Manuscript accepted on :15-03-2023
Published online on: 20-03-2023
Plagiarism Check: Yes
Reviewed by: Dr. Michael Jothirajan , Dr. Francisco Solano
Second Review by: Dr. Jagdish Joshi
Final Approval by: Dr. Patorn Promchai
Heba M. Abd el kareem1, 2 , Aiman I. Al-Qtaitat3, 4
, Aiman I. Al-Qtaitat3, 4 , Fadi S. Sawaqed5
, Fadi S. Sawaqed5 and Fardous S. Karawya3
and Fardous S. Karawya3
1Department of Medical Biochemistry and Molecular Biology, Faculty of Medicine, Benha University, Egypt
2Department of Biochemistry and Molecular Biology, Faculty of Medicine, Mutah Uiversity, Al-Karak, Jordan
3Department of Anatomy and Histology, Mutah University, Al-Karak, Jordan
4Dean of Faculty of Dentistry, Al zarqaa University, Jordan
5Department of Urology, Faculty of Medicine, Mutah Uiversity, Al-Karak, Jordan.
Corresponding Author E-mail: hebakareem@ymail.com
DOI : https://dx.doi.org/10.13005/bpj/2636
Abstract
Objectives: Noninvasive diagnosis of cancer bladder remains a challenge. The study aimed to evaluate the urinary gene expression of NDRG-2 (N-Myc downstream-regulated gene2) and MCM8 (the mini chromosome maintenance proteins) genes and their importance as novel urinary biomarkers for bladder cancer. In addition, to assess their diagnostic value in comparison with voided urine cytology is the focus of this work. Methods: the study included twenty healthy controls and fifty patients with bladder cancer. Quantitative real-time polymerase chain reaction (qRT-PCR) and voided urine cytology (VUC) were performed to demonstrate the NDRG2 and MCM-8 gene expression levels in the urine of healthy controls and bladder cancer patients. Results: There was a statistically significant decrease in NDRG-2 gene expression in bladder cancer group (4.38±0.66) compared to the control group (8.29±1.67). Gene expression of MCM-8 showed a statistically significant increase in bladder cancer group (5.57±0.79) in comparison to control group (4.55±1.39) with a significant negative correlation (ρ= -0.77) between NDRG-2 expression levels and tumor grade in cancer group (p<0.001), and a positive significant correlation (ρ=0.453) between MCM-8 expression levels and tumor grade in cancer group (p=0.001). NDRG-2 had the highest ability to predict bladder carcinoma (AUC of 1.0). In addition, the most precise differentiation between non–muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC) with AUC of 0.814. Conclusion: Expression of NDRG-2 and MCM-8 may be novel potential noninvasive biomarkers for diagnosis and prognosis of bladder cancer and a good tool for differentiation between NMIBC and MIBC with NDRG-2 is the most precise for diagnosis and differentiation over MCM-8, VUC and combined use of NDRG-2 and MCM-8.
Keywords
Bladder Carcinoma; Mini Chromosome Maintenance Proteins; NDRG-2; Polymerase Chain Reaction; Voided Urine Cytology
Download this article as:| Copy the following to cite this article: El-kareem H. M. A, Al-Qtaitat A. I, Sawaqed F. S, Karawya F. S. Urinary NDRG2 and MCM8 Gene Expression as New Noninvasive Biomarkers for Diagnosis and Differentiation between Muscle and Non-Muscle Invasive Bladder Cancer. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: El-kareem H. M. A, Al-Qtaitat A. I, Sawaqed F. S, Karawya F. S. Urinary NDRG2 and MCM8 Gene Expression as New Noninvasive Biomarkers for Diagnosis and Differentiation between Muscle and Non-Muscle Invasive Bladder Cancer. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3JqZozj |
Introduction
Bladder cancer (BC) is one of the most challenging expensive cancers to diagnose and treat. Its diagnosis depends on cystoscopy, which is invasive and expensive procedure, and voided urine cytology that lacks sensitivity in low-grade bladder cancer. Therefore, there is a challenge to identify highly sensitive noninvasive new biomarkers for BC1.
Muscle invaded BC (MIBC) is aggressive tumor with poor prognosis, a high risk of metastasis and recurrence. While Non-muscle invaded BC, (NMIBC) has a good prognosis. MIBC is usually treated by radical cystectomy and may be followed by postoperative chemotherapy, while NMIBC is commonly treated with transurethral resection. Therefore, an accurate early diagnosis of BC is a vital factor for clinical BC management especially when it can differentiate between MIBC and NMIBC2.
Interestingly, research revealed variable correlations between N-myc downstream-regulated gene 2 (NDRG2) and cancer pathogenesis. Collectively, the expression of NDRG2 is decreased within human tumors, while its overexpression suppresses the capacity of cancer cells to proliferate, invade and metabolize. Nevertheless, knowledge about the exact role of NDRG2 within bladder cancer is still restricted3.
Several studies suggested that NDRG-2 functions as cell stress-related gene and a candidate tumor suppressor. Recently, many studies concluded that NDRG-2 is down regulated in primary tumors and various human cancer cell lines through regulation of invasion and cell proliferation in bladder cancer4.
The mini chromosome maintenance proteins (MCMs) are ubiquitously expressed proteins. Previous studies have demonstrated that MCMs are implicated in DNA replication with eccentric expression with prognostic value in cancer. For instance, many recent studies suggested that MCM 2, 8, 10 were overexpressed in hepatocellular carcinom5. However, the role of MCMs family members remains unclear in the pathogenesis and progression of bladder cancer, suggesting that increased expression of MCM8 might be associated with increased malignancy6.
Consequently, we conducted our study to evaluate the urinary cell expression of NDRG-2 and MCM8 genes and their importance as novel noninvasive urinary biomarkers for bladder cancer and to assess their diagnostic value compared with voided urine cytology (VUC).
Materials and methods
The Committee of Ethics in the Faculty of Medicine, Mutah University, Jordan approved the study under number (882022). Signed informed written consent was obtained from the included study participants. The work has been carried out according to the Ethics of the World Medical Association (Declaration of Helsinki).
70 subjects of both genders chosen from the Urology Department, Al Karak governmental Hospital, Mutah, Jordan during the period from September 2019 to March 2022. The participants were in 2 groups. Fifty patients were diagnosed with bladder cancer with no history of chemotherapy or radiotherapy before sampling. Moreover, age sex matched 20 healthy control who attended hospital seeking medical treatment for bladder disease requiring cystoscopy with obtained renal biopsy during endoscopic removal of renal or ureteric stones to ensure that they are free from cancer.
Sampling
A naturally voided midstream single urine sample was collected in 100-mL sterile cups, put on ice immediately, and delivered to the laboratory within 1 hour.
-Voided urine cytology (VUC)
Approximately 10 ml were used for the preparation of the specimen. Then centrifugation for 30 minutes at 800 rpm was done and microscope slide was prepared from the precipitate. Then the slides were carefully submerged in 96% ethanol to fix the sediments for 30 minutes. Finally, they were stained using the Papanicolaou method7. A pathologist examined stained cytology slides.
The remaining urine specimen was processed as follows: centrifugation of urine samples at 4000 rpm for 10 min at 4 °C. Then the supernatant was removed without disturbing pellets. After that, twice washing the cell pellet with ice-cold buffer phosphate saline (PBS) was carried out. Then adding 1 ml PBS to the pellet, and the remaining ~ 0.5 ml of urine. The pellet was re-suspended by pipetting and were frozed at −80 °C to detect gene expression levels of NDRG-2 and MCM8 by qRT-PCR.
RNA extraction and quantitative RT-PCR
RNA-Spin total RNA extraction kit (iNtRON Biotechnology/South Korea) was used for extraction of total RNA from the collected urine samples. RNA purity and concentration were measured by Nanodrop (Biowave II, Germany). The extracted RNA was reverse transcribed using a one-step RT PCR-PreMix kit (iNtRON Biotechnology/South Korea). In brief, 8μl of ONE-STEP RT-PCR PreMix Kit were placed into PCR Eppendorfs. Then RNA templates and gene-specific primers were added to the PCR tubes. After that, tubes were completed to a total volume of 20μl using distilled H2O, and the mixture was mixed thoroughly. RT-PCR reaction of samples was performed using PCR thermal cycler (Perkin Elmer Cetus, Norwalk, CT, USA) as follows: a cycle of two steps, reverse transcription reaction at 45°C for 30 minutes. Followed by denaturation of RNA: cDNA hybrid at 94°C for 5 minutes followed by 40 cycles of 94℃ /60sec, 68℃ /60sec then 72℃ / 1min followed by Final Extension at 72℃ /5min. Integrated DNA Technologies/USA supplied Primers for both NDRG2 and MCM8 genes. Primers for NDRG2 were F-(5/-GCCCAGCGATCCTTACCTACC-3/) (R-5/ GGCTGCCCAATCCCAACC-3/). Primers for MCM8 were F-(5/-ATGGCTTTTCTTTGTGCTGC-3/) (R-5/ CCAGTCCATCGTAACTGTGAGA-3. We used β actin as a housekeeping control gene. Sequences of its primers were (F-5/-GGC ATC GTG ATG GAC TCC G-3/ and R -5/-GCT GGA AGG TGG ACA GCG A-3/). The relative mRNA gene expression fold changes of the studied genes were estimated by the method of 2-∆∆ct.
Statistical analysis
The data were analyzed using SPSS 20. Quantitative data were described as mean ± standard deviation and qualitative data as distribution and frequency. Mann-Whitney test and Student’s t-test were used to compare the mean of two groups of quantitative data of non-parametric and parametric data, respectively. The fisher exact and chi square test (X2-value) test compared categorical data. The correlation between NDRG-2 and MCM8 expression, tumor grade and size was assessed by Spearman correlation coefficient (rho; ρ) was used P-values <0.05 were considered statistically significant.
Results
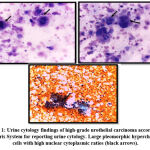
Fifty patients with diagnosed bladder cancer (BC) were included in this cohort. Figure (1) shows photos of urine cytology findings of one of those patients highlighting malignant features of high-grade urothelial carcinoma. As regards staging of BC participants, there were 56% MIBC (n= 28) and 44% NMIBC (n= 22), with non-significant statistical differences between MIBC and NMIBC groups regarding the voided urine cytology. (p-value > 0.05). Table 1.
Table 1: Voided urine cytology in bladder cancer group.
|
Voided urine cytology |
MIBC | NMIBC | p | ||
| No. | % | No. | % | ||
| Positive | 17 | 60.7 | 14 | 63.6 | 0.37 |
| Negative | 10 | 35.7 | 5 | 22.7 | |
| Missed | 1 | 3.6 | 3 | 13.6 | |
p≤0.05 significant p˃0.05 non-significant
The expression of NDRG2 and MCM8 in urinary samples by RT- PCR
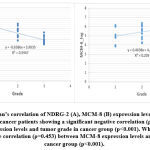
A statistically significant decline in NDRG-2 gene expression in the bladder cancer group (4.38±0.66) compared to the control group (8.29±1.67) (p<0.001). While gene expression of MCM-8 was significantly increased in the bladder cancer group (5.57±0.79) as compared to control group (4.55±1.39) (p<0.001) as shown in figure (2A).
Although VUC showed a non-significant statistical difference between MIBC and NMIBC groups (p > 0.05), gene expression of NDRG-2 and MCM-8 showed a significant decrease (p<0.001)and increase (p=0.001) between MIBC and NMIBC groups, respectively. Figure (2B).
Relationship between clinicopathologic parameters and urinary NDRG-2, MCM-8 gene expression of patients with bladder cancer
There was a statistically significant difference between NDRG-2 gene expression in the cancer group as regards the tumor stage (p<0.001) and grade (p<0.001). As regards MCM-8, there was also a statistically significant difference between MCM-8 gene expression in the cancer group regarding the tumor stage (p=0.001) and grade (p=0.004). However, for both genes, there was no statistically significant difference between cancer groups regarding sex, smoking, Bilharziasis, tumor type, tumor onset, tumor size, or voided urine cytology (p>0.05). Table 2.
Table 2: Relationship between urinary NDRG-2, MCM-8 gene expression and clinicopathologic parameters of patients with bladder cancer
|
Variable |
NDRG-2 | MCM-8 | |||
| Mean± SD | p | Mean± SD |
p |
||
| Sex | Females | 4.18±0.58 | 0.40 | 5.63±0.92 | 0.84 |
| Males | 4.41±0.67 | 5.56±0.77 | |||
| Smoking | smokers | 4.35±0.66 | 0.60 | 5.54±0.80 | 0.60 |
| Non-Smokers | 4.46±0.66 | 5.67±0.76 | |||
| Bilharzias | Positive | 4.26±0.71 | 0.77 | 5.69±0.50 | 0.79 |
| Negative | 4.38±0.66 | 5.57±0.80 | |||
| Type | TCC | 4.42±0.65 | 0.068 | 5.56±0.76 | 0.63 |
| SCC | 3.71±0.17 | 5.79±1.38 | |||
| Onset | Denovo | 4.40±0.66 | 0.54 | 5.52±0.82 | 0.28 |
| Recurrent | 4.24±0.68 | 5.85±0.51 | |||
| Stage | MIBC | 4.06±0.46 | <0.001** | 5.89±0.61 | 0.001** |
| NMIBC | 4.78±0.66 | 5.17±0.81 | |||
| Grade | I | 5.11±0.66 | <0.001** | 4.99±0.95 |
0.004** |
| II | 4.73±0.42 | 5.40±0.74 | |||
| III | 3.88±0.27 | 5.90±0.60 | |||
|
Tumor size |
<3cm | 4.58±0.59 | 0.18 | 5.59±0.60 | 0.93 |
| >3cm | 4.30±0.67 | 5.57±0.85 | |||
|
Voided urine cytology |
Positive | 4.36±0.65 | 0.97 | 5.49±0.82 | 0.46 |
| Negative | 4.41±0.73 | 5.79±0.64 | |||
| Missed | 4.33±0.52 | 5.42±1.09 | |||
To assess and compare the validity of VUC, NDRG-2, and MCM-8 as biomarkers for cancer bladder prediction and each biomarker’s ability to differentiate MIBC, a receiver operating characteristic (ROC) analysis for each area under the curve (AUC) was calculated. Our results revealed that NDRG-2 had the highest AUC of 1.0 (1.0-1.0), followed by the combined NDRG-2+MCM-8 with an AUC of 0.88 (0.802-958), followed by VUC with an AUC of 0.837 (0.743-0.930) while MCM-8 had the lowest AUC 0.744 (0.615-0.873). Moreover, NDRG-2 had the best to differentiate MIBC with an AUC of 0.814 (0.692-936), followed by MCM-8, which had an AUC equal to that of the combined markers (NDRG-2+MCM-8) 0.774 (0.637-0.912) and the least was VUC [AUC=0.554 (0.384-0.723]. Figure 4, 5A significant negative correlation (ρ= -0.77) between NDRG-2 expression level and tumor grade in the cancer group (p<0.001). While there was a significant positive correlation (ρ=0.453) between MCM-8 expression levels and tumor grade in the cancer group (p= 0.001) Figure 3
 |
Figure 4: show the validity of VUC, NDRG-2 and MCM-8 as biomarkers for prediction of cancer bladder. AUC of NDRG-2 gene expression in prediction of cases was the highest (1), |
 |
Figure 5: show the ability of VUC, NDRG-2 and MCM-8 biomarker to differentiate MIBC. NDRG-2 was the best to differentiate MIBC with AUC of 0.814 (0.692-936) followed by MCM-8 which had AUC of 0.774 |
Discussion
Previous studies found that NDRG2 regulates invasion and cell proliferation in bladder cancer8. In addition to its roles in DNA replication, MCM-8 reportedly indicated that it is related to several human tumors’ metastasis and invasion. The current study reported that MCM8 overexpression underlined prostate cancer aggressiveness 9. More importantly, the increased expression of MCM8 was considered as a valuable independent prognostic marker in pancreatic cancer10. However, the impact of MCM8 and NDRG2 on bladder cancer remains away from being fully demonstrated.
Consequently, we recruited 50 bladder cancer patients and 20 healthy controls. QRT-PCR and Voided urine cytology (VUC) were performed to measure the NDRG2 and MCM-8 expression in urine of healthy controls and patients with bladder cancer.
Our results demonstrated that MCM-8 was up-regulated in bladder cancer while NDRG-2 gene expression was statistically downregulated in bladder cancer group compared to control group, as shown in figure (2A). ROC curve analysis showed NDRG2 might be a good diagnostic marker, with an AUC of 0.888, with specificity and sensitivity were 81.4% and 85.5% respectively while in our study, the ROC curve showed AUC of 0.1 with the specificity and sensitivity were 100% and 98%, respectively indicating it could be a potential gold standard biomarker for non-invasive diagnosis of bladder cancer. Our results were in agreement with most of the published studies, such as the study of Zhang and his colleagues in 201711 and they reported that the relative NDRG2 expression was significantly decreased both at protein and mRNA levels in the urine of BC patients, and its low expression was dramatically correlated with tumor staging and grading.
Compared to other reported urinary biomarkers, Eissa et al. 12 reported that the Tissue inhibitor of metalloproteinase 2 (TIMP-2) and survivin might be considered urine biomarkers in early detection of BC. Survivin showed 95.3% specificity and 78.6% sensitivity in diagnosis of bladder cancer, while TIMP had 83.7% specificity and 93% sensitivity. Another study concluded that Vascular endothelial growth factor (VEGF) was an accurate urinary biomarker for bladder cancer, with 87% specificity and 83% sensitivity with an AUC of 0.88613.
In addition, and by using Spearman’s correlation, we further investigated whether NDRG2 and MCM-8 have a role in BC development, and we found a significant negative correlation (ρ= -0.77) between NDRG-2 expression levels and tumor grade in cancer group (p= 0.001). At the same time, a positive significant correlation (ρ=0.453) between MCM-8 expression levels and tumor grade in the cancer group (p= 0.001) was found. Our findings were supported by the results of Zhang and his colleagues in 201711. Their study reported that decreased expression of NDRG2 was associated with stage and grade. However, no statistically significant relationship between cancer groups regarding other clinicopathological features such as sex, smoking, Bilharziasis, tumor type, tumor onset, tumor size, or voided urine cytology (p>0.05), concluding that NDRG2 expression levels are closely associated with progression and pathogenesis of bladder cancer.
For more proof, in 2013, the Immunohistochemistry results of Li and his colleagues 14showed that the increased expression of NDRG2 in bladder carcinoma tissues was significantly decreased than in normal tissues. The expression of NDRG2 decreased as the degree of bladder carcinoma malignancy increased, and they conclusively reported that silencing of NDRG2 highly attenuates p53-mediated apoptosis. In addition, there was a report that NDRG2 could increase the expression of p53 in cells of breast cancer3. This information highly suggested that NDRG2 was a critical agent in regulating tumor apoptosis. While in 2015 the results of the study of Huang and his colleagues15 indicated that NDRG2 expression in mitochondria may arrest bladder cancer cells in S‑phase as well as decrease cell proliferation through inducing oncosis. It was therefore proposed that NDRG2 was not only a biomarker, but also a tumor suppressor for bladder cancer.
Recently, in 2021, Zhu et al. 16demonstrated that MCM8 expression in bladder cancer was up- regulated in association with advanced histologic stage with poor prognosis of BC patients. Furthermore, they found that MCM8 down regulation in bladder cancer cell lines decreased cell proliferation and induced apoptosis, concluding the substantial role of MCM8 in creating a rationale for the therapeutic potency of MCM8 inhibition in human bladder cancer therapy.
Recently in 2022 and in accordance to our results, Yu and his colleagues confirmed that mRNA and protein of MCM8 were significantly overexpressed in the tissues of hepatocellular carcinoma concluding that High MCM8 protein expression was an independent risk factor in HCC patients as MCM8 expression is altered in 60% of queried HCC patients and was associated with poor prognosis. 17previosly in 2020 Kang et al revealed that NDRG2 mRNA expression and protein levels were downregulated within both ovarian cancer tissues and cell lines. The overexpression of NDRG2 dramatically inhibited the cell viability and colony formation and tumor growth, whereas promoted the cell apoptosis, cell cycle arrest in G1 phase within ovarian cancer cells. More importantly, NDRG2 overexpression significantly enhanced the suppressive roles of cisplatin (DDP) in ovarian cancer cell viability18.
NDRG2 was repeatedly reported to be downregulated in a variety of cerebral tumors, including glioma and meningioma19. The transcription levels of human NDRG2 are significantly reduced in human glioblastoma tissues and human glioblastoma cell lines, and exogenous overexpression of NDRG2 repressed glioblastoma cell proliferation in vitro. Moreover, the expression level of NDRG2 was negatively correlated with the pathological grade of the brain tumors and positively correlated with survival in astrocytoma patients20. The above results suggest that NDRG2 may be a potential biomarker for predicting the prognosis of human brain tumors.
Analysis of differentially expressed genes in NMIBC versus MIBC phenotype is pivotal in understanding the molecular basis of bladder cancer progression21. Because pathogenesis and treatment of MIBC and NMIBC differ, and pathological evaluation is inadequate to precisely predict the prognosis of high-risk NMIBC. We found that NDRG-2 was the best to differentiate MIBC from NMIBC with an AUC of 0.814 followed by MCM-8 which had AUC equal to that of the combined markers (NDRG-2+MCM-8) 0.774 and the least was VUC AUC=0.554. Our study has a few limitations; the sample size was comparatively small. Research with a larger sample size is recommended, that remains a primary priority of our research team targets.
Conclusion
In summary, Expression of MCM-8 was increased in bladder cancer patients. NDRG-2 gene expression was decreased in bladder cancer patients and might be involved in its progression. NDRG2 might be a potential diagnostic and prognostic biomarker for bladder cancer. In addition to its pivotal role as a marker to differentiate MIBC from NMIBC. Research in this field would eventually provide novel methods for early diagnosis and new therapy for bladder cancer.
Acknowledgment
The authors acknowledge doctors and nurses in Al- Karak governmental hospital, Jordan for their support in collection of samples and technicians of medical biochemistry department, Mutah faculty of medicine, Jordan for their help in performing the biochemical assay.
Conflict of Interest
The authors declare that there are no conflict of interests.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not for- profit sectors.
References
- Song Y, Jin D, Ou N, Luo Z, Chen G, Chen J, Yang Y, Liu X. Gene expression profiles identified novel urine biomarkers for diagnosis and prognosis of high-grade bladder urothelial carcinoma. Frontiers in oncology. Mar 27;10:394 (2020). https://doi.org/10.3389/fonc.2020.00394
CrossRef - Zhu S, Yu W, Yang X, Wu C, Cheng F. Traditional classification and novel subtyping systems for bladder cancer. Frontiers in oncology:102 (2020). https://doi.org/10.3389/fonc.2020.00102
CrossRef - Hu W, Fan C, Jiang P, Ma Z, Yan X, Di S, Jiang S, Li T, Cheng Y, Yang Y. Emerging role of N-myc downstream-regulated gene 2 (NDRG2) in cancer. Oncotarget.;7(1):209–23 (2016) DOI: 10.18632/oncotarget.6228
CrossRef - Ma J, Liu W, Yan X, Wang Q, Zhao Q, Xue Y, Ren H, Wu L, Cheng Y, Li S, Miao L, Yao L, Zhang J. Inhibition of endothelial cell proliferation and tumor angiogenesis by up-regulating NDRG2 expression in breast cancer cells. PLoS One. 2012;7(2):e32368. Epub 2012 Feb 29. Retraction in: PLoS One. Oct 8;15(10): (2020) e0240574. PMID: 22393400; PMCID: PMC3290656. https://doi.org/10.1371/journal.pone.0032368
CrossRef - Liu Z, Li J, Chen J, Shan Q, Zheng S. MCM family in HCC: MCM6 indicates adverse tumor features and poor outcomes and promotes S/G2 cell cycle progression. BMC Cancer.18:200 (2018). DOI 10.1186/s12885-018-4056-8
CrossRef - Yang C, Wen Y, Li H, et al. overexpression of minichromosome maintenance 2 predicts poor prognosis in patients with gastric cancer. Oncol Rep. 27(1):135–142 (2012). DOI: 10.3892/or.2011.1473.
CrossRef - Palaoro LA, Angerosa M. Correlation between the cytology of urine sediment in fresh sample and smears stained by Papanicolaou and Giemsa methods. J Cytol. Jan;31(1):25-31 (2014). doi: 10.4103/0970-9371.130666
CrossRef - Huang J, Wu Z, Wang G et al: NMyc downstream regulated gene 2 suppresses the proliferation of T24 human bladder cancer cells via induction of oncosis. Mol Med Rep. 12(4): 5730–36 (2015). https://doi.org/10.3892/mmr.2015.4169
CrossRef - He DM, Ren BG, Liu S, et al. Oncogenic activity of amplified miniature chromosome maintenance 8 in human malignancies. Oncogene. Jun;36(25):3629-39 (2017). DOI: 10.1038/onc.2017.123
CrossRef - Li Q, Wang S, Wu Z, Liu Y. DDX11-AS1exacerbates bladder cancer progression by enhancing CDK6 expression via suppressing miR-499b-5p. Biomed Pharmacother.127:110164 (2020). https://doi.org/10.1016/j.biopha.2020.110164
CrossRef - Zhang M, Ren B, Li Z, Niu W, Wang Y. Expression of N-Myc downstream-regulated gene 2 in bladder cancer and its potential utility as a urinary diagnostic biomarker. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 23:4644 (2017). doi: 10.12659/MSM.901610
CrossRef - Eissa S, Shabayek MI, Ismail MF et al: Diagnostic evaluation of apoptosis inhibitory gene and tissue inhibitor matrix metalloproteinase-2 in patients with bladder cancer. IUBMB Life. 62(5): 394–99 (2010). https://doi.org/10.1002/iub.325
CrossRef - Urquidi V, Goodison S, Kim J et al: Vascular endothelial growth factor, carbonic anhydrase 9, and angiogenin as urinary biomarkers for bladder cancer detection. Urology. 79(5): 1185e1–6 (2012). https://doi.org/10.1016/j.urology.2012.01.016
CrossRef - Li R, Yu C, Jiang F, Gao L, Li J, Wang Y, Beckwith N, Yao L, Zhang J, Wu G. Overexpression of N-Myc downstream-regulated gene 2 (NDRG2) regulates the proliferation and invasion of bladder cancer cells in vitro and in vivo. PloS one. Oct 16;8(10):e76689 (2013). https://doi.org/10.1371/journal.pone.0076689
CrossRef - Huang, J., Wu, Z., Wang, G., Cai, Y., Cai, M., Li, Y.”N‑Myc downstream‑regulated gene 2 suppresses the proliferation of T24 human bladder cancer cells via induction of oncosis”. Molecular Medicine Reports 12, no. 4, 5730-5736. (2015): https://doi.org/10.3892/mmr.2015.4169
CrossRef - Zhu W, Gao F, Zhou H, Jin K, Shao J, Xu Z. Knockdown of MCM8 inhibits development and progression of bladder cancer in vitro and in vivo. Cancer Cell International. Dec;21(1):1-10 (2021). https://doi.org/10.1186/s12935-021-01948-2
CrossRef - Yu M, Wang H, Xu H, Lv Y, Li Q. High MCM8 expression correlates with unfavorable prognosis and induces immune cell infiltration in hepatocellular carcinoma. Aging (Albany NY). 2022 Dec 27;14(24):10027-10049. doi: 10.18632/aging.204440. Epub 2022 Dec 27. PMID: 36575045; PMCID: PMC9831725 (2022). doi: 10.18632/aging.204440
CrossRef - Kang, F., Wang, Y., Luo, Y. et al. NDRG2 gene expression pattern in ovarian cancer and its specific roles in inhibiting cancer cell proliferation and suppressing cancer cell apoptosis. J Ovarian Res 13, 48 (2020). https://doi.org/10.1186/s13048-020-00649-0
CrossRef - Zhang ZG, Li G, Feng DY, Zhang J, Zhang J, Qin HZ, et al. Overexpression of NDRG2 can inhibit neuroblastoma cell proliferation through negative regulation by CYR61. Asian Pacific Journal of Cancer Prevention: APJCP. 2014;15:239-244
CrossRef - Li L, Wang J, Shen X, Wang L, Li X, Liu Y, et al. Expression and prognostic value of NDRG2 in human astrocytomas. Journal of the Neurological Sciences;308:77-82 (2011) https://doi.org/10.1016/j.jns.2011.06.007
CrossRef - Carrasco R, Izquierdo L. van der Heijden AG, et al. Differential gene expression profile between progressive and de novo muscle invasive bladder cancer and its prognostic implication. Sci. Rep.11, 6132 (2021). https://doi.org/10.1038/s41598-021-85137-1
CrossRef