Manuscript accepted on :10-02-2023
Published online on: 22-02-2023
Plagiarism Check: Yes
Reviewed by: Dr. Harish M , Dr. Alvimaroof
Second Review by: Dr. Ayan Chatterjee
Final Approval by: Dr. Anton R Kiselev
Abdulrab Ahmed M. Alkhanjaf 1 ,Wala Eldin Osman Elradi2
,Wala Eldin Osman Elradi2 , Abdel Rahim Mahmoud Muddathir3
, Abdel Rahim Mahmoud Muddathir3 , Ream Elzain Abdelgadir4, Elharam Ibrahim Abdallah2
, Ream Elzain Abdelgadir4, Elharam Ibrahim Abdallah2 and Elhashimi E Hassan5*
and Elhashimi E Hassan5*
1Molecular Diagnostics, Clinical Laboratory Sciences Department, College of Applied Medical Sciences, Najran University, Najran, Saudi Arabia.
2Department of Hematology and Blood Transfusion, Faculty of Medical Laboratory Sciences, Alzaeim Alazhari University, Khartoum, Sudan.
3Department of Clinical Laboratory Sciences, Faculty of Applied Medical Sciences, Taibah University, Medina, Kingdom of Saudi Arabia.
4Hematology Department, Faculty of Medicine and Health Science- Kordofan University, Elobied- Sudan.
5Clinical Laboratory Sciences Department, College of Applied Medical Sciences, Najran University, Najran, Saudi Arabia
Corresponding Author E-mail: alhashimihassan2018@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2593
Abstract
Burkitt lymphoma, one of the two main types of B-cell non-Hodgkin lymphomas (B-NHL), is a cancer type that develops in the lymphatic system and is a very aggressive lymphoma. This study looked into the cytogenetic and molecular characteristics of Burkitt lymphoma in Sudanese individuals. Paraffin embedded tissue blocks associated to 34 people who had previously been diagnosed with burkitt's lymphoma and retained were studied as part of a retrospective cross-sectional study in Khartoum state, Sudan, in September 2017. The Soba Teaching Governmental Hospital and private histology laboratories provided these blocks. The analysis component included three translocations, including t(8;14) (q24;q32), t(8;22) (q24;q11), and t(2;8) (p12;q24) for 34 patients. We discovered that the majority of patients have t(8;14) (q24;q32), which was positive in 44.1% (15/34), while t(8;22) (q24;q11) verified in 17.6% (6/34) of patients. Only one (2.9%) displays a positive result for t(2; 8) (p12;q24). Although immune-phenotyping and morphological characteristics for BL were found in the study's 12 cases (35.3%), it is possible that these cases represent a different variety of Burkitt's lymphoma caused by different forms of translocation. According to the study's findings, t(8;14) (q24;q32) remains the most common chromosomal rearrangement among Sudanese individuals with BL. Nevertheless, translocation of BL variations may exist, necessitating the use of advanced tools like sequencing, as these variants may play a significant role in the development and prognosis of disease.
Keywords
BL: Burkitt’s lymphoma; Translocation
Download this article as:| Copy the following to cite this article: Alkhanjaf A. A. M, Elradi W. E. O, Muddathir A. R. M, Abdelgadir R. E, Abdallah E. I, Hassan E. E. Molecular Cytogenetic Characterization of Burkitt's Lymphoma Among Sudanese Patients. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Alkhanjaf A. A. M, Elradi W. E. O, Muddathir A. R. M, Abdelgadir R. E, Abdallah E. I, Hassan E. E. Molecular Cytogenetic Characterization of Burkitt's Lymphoma Among Sudanese Patients. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3mgLYhr |
Introduction
A set of disorders known as lymphomas are characterized by the overgrowth of one or more lymphatic system cell types, the invasion of malignant lymphocytes, and the devastation of healthy lymph nodes. 1 In 2009, Sudan’s first National Population Cancer Registry (NCR) was established, recording 6771 new malignancy cases. Lymphoma is the third most common cancer in men, and lymphoma in children under 15 is the second most common.2 Immunosuppression, both primary and acquired, autoimmune disorders, radiation exposure, occupational exposure, lifestyle variables, genetic factors, and infections like HIV, EBV, KSHV, HTLV-1, HCV, and H. pylori are some of the pathogenic factors for lymphoma. 3,4 Burkitt lymphoma (BL), a kind of B-cell non-Hodgkin lymphoma (B-NHL), is defined by an extranodal infiltration of tiny, non-cleaved malignant lymphoid cells that progresses quickly. Participation of c-myc genetic nonrandom gene translocations to either heavy or light immunoglobulin chain loci.5 According to the WHO (World Health Organization), there are three primary forms of BL: endemic, sporadic, and immunodeficiency-associated; they are related genetically, morphologically, and immunophenotypically.6 The term “BL” refers to the balanced, reciprocal translocation of genetic material from the long arm of chromosome 8 to the long arm of chromosomes 2, 14, and 22. Eighty percent of cases had the (8;14) (q24;q32). Additionally, it is known that translocations between chromosomes 8 and 22 t(8;22)(q24;q11) and 2 and 8 t(2; 8) (p12;q24) statistically occur in 15% and 5% of instances, respectively.7
Oncogene c-myc, in these three forms of translocations, show tight bond to the immunoglobulin heavy-chain locus IgH (14q32), the kappa light-chain locus IgK (2p12) or the lambda light-chain locus IgL (22q11) resulting in expression downregulation of c-myc, which normally act as a central player in the transcriptional regulation of diverse biological processes, including, but not limited to, cell cycle progression, differentiation, metabolism, telomerase activity, cell adhesion and apoptosis.8 For better detection of genetic alteration, the DNA polymerases has enhanced an amplifications of small pieces to gain longer targets from human genomic DNA. In terms of thermostability, the DNA polymerases used in Long distance polymerase chain reaction LD-PCR use polymerase that is identical to the one used in the standard Taq DNA polymerase, however, LD-PCR differs from Taq-PCR by possessing the 3’-5’ exonuclease or “proof reading” activity, thus, mis-incorporation of wrong nucleotides is highly controlled 9,10.
Burkitt’s lymphoma is caused by a chromosomal rearrangement called t(8; 14) (q24; q32). This rearrangement is found in about 90 to 95% of all Burkitt’s lymphoma cases. It is most commonly found in 2-5% of diffuse large B-cell lymphoma (DLBCL), which is a type of lymphoma that affects many different parts of the body.11. This study was geared towards identification of chromosomal rearrangements t(8; 14) (q24; q32) t(2;8)(p12;q24) t(8;22)(q24;q11) of Burkitt’s lymphoma among Sudanese patients.
Materials and methods
In 34 patients with confirmed diagnosis as Burkitt’s lymphomas, This was paraffin embedded tissue blocks were collected in retrospective manner from private histopathology laboratories and Soba teaching hospital laboratory. Paraffin embedded (PE) tissue blocks were carved, de-paraffinized, xylene removed, tissue lysed and for the DNA extraction from cell lysate, phenol-chloroform was used. Two primers for the c-myc gene and two primers for the IgH locus were combined for analysis of the rearrangement involving the c-myc gene, on chromosome 8, we designed primers in downstream orientation. On chromosomes 2 and 22, primers served annealing to the constant (C) regions of the kappa (k) and lambda (l) genes. For nested reactions, a lambda consensus primer derived from the joining (J) genes (Vasicek&Leder, 1990) was used .The two primers firstly used for t(8:14) .
Forward. MYC/M6:jH:5’ACAGTCCTGGATGATGATGTTTTTGATGAAGGTCT3’MYC/M9:5’GAGATCCTCTGGGGTTTGCGAGATAACCCATGG3`.
Reverse: jH:5’CTTACCTGAGGAGACGGTGACCGTGGTCCC3’C:5’GGTCACCACGCTGCTGAGGGAGTAGAGT3’.
The LD-PCR was performed using a mixture of Taq and Pwo polymerases.
 |
Figure 1: LD-PCR analysis of BL for t(8;14):, Lane 1: DNA Marker 0.65 Kb, lanes 2, 4, 5 and 7 represent positive group samples representing patients positive for t(8;14), |
 |
Figure 2: LD-PCR analysis of BL for t(8;22):, Lane M: DNA Marker 0.65 Kb, lane 7: PCR negative control, |
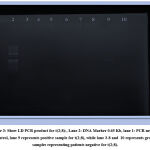 |
Figure 3: Show LD PCR product for t(2;8):, Lane 2: DNA Marker 0.65 Kb, lane 1: PCR negative control, lane 9 represents positive sample for t(2;8), |
Results
In this study, a total of 34 cases with BL were recruited, 21 (61.8%) were males and 13 (38.2%) were females, with ratio of Male: Female, 1.6:1. Their age range between 2 to 57 years old, 31 of patients (91.2%) were children their age ranged from 2 to 13 years old, while only four patients were adults, their ages were (35, 40, 48 and 57) years old, this last group was 3:1 ratio as female to male.
Using LD-PCR as shown in Figures 1, 2, and 3 assessing the three translocations {t(8;14) (q24;q32), t(8;22)(q24;q11) and t (2;8)(p12;q24) for 34 patients, we found that, most of patients have t(8;14) (q24;q32), which was positive in 44.1% (15/34), while t(8;22)(q24;q11), occur in approximately 17.6% (6/34). Of the patients with Burkitt’s lymphoma only one child male (2.9%) show positivity for t (2; 8)(p12;q24).
Discussion
Up to date, there is no clear evidences gathered from scientific research that explaining how the translocation that induces the change of functions in c-myc gene would promote the growth of BL.12 Incidence is encountered in childhood rather than adulthood.13 Central Africa has been reported as an endemic area for this disease14, therefore, this current paper is geared toward gathering more understanding about BL.
In the present study, we found that the frequency and distribution of translocations are similar to the literatures15,16, translocation t(8;14)(q24;q32) was detected in 44.1% of cases, which is in agreement with other published data on Burkitt’s lymphoma such as Burmeister T, 2005 (51.8%) 17 Katia Basso,`1999 (71%) 18, Küppers R & Dalla-Favera R, (2001) 80% 7 and ArezooKiaei ,etal (2016) 90–95%11. Contrary to Dalla-Favera R, etal (2001)7 , who found that the t(2;8)(p12;q24) and the t(8;22)(q24;q11), carried approximately in 15% and 5% of the cases, respectively. Our current study, demonstrated that t(8;22)(q24;q11), carried approximately in 17.6% (6/34), while (2.9%) show positivity for t(2; 8)(p12;q24). Variation in percentage might be related to deferent a variety of factors including genetic variation, age onset, predisposing cause of disease and geographical distribution.
The present study demonstrated 12 cases (35.3%) with negative cytogenetic, although the immunophenotyping and morphological characteristic for BL were present. And this in agreement to WHO classification of lymphoid neoplasm which mention that the diagnosis of Burkitt lymphoma still feasible even in the absence of c-myc rearrangement, if a proper correlation of clinical, morphological and immunophenotypic findings was followed to confirm or rule out that diagnosis.19 So it may be other variant of Burkitt’s lymphoma due to other types of translocation. Badr Mohammed S and Joda T (2016) found association between new translocation t(14;18) (q32;q21) with non-Hodgkin lymphoma and Burkett’s lymphoma.20
Conclusion
This study concluded that Burkitt’s lymphoma is the most common amongst Sudanese children with male predominance. The most involved sites are abdominal mass, t(8;14)(q24;q32) still the most common translocation among Sudanese patients with BL. Some patients demonstrate absence of c-myc rearrangement with the presence of immunophenotyping which may be associated with Epstein Bar Virus (EBV) and/or other factors, however, this link should be extensively studied to find out the clues and intermediate association with Bukitt’s lymphoma among the patients. Furthermore, Variants of translocation of BL may be present, so advance techniques like sequencing is required, as this variation may have important role in disease occurrence and prognosis.
Conflict of Interest
The authors claim no conflict of interest.
References
- Turgeon ML. Clinical Hematology: Theory and Procedures, 5th Edition. Vol 43.; 2011. https://journals.lww.com /acsm-msse/Fulltext/2011/ 11000/Clinical_ Hematology__Theory_and_Procedures,_5th.26.aspx
- Saeed IE, Weng HY, Mohamed KH, Mohammed SI. Cancer incidence in Khartoum, Sudan: First results from the Cancer Registry, 2009-2010. Cancer Med. 2014;3(4):1075-1084. doi:10.1002/cam4.254
CrossRef - Roman E, Smith AG. Epidemiology of lymphomas. Histopathology. 2011;58(1):4-14. doi:10.1111/j.1365-2559.2010.03696.x
CrossRef - Franchini G, Ambinder RF, Barry M. Viral Disease in Hematology. Hematology. 2000;2000(1):409-423. doi:10.1182/asheducation.v2000.1.409.409
CrossRef - Harris NL, Jaffe ES, Diebold J, et al. The world health organization classification-of hematological malignancies report of the clinical advisory committee meeting, Airlie House, Virginia, November 1997. Mod Pathol. 2000;13(2):193-207. doi:10.1038/modpathol.3880035
CrossRef - Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematol Am Soc Hematol Educ Progr. 2009;1:523-531. doi:10.1182/asheducation-2009.1.523
CrossRef - Küppers R, Dalla-Favera R. Mechanisms of chromosomal translocations in B cell lymphomas. Oncogene. 2001;20(40):5580-5594. doi:10.1038/sj.onc.1204640
CrossRef - Hecht JL, Aster JC. Molecular biology of Burkitt’s lymphoma. J Clin Oncol Off J Am Soc Clin Oncol. 2000;18(21):3707-3721. doi:10.1200/JCO.2000.18.21.3707
CrossRef - Hinnisdaels S, Del-Favero J, Vauterin M. Direct cloning of PCR products amplified with Pwo DNA polymerase. Biotechniques. 1996;20(2):186-188. doi:10.2144/96202bm05
CrossRef - Hopfner KP, Eichinger A, Engh RA, et al. Crystal structure of a thermostable type B DNA polymerase from Thermococcus gorgonarius. Proc Natl Acad Sci U S A. 1999;96(7):3600-3605. doi:10.1073/pnas.96.7.3600
CrossRef - Kiaei A, Onsori H, Alijani A, Andalib S, Ghorbian S, Sakhinia E. Detection of t(8;14) c-myc/IgH gene rearrangement by long-distance polymerase chain reaction in patients with diffuse large B-cell lymphoma. Hematol Oncol Stem Cell Ther. 2016;9(4):141-146. doi:10.1016/j.hemonc.2016.05.006
CrossRef - Kalisz K, Alessandrino F, Beck R, et al. An update on Burkitt lymphoma: a review of pathogenesis and multimodality imaging assessment of disease presentation, treatment response, and recurrence. Insights Imaging. 2019;10(1):56. doi:10.1186/s13244-019-0733-7
CrossRef - Mbulaiteye SM, Anderson WF, Ferlay J, et al. Pediatric, elderly, and emerging adult-onset peaks in Burkitt’s lymphoma incidence diagnosed in four continents, excluding Africa. Am J Hematol. 2012;87(6):573-578. doi:https://doi.org/10.1002/ajh.23187
CrossRef - Ogwang MD, Bhatia K, Biggar RJ, Mbulaiteye SM. Incidence and geographic distribution of endemic Burkitt lymphoma in northern Uganda revisited. Int J Cancer. 2008;123(11):2658-2663. doi:https://doi.org/10.1002/ijc.23800
CrossRef - Baris D, Zahm SH. Epidemiology of lymphomas. Curr Opin Oncol. 2000;12(5). https://journals.lww.com/co-oncology/Fulltext/2000/09000/ Epidemiology_of_lymphomas.2.aspx
CrossRef - Angi M, Kamath V, Yuvarani S, et al. The t(8;14)(q24.1;q32) and its variant translocations: A study of 34 cases. Hematol Oncol Stem Cell Ther. 2017;10(3):126-134. doi:10.1016/j.hemonc.2017.03.002
CrossRef - Burmeister T, Schwartz S, Horst H-A, et al. Molecular heterogeneity of sporadic adult Burkitt-type leukemia/lymphoma as revealed by PCR and cytogenetics: correlation with morphology, immunology and clinical features. Leukemia. 2005;19(8):1391-1398. doi:10.1038/sj.leu.2403847
CrossRef - Basso K, Frascella E, Zanesco L, Rosolen A. Improved Long-Distance Polymerase Chain Reaction for the Detection of t(8;14)(q24;q32) in Burkitt’s Lymphomas. Am J Pathol. 1999;155(5):1479-1485. doi:https://doi.org/10.1016/S0002-9440(10)65463-6
CrossRef - Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102(3):83-87.
- Badr Mohammed S, Joda T. Real time PCR detection Chromosomal Translocation in lymphoma by special Probe. J Cell Cancer. 2016;8(1):28-33.







