology and applied pharmacology 2009, 236 (3), 310-318.
CrossRef - Compton, C. C.; Nadire, K. B.; Regauer, S.; Simon, M.; Warland, G.; O’Connor, N. E.; Gallico, G. G.; Landry, D. B., Cultured human sole‐derived keratinocyte grafts re‐express site‐specific differentiation after transplantation. Differentiation 1998, 64 (1), 45-53.
CrossRef - Green, D. R., A Myc-induced apoptosis pathway surfaces. Science 1997, 278 (5341), 1246-1247.
CrossRef