Manuscript accepted on :15-03-2023
Published online on: 20-03-2023
Plagiarism Check: Yes
Reviewed by: Dr. Akmal El-Mazny , Dr. Loai Aljerf
Second Review by: Dr. Sherif Ramzy , Dr. Varsha Galani
Final Approval by: Dr. Ian James Martin
Aiman A Shoiab1* , Ahmed Gardouh1,2
, Ahmed Gardouh1,2 , Alia khwaldeh3
, Alia khwaldeh3 , Ali Alsarhan3
, Ali Alsarhan3 and Nazek Al-hamad1
and Nazek Al-hamad1
1Faculty of Pharmacy, Department of Pharmacy, Jadara University, Irbid, Jordan
2Faculty of Pharmacy, Department of pharmaceutics and industrial pharmacy, Suez canal university, Egypt
3Department of Pharmacy, Jadara University, Irbid, Jordan.
Corresponding Author E-mail: aiman.s@jadara.edu.jo
DOI : https://dx.doi.org/10.13005/bpj/2605
Abstract
Objective: Statins are one of the most effective drugs for reducing cholesterol and triglyceride levels, which main activity includes inhibiting 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase that is involved in cholesterol synthesis. However, statins are associated with several side effects; the most commonly reported ones are those related to the liver. This study was conducted to compare the impact of two formulas of Atorvastatin: Atorvastatin calcium (ATV), and Nanoparticles of Atorvastatin (NATV) on the liver. Methods: thirty Albino rats were randomly divided into three groups; control group (n=10) (standard diet), Atorvastatin group (ATV) 40 mgkg group, and Nanoparticle Atorvastatin group (NATV) 40 mgkg. After 30 days, all rat groups were sacrificed. Results: In comparison with the control group, the ATV and NATV groups had a significant increase in the activities of liver enzymes alkaline phosphatase (ALP), aspartate transaminase (AST), and alanine transaminase (ALT) (p< 0.05). Compared with the ATV group, the NATV group had a significant increase in the activities of liver enzymes alkaline phosphatase (ALP), aspartate transaminase (AST), and alanine transaminase (ALT) (p< 0.05). Furthermore, the NATV group significantly reduced LDL, VLDL, TC, and TG compared to the control and ATV groups (p< 0.05). The histopathological examination showed hepatocyte necrosis and sinusoidal vessel congestion, which was more significant in the NATS group than in the ATV group. Conclusion: NATV can cause a significant increase in the level of liver enzymes and has a more histopathological effect on the liver than ATV.
Keywords
Atorvastatin; Nanoparticle atorvastatin; Liver enzymes
Download this article as:| Copy the following to cite this article: Shoiab A. A, Gardouh A, khawaldeh A, Alsarhan A, Al-hamad N. The Comparison Between the Effect of Atorvastatin and Nanoparticle Atorvastatin on Rat Liver. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Shoiab A. A, Gardouh A, khawaldeh A, Alsarhan A, Al-hamad N. The Comparison Between the Effect of Atorvastatin and Nanoparticle Atorvastatin on Rat Liver. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3loT82T |
Introduction
Any change in plasma lipoproteins levels is considered a significant risk factor for coronary heart disease (CHD). So many therapies that lower LDL cholesterol and/or raise HDL cholesterol have been introduced to decrease CHD risk1. Statins are the drug of choice for managing elevated cholesterol in the primary and secondary prevention of atherosclerotic cardiovascular disease. However, they occasionally are associated with mild transaminase elevations. And they can also result in severe liver injury, which Atorvastatin mostly causes2.
Atorvastatin inhibits the enzyme hydroxy-3-methylglutaryl-CoA reductase, which produces cholesterol from HMG-CoA by converting it into mevalonate. As cholesterol synthesis in the liver is reduced, LDL receptors are upregulated, and LDL cholesterol is more readily taken up from the bloodstream. By reducing the production rate of apo B100-containing lipoproteins in the liver, statin therapy reduces cholesterol and triglyceride levels 3.
For better benefits, advanced nanoparticle carrier systems are used. A promising approach of formulated nanoparticles with self-emulsifying drug delivery systems (SNEDDs) has been confirmed to improve oral bioavailability and more remarkable ability to control serum cholesterol levels by enhancing the solubility and dissolution rate compared to traditional drugs and marketed products4. SNEDDS is an isotropic mixture of natural or synthetic oils, solid or liquid surfactants, or one or more hydrophilic solvents. 5,6.
Although Atorvastatin effectively monitors lipid profiles, it is essential to know that it is associated with several side effects, including effects on muscles and the liver. Among the most commonly reported side effects of statins are those related to the liver7. As a result of liver-related side effects, statins are often underused and unnecessarily discontinued8. The information about these adverse liver effects, their actual risks, monitoring requirements, and their safety for people with liver disease constantly evolves. This study aims to compare the effect of Atorvastatin and Nanoparticle Atorvastatin on lipid profile and their negative effect on the albino rat liver.
Materials and Methods
Male Albino rats weighing 250-300g were housed for one week under controlled environmental conditions at the Faculty of Pharmacy, Suez Canal University, before the experiment began (22 ± 3 C, 50 ± 5% relative humidity), where before the experiment, the subjects were kept in a regular dark/light cycle and fasted overnight. Following the institution’s research ethics committee, this study’s protocol and animal care procedures were approved [201710PHD1] by the Faculty of Pharmacy, Suez Canal University.
An effort was made to minimize the suffering of animals to the greatest extent possible. During the study, NIH guidelines for using and caring for laboratory animals (8th edition) were followed. Throughout the experiment, qualified personnel fed, handled, and delivered drugs to the animals, and feces and contaminated food were handled regularly. Atorvastatin calcium (ATV) was purchased from Medical Union Pharmaceuticals Co. (Ismailia, Egypt). The Nanoparticles of Atorvastatin were formulated in a previous study. Through in-vivo studies, the Nanoparticle Atorvastatin formula demonstrated improved oral bioavailability and an improved ability to control serum cholesterol levels over pure drug suspension 4. Thirty sexually matured male rats were randomly divided into three groups (10 in each group). Group I, as a control group, received no drug. Atorvastatin 40 mg/kg by gavage needle was given to group 2 for 30 days, and Nanoparticle Atorvastatin 40 mg/kg by gavage needle was given to group 3.
Collection of blood samples and histological assay
After dissecting the liver from the anesthetized rats with xylazine 0.2 ml and ketamine 0.1 ml, these livers were fixed in buffered formalin for 12 hours and then processed for histopathological examinations, including hepatocyte necrosis and sinusoidal vessel congestions. We used light microscopes (Nikon ECLIPSE E600W, Tokyo, Japan) and digital cameras (Microscope Digital Camera DP70, Tokyo, Japan) to examine mounted slides. The blood was drawn directly from the heart through a heart puncture to collect enough blood (≈ 5ml). All blood samples were kept in a refrigerator at (-20 C) until the time of biochemical analysis.
Three standard tests are performed to assess liver function: ALT, AST, and ALP using The ALT, ALP, and AST kits from Teco Diagnostics in Anaheim, California, USA. A UV/VIS single beam spectrophotometer (EMC-11D-V; EMCLAB) and an automated clinical chemistry instrument (Duisburg, Germany) was used to evaluate the LDH levels. We determined serum lipid profiles using Diamond Diagnostics kits (Total lipid concentration) and Reactivos Spinreact Company (Spain) kits (serum triglycerides, serum cholesterol, and serum HDL-cholesterol concentration).
Statistical analysis
All data were expressed as the mean ± standard error (SE). Differences between groups were assessed with one-way ANOVA before Tukey’s post hoc test was used for outcome evaluation.
Results
The Comparison between the effect of Atorvastatin and Nanoparticle Atorvastatin on lipid profile:
A significant difference was observed between the Atorvastatin group, the Nanoparticle Atorvastatin group, and the control group regarding the reduction in LDL, VLDL, TC, and TG (p0.05), as shown in Table 1.
Table 1: The comparison between ATV, NATV, and control groups regarding Lipid profile
| Parameters | ||||||
| Groups | LDL (mg\dl) | VLDL (mg\dl) | TC (mg\dl) | TG (mg\dl) | HDL (mg\dl) | P value |
| Control group | 49.10±0.40 | 14.11±0.57 | 92.1±1.30 | 73.30±0.64 | 36.11±1.62 | 0.02 |
| Atorvastatin group | 11.20±2.18 | 12.26±0.63 | 68.2±2.39 | 59.27±1.20 | 46.14±2.44 | 0.03 |
| Nanoparticle Atorvastatin group | 5.82±0.71 | 10.15±0.36 | 59.1±2.94 | 43.24±0.73 | 49.20±2.32 | 0.001 |
Value are Mean ± SD, * significant differences (p<0.05)
The Comparison between the effect of Atorvastatin and Nanoparticle Atorvastatin on liver enzymes:
There was a significant increase (p <0.05) in AST, ALT, and ALP after administration of ATV and NATV 40 mg\kg daily for 30 days compared with the control group. However, the elevation in liver enzymes was more significant in the NATV group than in the ATV group, as shown in Table 2.
Table 2: The comparison between ATV, NATV, and control groups regarding Liver enzymes.
| Groups | Parameters | P value | ||
| AST (U\L) | ALT (U\L) | ALP (U\L) | ||
| Control group | 45.2±2.11 | 72.3±2.64 | 41.0±2.20 | 0.00 |
| Atorvastatin group | 82.1±1.03 | 117.2±2.63 | 87.2±2.32 | 0.00 |
| Nanoparticle Atorvastatin group | 93.1±3.43 | 128.3±4.21 | 99.0±3.44 | 0.00 |
Value are Mean ± SD, * significant differences (p<0.05)
Histopathological examination results
A considerable difference in ATV and NATV groups was found in histopathological parameters, including hepatocyte necrosis, hepatocyte degeneration, and hepatic sinusoid congestion, as shown in Figures 1, 2, and 3. In ATV and NATV groups, according to changes in liver toxicity markers, rats had elevated injury scores. These results suggest that ATV and NATV cause severe liver damage. However, the severity of toxicity was elevated in the NATV group compared with the ATV group.
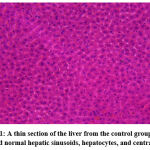 |
Figure 1: A thin section of the liver from the control group (5 μm) showed normal hepatic sinusoids, hepatocytes, and central veins. |
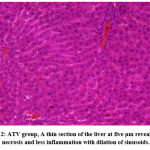 |
Figure 2: ATV group, A thin section of the liver at five μm reveals some necrosis and less inflammation with dilation of sinusoids. |
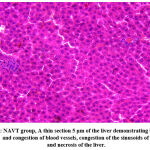 |
Figure 3: NAVT group, A thin section 5 μm of the liver demonstrating bleeding, dilation, and congestion of blood vessels, congestion of the sinusoids of the liver, and necrosis of the liver. |
Discussion
A major risk factor for coronary heart disease is dyslipidemia. According to several studies, atorvastatin lowers TC, TG, LDL, and VLDL while elevating HDL. The real concern with Atorvastatin is that it is not a very water-soluble antihyperlipidemic drug. Consequently, due to the extensive first-pass metabolism of Atorvastatin, it has limited oral bioavailability 9. According to the results presented in this study, nanoparticles of Atorvastatin are an excellent alternative to traditional Atorvastatin formulas in terms of bioavailability and the effects they have on lipid profiles, which is consistent with other studies 10,11.
There is some evidence that statins increase aminotransferase levels and cause hepatotoxicity, possibly due to changes in hepatocyte cell membranes rather than direct damage to the liver 12. An elevated level of ALT, AST, and ALP could indicate tissue damage and necrosis of the liver 13. Several studies have confirmed the current study’s results regarding the effects of Atorvastatin on liver enzymes and histopathological changes.14,15. However, the present study showed that Nanoparticle Atorvastatin significantly impacted liver enzymes and induced histopathological changes compared with Atorvastatin. This could be attributed to the increased bioavailability of Nanoparticle Atorvastatin.
The histopathological changes of the liver demonstrate bleeding, dilation, congestion of blood vessels, congestion of the sinusoids of the liver, and necrosis of the liver, which are more severe signs in rats that received nanoparticle Atorvastatin compared with Atorvastatin. Future studies could examine other factors that clarify the relationship between the bioavailability and hepatotoxicity of Nanoparticle Atorvastatin, such as the effect of size modulation on the distribution of nanoparticulate formula and the isotropic mixture used in nanoparticle formulations.
Conclusion
The present study has compared the effect of Atorvastatin and Nanoparticle Atorvastatin on lipid profiles, indicating that Atorvastatin nanoparticles show an excellent reduction in lipid profiles as an alternative to the traditional Atorvastatin formula. In addition, this study compares the negative effect of the mentioned formulation on the albino rat liver, showing that Nanoparticles of Atorvastatin could increase liver enzyme levels and have a more histopathological impact on the liver than Atorvastatin.
Conflict of interest
The authors declare no conflicts of interest or financial interest in any product or service mentioned in this article.
Funding Sources
No financial support was provided for this study.
References
- Schaefer EJ. Introduction to High-Density Lipoprotein, Dyslipidemia, and Coronary Heart Disease. Springer; 2010.
CrossRef - Saha A, Garg A. Severe liver injury associated with high-dose atorvastatin therapy. J Investig Med High Impact Case Reports. 2021;9:23247096211014050.
CrossRef - Wierzbicki AS. Atorvastatin. Expert Opin Pharmacother. 2001;2(5):819-830.
CrossRef - Gardouh AR, Nasef AM, Mostafa Y, Gad S. Design and evaluation of combined atorvastatin and ezetimibe optimized self-nano emulsifying drug delivery system. J Drug Deliv Sci Technol. 2020;60:102093.
CrossRef - Gershanik T, Benita S. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur J Pharm Biopharm. 2000;50(1):179-188.
CrossRef - Myers RA, Stella VJ. Systemic bioavailability of penclomedine (NSC-338720) from oil-in-water emulsions administered intraduodenally to rats. Int J Pharm. 1992;78(1-3):217-226.
CrossRef - Kon RH, Russo MW, Ory B, Mendys P, Simpson Jr RJ. Misperception among physicians and patients regarding the risks and benefits of statin treatment: the potential role of direct-to-consumer advertising. J Clin Lipidol. 2008;2(1):51-57.
CrossRef - Rzouq FS, Volk ML, Hatoum HH, Talluri SK, Mummadi RR, Sood GK. Hepatotoxicity fears contribute to the underutilization of statin medications by primary care physicians. Am J Med Sci. 2010;340(2):89-93.
CrossRef - Lennernäs H. Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet. 2003;42(13):1141-1160.
CrossRef - Arora A, Bhandari RK, Pandey AK, et al. Effect of atorvastatin nanoparticles compared to free atrovastatin on plaque properties in rabbit model of atherosclerosis. Int J Noncommunicable Dis. 2019;4(4):127.
CrossRef - Shahraeini SS, Akbari J, Saeedi M, et al. Atorvastatin solid lipid nanoparticles are a promising dermal delivery approach and an anti-inflammatory agent. AAPS PharmSciTech. 2020;21(7):1-10.
CrossRef - Davidson MH, Clark JA, Glass LM, Kanumalla A. Statin safety: an appraisal from the adverse event reporting system. Am J Cardiol. 2006;97(8):S32-S43.
CrossRef - Hu Z, Lausted C, Yoo H, et al. Quantitative liver-specific protein fingerprint in blood: a signature for hepatotoxicity. Theranostics. 2014;4(2):215.
CrossRef - Liu Y, Cheng Z, Ding L, et al. Atorvastatin-induced acute elevation of hepatic enzymes and the absence of cross-toxicity of pravastatin. Int J Clin Pharmacol Ther. 2010;48(12):798-802. doi:10.5414/cpp48798
CrossRef - Ashraf J, Ali Khan M, Minhaj S, et al. Statins and Abnormal Liver Function Tests: Is There a Correlation? Cureus. 2020;12(8):e10145. doi:10.7759/cureus.10145
CrossRef







