Km. Babita1* , Kapil Kalra1
, Kapil Kalra1 , Neetu Rajpoot1
, Neetu Rajpoot1 , Poonam Joshi2
, Poonam Joshi2 and Vandana Pokhriyal1
and Vandana Pokhriyal1
1Alpine college of management and technology, Dehradun Uttarakhand India.
2Uttaranchal Institute of Pharmaceutical Sciences, Uttaranchal University, Dehradun Uttarakhand India.
Corresponding Author E-mail: babitanegi495@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2621
Abstract
Advanced, easy and accurate UV spectroscopy technique (I & II) was developed for estimation of Ciprofloxacin and Tinidazole in bulk and tablet dosage form and validated using hydrotropic solubilization techniques. In Method I we focused on solving the simultaneous equation on the basis of absorbance measurement at 270nm and 320nm wavelength of Ciprofloxacin and Tinidazole individually in 2M urea solution which results in enhancement of solubility of CPH and TNZ to about 15 folds as compared to distilled water.Strategy II is lay out idea of Q-analysis in which absorbance was estimated at 288nm (iso-absorptive point) 270nm (λmax of CPH) in 2M urea. Ciprofloxacin and Tinidazole display linearity at chose frequencies and comply with Beer's regulation in the focus scope of 0.2-1.2μg/mL and 10 to 60μg/mL separately. Recovery reads up for CPS and TNZ were completed and rate recuperation of the medications gotten in the scope of 99.2 - 99.6% (Method A) and 98.0-100.8% (Method B) affirming the exactness of the proposed strategy. Both the techniques showed great reproducibility and recuperation with %RSD less than 2. Factual approval of the information shows that the proposed strategies can be effectively applied for the standard examination of medications in business tablets.
Keywords
absorbance ratio method; hydrotropic agent; Simultaneous estimation method
Download this article as:| Copy the following to cite this article: Babita K, Kalra K, Rajpoot N, Joshi P, Pokhriyal V. Simultaneous Estimation of Ciprofloxacin and Tinidazole by U.V Spectrophotometer using a Hydrotropic Solubilization Technique. Biomed Pharmacol J 2023;16(1) |
| Copy the following to cite this URL: Babita K, Kalra K, Rajpoot N, Joshi P, Pokhriyal V. Simultaneous Estimation of Ciprofloxacin and Tinidazole by U.V Spectrophotometer using a Hydrotropic Solubilization Technique. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3DNaY5T |
Introduction
Hydrotropic is solubilization process, where the adding of huge quantity of a subsequent solute outcome in the rise of aqueous solubility of insoluble and slightly solvable drugs.A poorly water- soluble drug refers to a “practically insoluble” [1]. Poor solubility of drugs is a crucial factor in dosage form designing and due to which drugs show low bioavailability. Hydrotropic agent like, nicotinamide, lysine, ß-cyclodextrinand urea, can be utilized for the solvency upgrade. [2]. synthetically ciprofloxacin be in to the gathering of manufactured fluoroquinolone anti-infection agents with strong action against wide range microscopic organisms. Tinidazole is dynamic against both gram+ and gram – microbes.
Material and Methods
Chemical and Reagent
Analytical grade of reagents and solvents take place used for the quantitative estimation of Ciprofloxacin and Tinidazole from Himgiri traders, whereas gift sample of Ciprofloxacin and Tinidazole was obtained from east African overseas. 2.0M urea was used as hydrotropic agent.
Instrumentation
Double beam UV-Visible Spectrophotometer of Agilent Technologies (Model No- G6860A) take placed. All weighing done on electronic balance.
Selection of solvent
solubility of the two not set in stone at 28 ± 2C. An immense measure of medications was added to the recepticle containing different hydrotropic arrangement. The measuring glasses were shaken precisely for 12 hrs.’ at 280C ± 10 C in a mechanical stirrer. Sample were permitted to get stable within 24 hrs. and afterward agitate for 5min at 2000 rpm. The supernatant fluid taken for proper dilution after separated through filter paper (whatmann) and investigated spectrophotometric partner against reference. After examination it was observed that the expansion in the dissolvability of CPH and TNZ was viewed as in excess of 15 folds in 2.0M urea arrangement when contrasted with solvency in refined water [5, 6].
Preparation of Stock Solution (1000μg/ml)
Accurately weigh 50mg CPH and TNZ independently and moved to two separate 50ml volumetric, dissolve with the 2M urea arrangement and last volume left up to mark with distilled water get stock soln. of CPH (1000µg/ml) and TNZ (1000µg/ml).
Preparation of Working Standard (100µg/ml)
From the above stock solution, 5ml every one of CPH and TNZ was taken more time, to isolate 50ml volumetric carafe and volume was up to 50ml D.W water to acquire working norm of CPH(100µg/ml) and TNZ(100µg/ml).
Method I: Simultaneous Equation Method
Assuming an example contain two absorbing drug (X and Y) all absorbed at different λmax. it very well may be practicable to choose the two drugs by the simultaneous equation [7, 8].
Determination of wavelength of maximum absorbance of Ciprofloxacin and Tinidazole
From the working standard, 10µg/ml soln. of CPH and TNZ independently prepared in D.W and the solutions were scanned under spectrum mode for 200-400nm wavelength range against blank to analyses the λmax .Sharp peaks were detected at 270nm for CPH and 320nm for TNZ.
Preparation of Calibration curve of CPH and TNZ
From the working norms of CPH(50µg/ml) 0.2, 0.4, 0.6, 0.8, 1.0, 1.2 and TNZ(50µg/ml) 10, 20, 30, 40, 50, 60 ml were moved to 10ml volume made up in volumetric flask to mark with D.W. Calibration curve of absorbance versus concentration was plotted and the regression coefficient was determined as shown in fig 1 and 2.
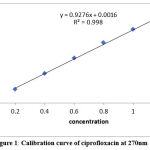 |
Figure 1: Calibration curve of ciprofloxacin at 270nm |
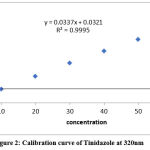 |
Figure 2: Calibration curve of Tinidazole at 320nm |
Both the drugs obey’s Beer-Lambert’s law in the conc. of0.2- 1.2µg/ml for Ciprofloxacin and 10
– 60µg/ml for Tinidazole. The correlation coefficient were found to be R²>0.999. The absorptivity values of CPH and TNZ are calculated at their respective wavelengths and presented in table 1.
Derivation of equation [9]: from the absorptivity values obtained for CPH and TNZ, simultaneous equation is derived for determination of these two drugs in combination in their pharmaceutical formulations.
A1 = 662CCPH + 206CTNZ
A2 = 445CCPH + 253CTNZ
A1 and A2 are the absorbance of mixture at chosen wavelength 270nm and 320nm [10].
Method II: Absorbtion Ratio Method
Selection of Iso-absorptive point
From the overlay spectrum of CPH and TNZ two wavelength were selected, one at 288nm (λ1) isoabsorptive point for both drugs and other at 270nm(λ2) wavelength of ciprofloxacin for the study. Overlay spectrum is shown in figure 2 and the absorptivity values for CPH and TNZ at selected wavelength are presented in table 1.
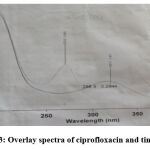 |
Figure 3: Overlay spectra of ciprofloxacin and tinidazole. |
Derivation of equation [13]: In Q analysis method, from overlay spectra of CPH and TNZ absorbances were detected at chosen wavelength i.e. at 270nm (λmax of CPH ) and 288nm (isoabsorptive point). The concentration of each drug in the tablet is determined by substituting the absorbances and absorptivity in the equation
CCPH = (1.97-1.33)/ (4.21-1.337) x A1/ax1
CTNZ = (1.97-4.21)/ (1.337-4.21) x A2/ ay1
A1 and A2 absorbance of samples at 270nm and 288nm. ax1 and ay1 are the absorptivity of CPH at 270nm and TNZ at 288nm.
Analysis of The Tablet Formulation
On concurrent evaluation of CPH and TNZ in retail tablet dosage form (CIPLOX-TZ) containing retail product of CPH-500 mg and TNZ-600mg was completed. In this assay methodology, 20 tablets were squashed and ground to a fine powder. Powder comparable to 25 mg of CPH and 30 mg of TNZ was moved to a 50 ml volumetric flask containing around 50 ml of urea solution broke up and sonicated for 10 min. The solution was sifted through Whatmann filter paper. The solution was additionally dilution with D.W to get conc. of 10µg/ml for ciprofloxacin and 12µg/ml for tinidazole. Absorbance of these solution were estimated at 270nm(ciprofloxacin) and 320nm(tinidazole) as λ1 and λ2 and centralization of these two medications in the tablet were determined. The consequences of investigation were in table 3.
Validation of Developed Method 14
The developed U.V spectrophotometric was validated as per (International council for harmonization) guidelines in terms of linearity, range, specificity, precision, Accuracy, LOD & LOQ for estimation of ciprofloxacin and tinidazole in bulk & tablet dosage form.
Linearity15
From working standard of CPH(50µg/ml) 0.2, 0.4, 0.6, 0.8, 1.0, 1.2 and TNZ (50µg/ml) 10, 20, 30, 40, 50, 60 ml transferred to 10ml volumetric flask and volume was made up to mark with distilled water. These solution were then scanned in the range of 400- 200nm against solvent as blank at their respective wavelength and then calibration curve was plotted.
Precision16
Repeatability (inter day) and (intraday). In precision for interday we are required to analyses three samples per day for consecutive three days and for intraday one sample was analysed for three days.
Accuracy17
Known amount of standard of CPH and TNZ were added to the pre-analyzed sample solutions of tablet dosage forms. Results are shown in table 2.
LOD and LOQ 18,19: LOD and LOQ can be calculated as:
LOD = 3.3σ/S
LOQ = 10σ/S
Result and Discussion
Table 1: Absorptivity values (A 1 %, 1 cm) of CPH and TNZ by method A and B.
| METHOD I | METHOD II | ||||||||||
| Conc.
μg/ml |
CPH | Conc.
μg/ml |
TNZ | Conc.
μg/ml |
CPH | Conc.
μg/ml |
TNZ | ||||
| 270nm | 320nm | 270nm | 320nm | 270nm | 288nm | 270nm | 288nm | ||||
| 0.2 | 865 | 471 | 10 | 354 | 347 | 0.2 | 771 | 180 | 10 | 431 | 161 |
| 0.4 | 680 | 397 | 20 | 226 | 315 | 0.4 | 646 | 197 | 20 | 256 | 132 |
| 0.6 | 646 | 433 | 30 | 188 | 262 | 0.6 | 590 | 170 | 30 | 223 | 108 |
| 0.8 | 614 | 467 | 40 | 163 | 207 | 0.8 | 578 | 133 | 40 | 195 | 146 |
| 1.0 | 598 | 454 | 50 | 159 | 188 | 1.0 | 564 | 100 | 50 | 168 | 194 |
| 1.2 | 578 | 469 | 60 | 149 | 204 | 1.2 | 562 | 167 | 60 | 150 | 183 |
| mean | 662 | 445 | 206 | 253 | mean | 618 | 157 | mean | 237 | 154 | |
Table 2: Determination of accuracy of CPH and TNZ
| Drug | Level of recovery
(%) |
Quantity taken
(µg/ml) |
Quantity added
(µg/ml) |
Total (µg/m
l) |
Quantity
recovered |
% Recovery(n=3) |
||
| Method I | Metho d II | Method I | Method II | |||||
| CPH | 80 | 5 | 4 | 9 | 9.1 | 9.2 | 101.1 | 102.2 |
| 100 | 5 | 5 | 10 | 9.8 | 9.6 | 98 | 96 | |
| 120 | 5 | 6 | 11 | 10.8 | 10.5 | 98.1 | 95.45 | |
| TNZ | 80 | 6 | 4.8 | 10.8 | 10.7 | 10.6 | 99.07 | 98.1 |
| 100 | 6 | 6 | 12 | 12.2 | 12.1 | 100 | 100.8 | |
| 120 | 6 | 7.2 | 13.2 | 13.0 | 13.1 | 98.4 | 99.2 | |
Table 3: Analysis of tablet formulation for method A & Method
|
METHOD |
LABEL CLAIM | AMOUNT FOUND | % RECOVERY | |||
| CPH | TNZ | CPH | TNZ | CPH | TNZ | |
| A | 500 | 600 | 496 | 598 | 99.2 % | 99.6 % |
| B | 500 | 600 | 490 | 605 | 98 % | 100.8 % |
Table 4: Optical characteristics Data
| Parameters | Method A | Method B | ||
| CPH | TNZ | CPH |
TNZ |
|
| Analytical
wavelength |
270nm and 320nm | 270nm and 288nm | ||
| Beer’s range | 0.2 –1.2µg/ml | 10–60µg/ml | 0.2 –1.2µg/ml | 10-
60µg/ml |
| Slope | 0.52 | 0.01 | 0.51 | 0.009 |
| Intercept | 0.06 | 0.023 | 0.05 | 0.06 |
| Correlation
coefficient |
0.9995 | 0.997 | 0.999 | 0.998 |
| Intra-day
precision |
0.366 | 0.951 | 0.350 | 0.421 |
| Inter-day
precision |
0.433 | 0.853 | 0.465 | 0.243 |
| LOD | 0.310 | 0.180 | 0.101 | 0.212 |
| LOQ | 0.718 | 0.821 | 0.103 | 0.991 |
| Tablet assay | 99.2% | 99.6% | 98% | 100.8% |
Discussion
Solvency of ciprofloxine and Tinidazole was viewed as in excess of 15 folds in 2.0M urea solution when compared with dissolvability in D.W. It is apparent from table 3 that the percent drug assessed in tablet definition was 99.2% for CPH and 99.6% for TNZ by technique An and 98% for CPH and 100.8% for TNZ by method II The optical characteristic such as, t , regression equation, beer’s range and Correlation coefficient also determined in table 4.
Conclusion
The analytical study of Ciprofloxacin and Tinidazole by U.V Visible Spectrophotometry was successfully performed. Developed method enhances solubility of both water insoluble drugs and here was no involvement generated by urea in the estimation, therefore we analyzed that two UV spectroscopy techniques were established to be easy, accurate, inexpensive and speedy for concurrent estimation of CPH and TNZ in bulk and tablet dosage forms. So different dosage form containing CPH and TNZ can be easily analyzed by utilizing these techniques of spectroscopy. Ciprofloxine belongs to Class IV and Tinidazole belongs to Class II of BCS Classification.
Conflict of Interest
There is no conflict of interest
References
- Nidhi K, Indrajeet S, Khushboo M, et al, Hydrotropy: A promising tool for solubility enhancement: A review. Int. J. Drug Dev. 3(2), Pages- 26-33.
- Sherje AP, Desai KJ, 2011. Spectrophotometric determination of poorly water soluble drug rosiglitazone using hydrotropic solubilization Indian journal of pharmaceutical sciences.73(5), Pages- 579.
CrossRef - G, 1979. A reviewson the hydrptropes. J Pharm Sci.68, Pages- 728-729.
CrossRef - Maheshwari RK, Mixedhydrotropy in spectrophotometric analysis of poorly water- soluble drug. Indian Pharmacist. Sci.68, Pages- 728-9
- Jain P, Goel A, Sharma S, Parmar M, 2010. Solubility enhancement techniques with special emphasis on International Journal of Pharma Proffessional’s Research. Pages- 34-45
- Shalu Verma, Mohini Rawat, 2019. Novel Approach on Solubility Enhancement: Solid Dispersion a Brief Overview. International Journal of Pharmacy and Biological Sciences. 9(3), Pages- 1354-3272.
- Mishra GP, Joshi HM, Quantitative estimation of Ciprofloxacin in marketed formulation by hydrotropic techniques. International Journal of Pharmacy & Life Sciences, Pages- 76.
- Maheshwari RK, Shukla RS, Quantitative spectrophotometric estimation of famotidine using hydrotropic solubilization technique. Asian Journal of Chemistry. 20(6), Page- 4221.
- Maheshwari RK, Solid dispersion and syrup formulation of poorly water soluble drug by hydrotropy. The Indian Pharmacist. 5(50), Pages- 87-90.
- Bhatia MS, Kaskhedikar SG, Chaturvedi SC.1999; 61(5):311 High performance liquid chromatographic estimation of ciprofloxacin hydrochloride and tinidazole from tablets. Indian Journal of Pharmaceutical
- Panzade PD, Mahadik KR, 2001. Simultaneous estimation of ofloxacin and tinidazole in tablet dosage form. Indian Drug 38(7), Pages- 368-70.
- Tatane S, 2011. Development of UV spectrophotometric method of telmisartan in tablet Journal of Advances in Pharmacy and Healthcare Research. 1, Pages- 23-6.
- Rathod SD, Patil PM, Waghmare SS et al, 2012. UV-spectrophotometric method for estimation of telmisartan in bulk and tablet dosage International Journal of Pharmaceutical Sciences and Research. 3(10), Pages- 3936
- Kamberi M, Tsutsumi K, Kotegawa T et al, 1998. Determination of ciprofloxacin in plasma and urine by HPLC with ultraviolet detection. Clinical chemistry. 1; 44(6), Pages- 1251-5.
CrossRef - Wu SS, Chein CY, Wen YH, 2008. Analysis of ciprofloxacin by a simple high- performance liquid chromatography method. Journal of chromatographic science. 46(6), Pages- 490-5.
CrossRef - Krishna JR, Naga B, Sandhya SH et al, 2014. Development and Validation of RP-HPLC method for the Simultaneous estimation of Ciprofloxacin Hydrochloride and Ornidazole in Combined Pharmaceutical Dosage Form. Journal of Advanced Pharmacy Education & 4(4), Page- 44.
- Grewal AS, Patro SK, Kanungo SK et al, Simultaneous Spectrophotometric estimation of Ciprofloxacin and Ornidazole in tablet dosage form. International Journal of Pharmaceutical Sciences and Research. 3(8), Pages- 2716.
- Ravisankar P, Navya CN, Pravallika D et al, 2015. A review on step-by-step analytical method IOSR J Pharm. 5(10), Pages- 7-19.
- Virani P, Jain P, Raj H et al, 2014. Updated review: validation and method validation PharmaTutor.2(10), Pages- 27-37.








