Manuscript accepted on :14-03-2023
Published online on: 17-03-2023
Plagiarism Check: Yes
Reviewed by: Dr. Hiren Patel
Second Review by: Dr. Anjaneyulu Vinukonda
Final Approval by: Dr. Jihan Seid Hussein
Department of Pharmacology; Gr T Popa University of Medicine and Pharmacy; Universitatii 16 Iasi 700115 Romania.
Corresponding Author E-mail: mihainechif@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2582
Abstract
Bipolar disorder ( BD ) is a severe and recurrent mood disorder. The influence of magnesium and zinc on animal behavior is certain and this has been experimentally demonstrated repeatedly. However, some clinical studies have positively correlated the decrease in the concentration of the two cations with a more severe symptomatology of BD, but in other cases no modified values of the concentration of magnesium and zinc were found or no relationship was identified between these concentrations and the clinical manifestations of the disease. This diversity of results has various causes but the most important of these are: problems regarding the diagnosis of BP and the diagnostic criteria used; the phases of the disease in which the cationic concentrations were determined were different; determination of intracellular magnesium was rarely done; the different ages of the patients and different associated diseases influenced the interpretation of the results. In some studies, the administration of some mood modulators (sodium valproate, carbamazepine or quetiapine) in BD type I hospitalized adult patients during the maniacal episode has increased plasma zinc and erythrocyte magnesium concentration . Missing correlations between how long is the evolution of the disease and the levels of these cations. Existing date support the idea that a low level of magnesium and zinc play a role in pathogenesis of BD. The assertion of a definite beneficial role of the association of magnesium and zinc with mood modulators in BD therapy requires more clinical studies.
Keywords
Bipolar Disorder; Magnesium; Pathogenic Theories; Zinc
Download this article as:| Copy the following to cite this article: Nechifor M. Magnesium and Zinc in Bipolar Disorders. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Nechifor M. Magnesium and Zinc in Bipolar Disorders. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3JKsc7g |
Introduction
Mood disorders, including depression and BD, are complex and multifactorial mood disorders which have an important impact on the quality of life. BD is a chronic, severe and recurrent mood disorder with repeated relapses which affects about 1% of the world’s population1. The lifetime prevalence of BD is approximately 1%-4%. There are two types of BD. Bipolar I disorder (BD-I) is characterized by alternating episodes of mania and depression. Bipolar II Disorder (BD-II)) is characterized by the association of hypomanic states with depressive episodes. BD is more common in women than in men2.This disease is an important psychiatric disease associated to an important morbidity . In both types of BD, the active life expectancy is significantly reduced and mortality is increased.
Zinc is the second most abundant bivalent cation in the central nervous system. This element is a cation that is unlike magnesium predominantly extracellular localized. There are over 300 zinc-dependent enzymes in the human body. Numerous transcription factors require zinc for their activities. This cation is important for the biological signal transduction at the cellular level 3. Zinc is found in all parts of the central nervous system, but the highest concentrations of this element are found in the hippocampus, cortex and amygdala.
The highest concentration of zinc in the brain is found in synaptic buttons4. About 10% of total brain zinc is found in the synaptic vesicles. Zinc is involved in the synaptic transmission . It acts as a neuromodulator in some synapses. In some synapses from the hippocampus the concentration of this transition metal is higher than 150 microM. Most of the neuronal zinc is found in metalloproteins. About 85-90% of synaptic zinc is bound to these proteins 5. The normal intra-neuronal cytosolic free concentration of zinc is low being below 1 nanoM. Zinc-releasing neurons exist in some areas of the brain. These neurons which release zinc release also glutamat , the most important excitatory amino acid from brain. Zinc-rich neurons are in the cortex and hippocampus. Zinc is also co-located in some gamma aminobutyric acid GABA neurons6.
This element is required by all cells ( including neurons ) for DNA transcription, it is involved in cell proliferation and differentiation and plays important roles in immunity. The DNA binding of some transcription factors is also zinc dependent. This trace element is important for brain development ,neurogenesis and neuronal differentiation 7. Zinc is a modulator of neuronal excitability and plays a role in neuronal plasticity.
The imbalances of zinc concentration are involved in some degenerative diseases8. Magnesium, the second most abundant intracellular bivalent cation9. This is an element located largely inside the cell where it stores 99% of the magnesium in the human body. About 90-95% of intracellular magnesium is bound to various molecules in the cell, mostly ATP( adenosine triphosphate) . Magnesium is a cofactor in about 600 enzymatic reactions and plays many roles in the brain. It is involved in the presynaptic release of neurotransmitters, in the functioning of receptors for neurotransmitters, in the regulation of neuronal excitability. An important magnesium role regarding his involvement in BD is the action at the level of excitatory and inhibitory systems in the central nervous system ( CNS). A low concentration of magnesium has led to a decrease in GABA activity in neocortex slices but also to an increased response to glutamate by activating N-methyl-D-aspartate( NMDA) receptors 10. The influence of magnesium and zinc on behavior has been demonstrated by numerous studies. However, the data on the implications of these two cations in the pathogenesis, clinical evolution and treatment of BD are much more heterogeneous.
Magnesium and zinc concentration in BD
The existing data regarding the involvement of the two cations in bipolar disorder (BD) are different and sometimes even contradictory. There are studies that have shown a low concentration of the two bivalent cations in patients with severe clinical manifestations of BD, but also results that show the lack of a relationship between the cationic concentrations and the clinical manifestations of this disease.
There are conflicting but few data on the erythrocyte and plasma concentration of magnesium in mood disorders. Magnesium is a cation located mostly intracellularly and therefore the determination of erythrocyte concentration should be done frequently. A low plasma or erythrocyte magnesium concentration in patients with mood disorders has been reported by some researchers 11,12,13,14,15. The decrease in magnesium levels was positively correlated with the intensity of symptoms in depressive states measured with psychometric scales(15).The concentration of magnesium in the cerebrospinal fluid (CSF) in patients with mood disorders has been little studied. The ratio of calcium / magnesium was increased in CSF and serum in both patients with major depression and patients in a depressive episode of BD 16. An increased calcium/magnesium ratio was correlated with maniac agitation 17. The other study showed that in patients with suicide attempts in depressive states , the concentration of magnesium in CSF was lower compared to normal control , but there were no significant differences in magnesium concentration in CSF in depressed patients without suicide attempts18. However, there are some contrary data. In another study a higher level of erythrocyte and plasma magnesium in patients with mood disorders than in normal subjects was identified 19. There are also a number of works that did not identify changes in the concentration of magnesium in BD. Some authors analyzed CSF in patients with both types of BD and found no significant changes in the concentration of this cation compared to healthy controls20. They also found no changes in magnesium concentration after lithium or carbamazepine.
Sometimes it not observe differences in the intra-erythrocyte concentration of magnesium in patients with active depressive states in patients but only in the remission phase of depressive states compared to normal subjects21. In other studies, no statistically significant changes in plasma magnesium and other electrolytes concentrations were detected in either monopolar or bipolar disorders22. During the aging process, the level of intracellular magnesium is reduced in most people and an increase in the frequency of mood disorders was observed.
Chronic magnesium deficit increases insulin resistance, typeII diabetes mellitus and depressive states incidence 23. Major depression and depressive episodes of patients with BD are associated with increased insulin resistance 24. Magnesium supplementation has reduced both insulin resistance and depression25. The biochemical link between insulin resistance and depression is not yet fully elucidated but a number of factors such as glutamate, brain derived neurotrophic factor ( BDNF)and peroxisome proliferator-activated receptor gamma (PPAR -γ )certainly have a role 26. Decreased Na+K+ ATP activity was observed in BD. This leads to a decrease in neuronal transmembrane ion exchange and is followed by a decrease in the intraneuronal concentration of magnesium and an increase intracellular calcium level27.
Magnesium is an insulin sensitizer and increases tissue sensitivity to insulin. Hypomagnesaemia increases insulin resistance, inhibits glucose transporter4 (glut4) translocation, and is therefore implicated in the pathogenesis of diabetes28. There are several studies showing the association of diabetes with mood disorders (especially depressive states). Hypomagnesaemia is involved in both the pathogenesis of diabetes and mood disorders. Magnesium supplementation (6 weeks) increased PPAR-γ and glucose transporter-1(GLUT-1) genes expression29. Pioglitazone, which is a widely used oral antidiabetic drug, is also an insulin sensitizer. Some oral antidiabetic drugs such as pioglitazone have reduced the intensity of depressive manifestations in mood disorders.
Low plasma levels of zinc in bipolar depression and monopolar depression have been reported in several studies30,31. In some studies the level of plasma zinc was low in the depressive phase of BD but was normal in the manic phase. The plasma zinc concentration was significantly reduced in the manic phase and also in depressive phase of type I BD compared to healthy controls 32 and also in major depression.
Magnesium, zinc and BD pathogenic theories of BD
The pathophysiology of BD is complex and incompletely known. It includes both genetic and nongenetic factors. The risk of suicide and suicidal behavior is higher in patients with BP than in the general population. There are the most important theories that try to explain the pathogenesis of mood disorders: glutamateric theory, BDNF and cAMP Response Element-Binding Protein ( CREB)theory , immuno-inflammatory theory and oxidative stress theory, neuronal neuroplasticity theory , monoaminergic (serotonin / norepinephrine) theory, endocannabinoids theory , Hypothalamic-Pituitary-Adrenal (HPA) axis disturbances theory and nitric oxide theory. There is also the possibility of the simultaneous involvement of several pathogenic mechanisms in both BD and major depression .
The main causes of the differences between the results of clinical studies are the following:
Differences regarding the diagnosis of the type of BD and the phase of the disease in which the research took place
Lack of intracellular magnesium dosages in most studies
Differences regarding the sex and age of the patients
The lack of reporting in many cases of the diseases associated with BD and the medication of these diseases (with possible influences on the concentration of magnesium and zinc.
The number of studies related to magnesium and zinc in BD is still small
Differences regarding the supervision of the diet and the administration of food supplements during the study and in the period immediately preceding the research
An important difficulty in clearly establishing the role of magnesium and zinc in BD is that some studies assess the imbalance of these cations during manic episodes, and others during depressive periods.
Glutamatergic theory
The involvement of excessive glutamate synthesis in the brain and the imbalance between its excitatory action and the activity of GABA-ergic inhibitory systems is the most discussed and accepted pathogenic theory of mood disorders.
The increased activity of the glutamatergic systems has a major role in the mechanism of depression33 and also of BD. Zinc is a modulator of both excitatory and inhibitory neutotransmission in brain. This cation is important for the balance between the activity of the glutamateric and GABA-ergic systems in the brain.
This cation acts in several directions including NMDA receptors 34. Zinc, by binding to a subunit of NMDA receptors, reduces this action of glutamate. The NR2A subunit of NMDA receptors has an increased sensitivity to the action of zinc. By acting on this subunit, zinc causes an allosteric inhibition of NMDA receptors and reduces the excitatory action of glutamate33.
The role of glutamate in depression correlates with data showing a higher than normal level of glutamate in the frontal cortex in cases of suicide35. Chronic zinc exposure reduces not only the activity of NMDA receptors but also the neuronal surface expression of NR2A-containing NMDA receptors 36. The activity of systems based on glutamatergic transmission in the hippocampus ,amygdala and in the other cerebral regions is exacerbated and the activity of GABA-ergic systems is reduced by dietary zinc deficiency in rats37.The excitability of glutamatergic neurons is enhanced by experimental dietary zinc deficiency in rats .
Experimental infusion of the hippocampus with ZnCl2( 10-300micro M )induced a decrease in glutamate concentration and an increase in GABA level in the perfuzate38. This effect of zinc could be essential for its action in BD. Breaking the balance between the action of glutamatergic systems and the neurotransmission of GABA in favor of the action of glutamate is found in all mood disorders. Inhibitory neurotransmission is increased by zinc because this element enhances presynaptic GABA release39.By this way zinc tends to restore normal balance between excitatory and inhibitory system in brain.
In experimental studies40 the antidepressant action of zinc has been antagonized by the administration of N-methyl-D-aspartate (NMDA), a fact that involves NMDA receptors in this effect. Glutamate α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid ( AMPA) receptors cannot be omitted from this anti-depressant mechanism of zinc either because NBQX (an antagonist of AMPA receptors) has reduced the antidepressant action of zinc.
Magnesium has an action by blocking the calcium channel coupled with NMDA receptors and reduces the release of glutamate into the brain41. Some drugs used to treat mood disorders, such as lamotrigine, also reduce the activity of glutamate in the brain42.
BDNF and CREB theory
BDNF is involved in brain activity and also in the growth and development of neurons. CREB-BDNF plays a role in hippocampal neurogenesis increase and nervous cells survival. Apoptosis is involved in ethiopathogenesis of mood disorders more in the pathogenesis of depressive states. BDNF level is decreased in mood disorders43.
A decreased concentration of BDNF is associated with increased suicidal ideation in depressive states .Other ways in which zinc could be implicated in mood disorders are: stimulating BDNF synthesis .Zinc monotherapy ( 30mg/day 12 weeks )increased serum BDNF levels and improved depression in obese subjects44. There are also contrary data according to which zinc supplementation in diabetics, obese and premenstrual women with moderate depression has not significantly increased serum BDNF levels45. In a large meta-analysis, it was confirmed that there was a low level of BDNF in patients with depression and an increase in the level of this factor after antidepressant treatment46. Such a study does not exist for patients with BD.
The level of BDNF in the brain plays a central role in the neurotrophic hypothesis of depression, which believes that increased neuroplasticity plays a major role in reducing depressive states and that the response to antidepressants depends on neuronal plasticity. In animal studies , a low BDNF brain expression is positively correlated to depressive-like states47. CREB is a transcriptional factor. This factor is also implicated in neurogenesis and neuroplasticity. There are differences in cAMP-CREB signaling between patients with BD and normal subjects. In the brain, the action of BDNF correlates positively with cAMP – CREB signaling. In the lung, increased intracellular zinc concentration increased CREB-mediated transcriptional activity 48. In some experimental studies, chronic administration of magnesium (15 and 20 mg / kg) significantly increased serum BDNF levels49. Mood modulators such as lithium and sodium valproate activate the BDNF promoter in some neurons .But there are also contrary data. In a double blind randomized clinical trial , administration of magnesium ( 500mg/day daily 8 weeks)to people with depressive syndromes did not significantly alter serum BDNF50.
Inflammatory and oxidative stress theory
Another pathogenic theory of mood disorders is the inflammatory theory. This theory is based on observations that show that in mood disorders and even in recurrent depressive states the level of inflammatory cytokines and oxidative stress is higher than in normal subjects51,52.Increased oxidative stress is involved in both the pathogenesis of BD and that of major depression53. The level of glutathione in the brains of these patients is significantly lower compared to healthy controls and the level of free radicals is higher. This is associated with mitochondrial lesions and increased RNA oxidation in the hippocampus from patients with BD. In patients with an acute depressive episode the level of total antioxidant capacity ( TAS ) is lower54. Reducing oxidative stress is a possible way to treat patients with mood disorders. Zinc and magnesium have antioxidant action, should be given to these patients.
Both zinc and magnesium have anti-inflammatory action, reduce the synthesis of proinflammatory cytokines and reduce oxidative stress. Zinc modulates cell immunity. This cation down regulated some cytokines ( such as IL-1β, IL-6, TNF-α)synthesis and action55. The anti-inflammatory effect of zinc is more intense than that of magnesium. There are data that associate a proinflammatory diet with a higher risk of psychiatric illness including mood disorders56. Zinc and magnesium have an antioxidant action and we believe that the two cations could be involved in this way in reducing the risk of occurrence and / or in improving the clinical symptoms in mood disorders.
Neuronal neuroplasticity theory
The reduction of neuronal and glial neuroplasticity has been highlighted in BD and has a role in the pathogenesis of this disease57.
Zinc has a modulating role in neurogenesis and neuroplasticity. This role exists both in neonatal period and in adulthood 58 .An increased apoptosis in some regions of the brain especially in in the hypothalamus and hippocampus has been evidenced in mood disorders. Zinc has an antiapoptotic action and an important role in cell cycle. Neuronal precursor survival and stem cells proliferation is also zinc dependent. This action of zinc is present at the both cortical and subcortical levels. The volume of hippocampus is significantly reduced in BD patients compared to normal people 59. There are also differences in the volume of hippocampus between BD type I and BD type II patients 60 Prolonged lithium treatment increases the hippocampus volume in BD patients61. This increases the importance of the factors that reduce apoptosis and increase neuronal plasticity in reducing the risk of BD and improving the evolution of this disease. Zinc deficiency increases apoptosis and reduced neurogenesis in hippocampus62.
In apoptosis induced by hypoxic-ischemic brain damage in newborn rats , magnesium sulphate reduced Apoptosis Index in hippocampus63. This metal also reduces neuro degeneration and apoptosis indirectly by blocking the activity of NMDA receptors. In this way , magnesium and zinc deficiency could be essential in the development of mood disorders.
Monoamines theory
This theory involves disruption of serotonergic and norepinephrine mediation in pathogenesis BD and major depression.
Serotonin increases the mobilization of intracellular calcium and thus alters the ratio of calcium to intracellular magnesium.
Serotonin causes a more intense intracellular calcium response in BD and major depression.
Signal transduction, mediated by the 5-HT2A receptor is significantly increased in patients with BD and this process could be specific to patients with BD being part of the pathogenic mechanism of the disease64. 5HT2A receptor Bmax was increased in platelets obtained from drug-free BD patients as compared with normal subjects65. In suicidal patients with depression or BD, number of 5-HT2A receptors and their activity are increased in both platelet and postmortem brain.
Lithium carbonate treatment has a biphasic effect on the action of serotonin on platelet serotonin receptors in patients with BD in both manic and depressive patients. A short treatment with lithium carbonate (2-3 weeks) decreased maximum velocity (Vmax) of serotonin (5-HT) uptake while prolonged treatment (at least 1 year) significantly increases in Vmax.
On the other hand, in patients with BD, the concentration of the serotonin transporter is 16-26% lower than in normal subjects in the hypothalamus, amygdala and previously cingulate cortex66. At platelet level, serotonin uptake (Vmax) is significantly reduced in BD patients compared to normal subjects, but there is no evidence that the same thing happens in the brain67. In an experimental study on mice , inhibition of serotonin synthesis and also 5-HT1 receptor antagonists reduced the antidepressant-like effect of magnesium 68. Magnesium in experimental studies has reduced serotonin turnover in the brains of animals exposed to noise stress. Antidepressant –like magnesium effect in the mouse forced swimming test was potentiated by sub-effective doses of fluoxetine (10 mg / kg, i.p.( a serotonin reuptake inhibitor)and by imipramine (5 mg / kg, i.p.) 69. Serotonin still enhances GABA release in rat entorhinal cortex by activating 5-HT2A receptors on GABA-ergic neurons. By increasing the concentration of cerebral serotonin, magnesium increases the release of GABA and can help restore the normal balance between glutamatergic and GABA actions. This balance is always disturbed in mood disorders.
Zinc also increased the concentration of serotonin in the hypothalamus 70. Modulation of brain serotonergic systems by different ways is a common point of the action of magnesium and zinc in mood disorders.
Endocannabinoids theory
There are few studies on the implications of endocannabinoids in mood disorders, but disturbances in the concentration of these cannabinoids in both major depressive disorders and some psychotic disorders have been observed in CSF. There are also changes in the density and activity of cannabinoid receptors in both BD and depression71. CB1 cannabinoid receptors are present at the axonal level in both the glutamatergic synapses and the GABA- ergic and serotonergic synapses. Thus, the activity of the endocannabinoid system could influence the major neurotransmission systems involved in mood disorders. Stimulation of cannabinoid receptors with anandamide, tetrahydrocannabinol (THC), cannabidiol (CBD) or anandamide has been shown to reduce depression and other related mood disorders72.
The interactions between zinc and magnesium with the endocannabinoid system could also be involved in the mechanism of action of the two biometals in mood disorders. In an experimental study the antidepressant like effect of zinc and magnesium was augmented by CB1 cannabinoid receptor stimulation after administration of oleamide73.
Hypothalamic-Pituitary-Adrenal (HPA) axis disturbances theory.
There are many studies that show that cortisol and adrenocorticotrope hormone( ACTH) levels are significantly increased in BD74. This increase is present both in the manic phase and in the depressive phase of BD.The cortisol level was higher in BD compared to major depression patients and always significantly higher than healthy controls75.In BD patients, ACTH basal and peak concentration is also higher than control patients76. Corticotropin releasing factor level is higher in BD patients 77. Valproic acid used as a mood stabilizer in the treatment of BD inhibits the synthesis and release of corticotropin-releasing factor. Part of the therapeutic effect of valproic acid in BD also occurs through this decrease in CRF secretion followed by decreased ACTH production. Decreased magnesium concentration causes hypothalamic-pituitary adrenal (HPA) axis disorder and increases the synthesis of ACTH and cortisol. Magnesium has a modulating role of this axis78. This cation reduces the secretion of ACTH and cortisol.
Nitric oxide theory
Nitric oxide synthase (NOS) is present at the neuronal level and nitric oxide is involved in both synaptic transmission and neuroplasticity. Nitric oxide (NO) is unique gaseous neurotransmitter from the CNS. There is evidence of imbalances in the functioning of neuronal NOS and in the concentration of nitric oxide in the pathogenesis of mood disorders( 79) and in degenerative diseases such as Parkinson’s disease and Alzheimer’s disease.
Some authors believe that nitric oxide in the hippocampus plays an important role in the modulation of mood and imbalances related to this action are involved in mood disorders80. NO plays an important role in the pathogenesis of major depression81 and probably plays the same role in depressive states in BD. It modulates the action of antidepressant drugs including the activity of selective serotonin reuptake inhibitors.
NO also plays an important role in the inflammatory processes involved in the pathogenesis of mood disorders79. Zinc is an important structural element of NOS . This element down-regulated the expression of inducible NO synthase( iNOS )(mRNA+protein) and decreased cytokine-mediated activation of the iNOS promoter.
Zinc involvement in BD pathogenesis is presented in Figure I
Magnesium involvement in BD pathogenesis in Figure II
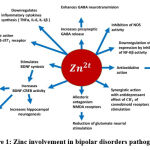 |
Figure 1: Zinc involvement in bipolar disorders pathogenesis |
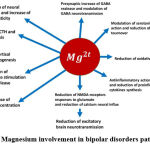 |
Figure 2: Magnesium involvement in bipolar disorders pathogenesis. |
Nutritional studies on the implications of magnesium and zinc in BD
Few studies have shown a correlation between zinc and magnesium amount in the diet and BD. Some results from clinical trials show that the type of diet has an implication in the risk of developing mood disorders82.A limited number of nutritional studies showed a significant inverse correlation between magnesium dietary intake and depressive states83.There are data that show that magnesium and zinc have a beneficial effect on depressive disorders and can reduce suicidal tendencies. The effect of these two cations is not limited to their antidepressant action but also includes an improvement in cognitive functions. A beneficial effect of zinc-rich diet in depression and anxiety was observed 84. and another study 85 showed that daily administration of 25 mg zinc as an adjuvant treatment to selective serotonin reuptake inhibitors (SSRI) therapy has a beneficial effect. Few cross-sectional study supports the inverse association between dietary zinc intake and mood disorders 86. Serum zinc levels were inversely correlated with mood disorders in adolescent female87. Since the serum concentration of zinc correlates positively with the dietary intake of zinc, the logical conclusion is that an increased dietary intake of zinc reduces the risk of mood disorders and / or improves their evolution. Contrary, in a recent study, plasma zinc levels were higher in patients with BD who are in a stable phase of the disease compared to normal subjects88. In a double-blind, randomized and placebo-controlled study zinc supplementation( 10 weeks) improved mood state in young women89. Magnesium and dietary supplements containing this cation may be appropriate for controlling bipolar disorder90.
Magnesium reduced mania in BD patients. Dietary magnesium intake also reduced the risk of developing depressive states in hospitalized patients.
Regarding the different results of the studies regarding the influence of diets rich in zinc or magnesium administered to patients with BD on the evolution of the disease and the effectiveness of the treatment, we consider that the following aspects should be considered:
Lack of mentions regarding the time elapsed from the onset of the disease to the hospitalization and the various problems to establish the onset of disease( especially in patients with BD type II).
Difficulties in tracking the actual amount of food (or food supplements) really ingested by the patient for a longer time.
Sometimes the absence of mentions related to possible diseases or treatments that can reduce the absorption or increase the elimination of the two cations administered through the diet.
Associated pathological factors such as some chronic kidney disease, malabsorption syndromes, some liver diseases may alter the nutritional intake of zinc or magnesium in BD
Implications of zinc and magnesium in BD therapy
Mood stabilizers are the treatment of choice for bipolar disorders. Use of a mood stabilizer is recommended in all subtypes and in all phases of BD. Some antiepileptic and anticonvulsant drugs have been used as mood modulators in BD therapy. The most used drugs in this group are: carbamazepine, sodium valproate, clozapine and others. Lithium is an old mood stabilizer drug which has been and still is used in the treatment of BD. In the brain, lithium enhances inhibitory GABA neurotransmission ,but reduces glutamatergic and dopaminergic activity.
There are data that show an increase of intracellular magnesium concentration by competition between magnesium and lithium for some intracellular binding sites91. This competition occurs at therapeutic concentrations of lithium that are reached in patients during therapy. Increasing the intracellular concentration of lithium also increases the intracellular concentration of free magnesium. At an intracellular lithium concentration of 15 mM the increase in free magnesium concentration is 158% .The increase in intraneuronal free magnesium concentration was observed experimentally after chronic exposure of neurons to concentrations of 1-2 mM lithium for at least 72 hours92. This experimental data is consistent with the observation that therapeutic results after lithium administration are obtained after a period of treatment. There are conflicting data on the influence of lithium salt treatment on the plasma concentration of magnesium.
Lithium and other mood stabilizers used in the treatment of BD inhibit the transformation of arachidonic acid into some proinflammatory icosanoids. Because some icosanoids synthesized from arachidonic acid have proinflammatory action and stimulate the synthesis of proinflammatory cytokines, lithium may also have a therapeutic effect in BD by inhibiting their synthesis. It is unclear whether major depression and depressive periods in BD are pathologically exactly the same, but there are certainly many similarities between them. The strong argue that monopolar depression and BD depression phase have similar pathogenesis is the great resemblance between the clinical symptoms including the existence of suicidal tendencies in depressive states in BD. Therapeutic results argue against a similarity in the pathogenic mechanism, showing that the antidepressant drugs that are effective in major depression have few therapeutic results in BD therapy and often have no results regardless of the duration of treatment. In some cases, antidepressant medication increased cycle frequency and mood episode severity in patients with BD. Mood stabilizers were much more effective in these patients than antidepressant medication93. Although some small clinical differences can be discussed between the depressive phases of BD and monopolar depression, there are certainly many similarities and the boundary between the two pathological conditions is not clearly established.
Ketamine showed an antidepressant action in monopolar and also in bipolar depression. Magnesium and ketamine have a synergistic action regarding the antidepressive effect. The both reduced the NMDA receptor activity and the both increase the BDNF activity in brain94. The mechanism of reduction in NMDA receptor activity is not the same for ketamine and magnesium. Regarding ketamine, the critical point of action is the GluN2B subunit from NMDA receptors. For magnesium, essential is the effect on the calcium channel coupled with NMDA receptors. Ketamine has a therapeutic effect in both forms of monopolar and bipolar depression (depressive symptomatology, including suicidal ideation, was reduced in 69% of patients after ketamine (5mg/kg), but no patient had a symptomatology reduction after placebo administration) 95. The synergistic action of ketamine and magnesium in the both forms of depressive states is an strong argue for the involvement of magnesium deficit in pathogenesis of depressive states.
The antidepressant mechanism of action of ketamine is that of noncompetitive inhibition of NMDA receptors. Because zinc and ketamine both act by noncompetitive inhibition of activity of NMDA receptors but in different sites it is possible that there is a potentiating relationship between them. The same potentiating relationship is true for magnesium.
Mood stabilizers such as lithium carbonate and sodium valproate have anti-apoptotic and neuroprotective action. Lithium and sodium valproate protect against glutamate-induced NMDA receptor mediated neuronal exocytosis and against apoptosis. Increasing the concentration of intracellular magnesium and plasma zinc by mood stabilizer therapy may be involved in reducing the activity of NMDA receptors and in the antiapoptotic action of these drugs.
A strong argument for the involvement of the imbalance between calcium and magnesium concentrations in BD pathogenesis is the favorable therapeutic effect of the administration of nimodipine and other calcium channel blockers with dihydropyridine structure in patients with BD during manic periods.
Mood stabilizers are the treatment of choice for bipolar disorders. Use of a mood stabilizer is recommended in all subtypes of BD. The effect of therapy with sodium valproate 900 mg / day, carbamazepine 600 mg / day and quetiapine 600 mg / day, respectively, was followed for 4 weeks on three groups of adult patients with BD type I hospitalized in the manic phase of the disease. In all three groups, at the hospital admission, the erythrocyte concentration of magnesium and the plasma concentration of zinc were significantly reduced compared to healthy controls. There were no significant differences in plasma magnesium concentration compared to the control group. After 4 weeks of treatment, the erythrocyte magnesium and plasma zinc concentrations increased significantly(erythrocyte concentration of magnesium (56.9 +/- 5.22 mg/L after sertraline vs 44 +/- 2.7 mg/L before sertraline, p < 0.01). The treatment did not produce significant changes in the plasma concentration of magnesium. There was a positive correlation between an increase in erythrocyte magnesium and plasma zinc levels and an improvement in patients’ clinical condition96. The increase in the intracellular concentration of magnesium by various mood modulators (carbamazepine, lithium sodium valproate and others) used in the treatment of BD that have different mechanisms of action indicates that this increase is an important component of the mechanism of action of these drugs in this disease.
The level of serum magnesium was not significantly different in patients with treated mood disorders compared to those who did not receive treatment 97. In favor of the involvement of magnesium deficiency in the pathogenesis of BD, clinical data argue that the administration of magnesium sulfate (200mg/h in iv continuous infusion )in patients with BD type I during a manic episode reduces the manic agitation and decreases the need for psychotropic drugs. Magnesium aspartate administration in rapid cycling BD type I has improved the clinical condition of patients98.
This cation may be beneficial for reducing maniac symptoms of type I BD99. Low magnesium levels after experimental brain trauma are associated with depression. Because BD is a recurring disease, one of the problems is the presence of repeated relapses. A study showed that in patients with BD type I( treated with mirtazepin or sodium valproate) who relapsed in the first two years after the first hospitalization, the level of erythrocyte magnesium and plasma zinc was lower at the time of relapse than at hospital discharge and also significantly lower than in patients without relapse100.
Biomarkers problem in mood disorders
One problem is the absence of biological markers to monitor the evolution and effectiveness of treatment of patients with BD. In order to assess the evolution of psychiatric diseases as well as to evaluate the effectiveness of the treatment, many authors consider it important that in addition to the clinical evaluation of the patient’s behavior, there are also biological markers. Plasma zinc concentration was proposed as a biological marker of major depression and associated a low zinc concentration with treatment resistance in depressed patients. Serum magnesium was proposed as a marker in BD101.
We believe that plasma zinc and erythrocyte magnesium should be considered as biological state markers and used as such in both major depression and BD. Of course, this does not exclude the use of other biological markers and it is recommended to use the determination of these markers together with other methods for assessing the behavior of patients with BD.
Conclusions
Despite the heterogeneity of clinical studies results, existing data support the idea of a therapeutic and prophylactic potential of both magnesium and zinc in BD .
The time when the administration of the two cations should be started, the duration of administration and the doses required for the best possible effect should be determined. In this direction more clinical studies are surely needed.
Conflict of Interest
There are no conflict of interest.
Funding Sources
There is no funding sources.
References
- Grande I, Berk , Birmaher B, Vieta E. Bipolar .disorder. Lancet, 2016; 387:1561-1572.
CrossRef - Benazzi E. Is there a continuity between bipolar and depressive disorders? Psychother. Psychosom., 2007;76:70-76.
CrossRef - Portbury S D, Adlard P A.Zinc Signal in Brain Diseases. Int. J. Mol .Sci 2017; 18:2506.
CrossRef - Krall R F , Tzounopoulos T , Aizenman E.The Function and Regulation of Zinc in the Brain. Neuroscience., 2021;457:235-238.
CrossRef - Weiss J H , Sensi S L , Koh J Y. Zn2+: a novel ionic mediator of neural injury in brain disease. Trends Pharmacol. , 2000 ;21:395-401.
CrossRef - Frederickson CJ, Suh SW, Silva D, Frederickson CJ, Thompson RB. Importance of zinc in the central nervous system: the zinc-containing neuron. Nutr., 2000; 130(5S Suppl): S1471-1483.
CrossRef - Brion LP , Heyne R , Lair C Role of zinc in neonatal growth and brain growth: review and scoping review. Pediatr. Res., 2021; 89:1627-1640.
CrossRef - Xie Z , Wu H , Zhao J. Multifunctional roles of zinc in Alzheimer’s disease. Neurotoxicology ,2000; 80:112-123.
CrossRef - DentA , Selvaratnam R. Measuring magnesium – Physiological, clinical and analytical perspectives. Clin Biochem ., 2022;105-106:1-15.
CrossRef - el-Beheiry H , Puil E. Effects of hypomagnesia on transmitter actions in neocortical slices. Br. J. Pharmacol., 1990; 101:1006-1010.
CrossRef - Herzberg L, B Herzeberg B. Mood change and magnesium. A possible interaction between magnesium and lithium? J. Nerv. Ment. Dis. 1977 ; 165:423-426.
CrossRef - Kirov G K , Tsachev K N. Magnesium, schizophrenia and manic-depressive disease.Neuropsychobiology, 1990; 23:79-81.
CrossRef - Nechifor, M., Vaideanu, C., Mandreci, I., Palamaru, I., Boisteanu P.Research on plasma and erythrocyte concentration of some bivalent cations in patients with bipolar disorders. In : Ermidu-Pollet S, Pollet S. editors Proceedings Book of 4th International Symposium on Trace Elements in Human –New Perspectives;2003Oct 9-11 ;Athens Greece .Athens :Athens Entipossis 2005.p.150-159.
- Siwek , Sowa-Kućma M , Styczeń K , Szewczyk B , Reczyński W, Misztak P,et al. Decreased serum zinc concentration during depressive episode in patients with bipolar disorder. J. Affect. Disord .,2016; 190: 272–277.
CrossRef - Botturi A , Ciappolino V, Delvecchio G , Boscutti A , Viscardi B ,Brambilla P. The Role and the Effect of Magnesium in Mental Disorders: A Systematic Review. Nutrients 2020 ;12:1661.
CrossRef - Levine J , Stein D , Rapoport A , Kurtzman L. High serum and cerebrospinal fluid Ca/Mg ratio in recently hospitalized acutely depressed patients. Neuropsychobiology, 1999; 39:63-70.
CrossRef - Carman J S, Wyatt RJ. Calcium: bivalent cation in the bivalent psychoses. Biol .Psychiatry ,1979 ; 14:295-336.
- Banki C M , Arató M , Kilts C D.Aminergic studies and cerebrospinal fluid cations in suicide. Ann. N. Y. Acad. Sci.,1986 ;487:221-230.
CrossRef - Widmer J , Henrotte J G , Raffin Y , Bovier P , Hilleret H , Gaillard J M. Relationship between erythrocyte magnesium, plasma electrolytes and cortisol, and intensity of symptoms in major depressed patients . J . Affect. Disord., 1995 ;34:201-209.
CrossRef - George M S, Rosenstein D, Rubinow DR, Kling M A, Post R M. CSF magnesium in affective disorder: lack of correlation with clinical course of treatment. Psychiatry Res., 1994; 51:139-146.
CrossRef - Kamei K, Tabata O, Muneoka K , Muraoka S I, Tomiyoshi R, Takigawa M. Electrolytes in erythrocytes of patients with depressive disorders. Psychiatry Clin. Neurosci., 1998; 52:529-533.
CrossRef - Ramsey TA, Frazer A , Mendels J . Plasma and erythrocyte cations in affective illness. Neuropsychobiology ,1979; 5:1-10.
CrossRef - Barbagallo M ,Belvedere M, Dominguez LJ. Magnesium homeostasis and aging. Magnes .Res., 2009; 22:235-246.
CrossRef - Kan C , Silva N, Golden S H, Rajala U, Timonen M, Stahl D, et al. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care ,2013; 36:480-489.
CrossRef - Tarleton E K , Littenberg B , MacLean C D, Kennedy AG, Daley C. Role of magnesium supplementation in the treatment of depression: a randomized clinical trial. PLoS One, 2017 ; 12: e0180067.
CrossRef - Jeremiah O J, Cousins G, Leacy FP, Kirby B P, Ryan B K. Evaluation of the effect of insulin sensitivity-enhancing lifestyle- and dietary-related adjuncts on antidepressant treatment response: protocol for a systematic review and meta-analysis. Syst. Rev., 2019; 8: 62.
CrossRef - Kumar A R , Kurup P A. Inhibition of membrane Na+-K+ ATPase activity: a common pathway in central nervous system disorders. J. Assoc. Physicians India, 2002; 50:400-406.
- Feng J ,Wang H , Jing Z, Wang Y, Cheng Y, Wang W, et al.Role of Magnesium in Type 2 Diabetes Mellitus. Biol. Trace. Elem. Res., 2020;196:74-85.
CrossRef - Jamilian M , Samimi M , Faraneh A E, Aghadavod E, Shahrzad H D, Chamani M., et al.Magnesium supplementation affects gene expression related to insulin and lipid in patients with gestational diabetes. Magnes. Res., 2017 ; 30:71-79.
CrossRef - Grønli O, Kvamme J M, Friborg O, Wynn R. Zinc Deficiency Is Common in Several Psychiatric Disorders. PLoS One ,2013 ; 8: e82793.
CrossRef - Młyniec K , Davies C L , de Agüero Sánchez I G, Pytka K, Budziszewska , Nowak,G. Essential elements in depression and anxiety. Part I. Pharmacol. Rep., 2014; 66:534-544.
CrossRef - Nechifor, M., Vaideanu, C., Mândreci, I., Palamaru ,I., Boişteanu, P. The influence of bipolar disorders treatment on plasmatic and erythrocyte levels of some catios . In: Alpoim M C , Vasconcellos P , Santos M A , Cristovao A J , Centero J A , Colley P.editors.Proceedings of the Ninth International Symposium on Metal ions in Biology and Medicine;2006 ;Paris, France. Paris :John Libbey Eurotext;2006.560-564.
- Mlyniec K .Zinc in the Glutamatergic Theory of Depression . Curr. Neuropharmacol., 2015; 13:505-513.
CrossRef - PaolettiP, Vergnano A M , Barbour , Casado M .Zinc at glutamatergic synapses. Neuroscience, 2009; 158:126-136.
CrossRef - Hashimoto K, Sawa A , Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Psychiatry, 2007; 62:1310–1316.
CrossRef - Zhu J, Shao C Y , Yang W, Zhang X M, Wu Z Y , Zhou L,et al. Chronic zinc exposure decreases the surface expression of NR2A-containing NMDA receptors in cultured hippocampal neurons. PLoS One, 2012; 7:e46012.
CrossRef - Takeda A , Itoh H , Imano S, Oku Impairment of GABAergic neurotransmitter system in the amygdala of young rats after 4-week zinc deprivation. Neurochem. Int., 2006; 49:746-750.
CrossRef - TakedaA , Minami A , Seki Y, Oku Differential effects of zinc on glutamatergic and GABAergic neurotransmitter systems in the hippocampus. J. Neurosci. Res., 2004 75:225-229.
CrossRef - Smart T G , Hosie A M , Miller PS. Zn2+ ions: modulators of excitatory and inhibitory synaptic activity. Neuroscientist, 2004 ;10:432-442.
CrossRef - Szewczyk B , Poleszak E , Sowa-Kućma M., Wróbel A, Słotwiński S, Listos J,et al.The involvement of NMDA and AMPA receptors in the mechanism of antidepressant-like action of zinc in the forced swim test. Amino Acids 2010; 39:205-217.
CrossRef - Kang SW, Choi S K , Park E , Chae S J, Choi S , Joo H J, et al. Neuroprotective effects of magnesium-sulfate on ischemic injury mediated by modulating the release of glutamate and reduced of hyperreperfusion. Brain Res., 2011; 1371:121-128.
CrossRef - Kugaya A, Sanacora G. Beyond monoamines: glutamatergic function in mood disorders. Spectr., 2005;10:808–819.
CrossRef - Dwivedi Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr. Dis. Treat., 2009;5:433-449.
CrossRef - Solati Z , Jazayeri S , Tehrani-Doost M , Mahmoodianfard S, Gohari M R. Zinc monotherapy increases serum brain-derived neurotrophic factor (BDNF) levels and decreases depressive symptoms in overweight or obese subjects: a double-blind, randomized, placebo-controlled trial. Nutr. Neurosci., 2015;18:162-168.
CrossRef - Jafari F , Mohammadi H , Amani R. The effect of zinc supplementation on brain derived neurotrophic factor: A meta-analysis. J . Trace. Elem . Med. Biol., 2021;66:126753.
CrossRef - Molendijk M L , Spinhoven P, Polak M , Bus B A A , Penninx W J H , Elzinga B M.Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol. Psychiatry ,2014;19:791-800.
CrossRef - Bus B A A, Molendijk M L .The neurotrophic hypothesis of depression. Tijdschr Psychiatr 2016; 58:215-22.
- Xiao G , Lian G , Wang T , Chen W, Zhuang W , Luo L , et al.Zinc-mediated activation of CREB pathway in proliferation of pulmonary artery smooth muscle cells in pulmonary hypertension. Cell Commun Signal 2021; 19: 103.
CrossRef - Pochwat, B., Sowa-Kucma,M., Kotarska,K., Misztak,P., Nowak,G., Szewczyk,B.Antidepressant-like activity of magnesium in the olfactory bulbectomy model is associated with the AMPA/BDNF pathway. Psychopharmacology (Berl), 2015; 201232:355-367.
CrossRef - Afsharfar M, Shahraki M ,Shakiba M , Asbaghi O , Dashipour The effects of magnesium supplementation on serum level of brain derived neurotrophic factor (BDNF) and depression status in patients with depression. Clin. Nutr. ESPEN., 2021;42:381-386.
CrossRef - Maes M , Fišar Z, Medina M, Scapagnini G, Nowak G, Berk M. New drug targets in depression: inflammatory, cell-mediated immune, oxidative and nitrosative stress, mitochondrial, antioxidant, and neuroprogressive pathways. And new drug candidates–Nrf2 activators and GSK-3 inhibitors. Inflammopharmacology, 2012; 20:127–150.
CrossRef - Gałecki P ,Talarowska Inflammatory theory of depression. Psychiatr .Pol. 2018; 52:437-447.
CrossRef - Gawryluk ,J.W., Wang,J. F., Andreazza,A.C., Shao,L., Young L T. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int. J. Neuropsychopharmacol .,2011; 14:123-130.
CrossRef - Sarandol A , Sarandol E , Eker S S, Erdinc S , Vatansever E., Kirli . Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum. Psychopharmacol., 2007; 22:67-73.
CrossRef - Szewczyk B , Kubera M , Nowak G. The role of zinc in neurodegenerative inflammatory pathways in depression. Prog . Neuropsychopharmacol. Psychiatry ,2011; 35: 693–701.
CrossRef - Haghighatdoost F , Feizi A , Esmaillzadeh A ,Feinle-Bisset C , Keshteli A H , Afshar H ,et al. Association between the dietary inflammatory index and common mental health disorders profile scores. Clin. Nutr., 2019; 38:1643-1650.
CrossRef - McIntyre R S , Berk M , Brietzke E , Goldstein B I , López-Jaramillo C L , Vedel L, et al. Bipolar disorders Lancet 2020; 396:1841-1856.
CrossRef - Kumar V , Kumar A , Singh K , Avasthi K , Kim J J. Neurobiology of zinc and its role in neurogenesis. Eur. J. Nutr., 2021;60:55-64.
CrossRef - Haukvik U K , Gurholt T P , Nerland S , Elvsåshagen T , Akudjedu T N , Alda M, ENIGMA Bipolar Disorder Working Group. In vivo hippocampal subfield volumes in bipolar disorder—A mega‐analysis from The Enhancing Neuro Imaging Genetics through Meta‐Analysis Bipolar Disorder Working Group. Hum. Brain. Mapp., 2022; 43:385-398.
- Cao B J , Peng N A. Magnesium valproate attenuates hyperactivity induced by dexamphetamine-chlordiazepoxide mixture in rodents. Eur. J. Pharmacol. 1993;237:177-81.
CrossRef - Simonetti A , Sani G , Dacquino C , Piras F , De Rossi P , Caltagirone C, et al.Hippocampal subfield volumes in short- and long-term lithium-treated patients with bipolar I disorder . Bipolar Disord., 2016;18:352-362.
CrossRef - Gao H L , Zheng W , Xin N , Chi Z H , Wang Z Y , Chen J , et al.Zinc deficiency reduces neurogenesis accompanied by neuronal apoptosis through caspase-dependent and -independent signaling pathways. Neurotox. Res., 2009;16:416-425.
CrossRef - Türkyilmaz C , Türkyilmaz Z , Atalay Y, Söylemezoglu F, Celasun B. Magnesium pre-treatment reduces neuronal apoptosis in newborn rats in hypoxia-ischemia. Brain Res., 2002; 955:133-137.
CrossRef - Suzuki K , Kusumi I , Sasaki Y , T Koyama T. Serotonin-induced platelet intracellular calcium mobilization in various psychiatric disorders: is it specific to bipolar disorder? J. Affect. Disord. 2001; 64:291-296.
CrossRef - Pandey G N , Pandey S C, Ren X , Dwivedi Y, Janicak P G. Serotonin receptors in platelets of bipolar and schizoaffective patients: effect of lithium treatment. Psychopharmacology (Berl),2003; 170:115-123.
CrossRef - Oquendo M A ,Hastings R S ,Huang Y Y, Simpson N ,Ogden R T ,Hu X Z ,et al.Brain serotonin transporter binding in depressed patients with bipolar disorders using positron emission tomography.Arch. Gen. Psychiatry, 2007;64:201-208.
CrossRef - Marazziti D , Lenzi A , Galli L , Martino S S , Cassano G B. Decreased platelet serotonin uptake in bipolar I patients. Int. Clin. Psychopharmacol. , 1991;6:25-30.
CrossRef - Poleszak E.Modulation of antidepressant-like activity of magnesium by serotonergic system. J . Neural. Transm . (Vienna) ,2007; 114:1129-1134.
CrossRef - Cardoso C C , Lobato K R, , Binfaré R W., Ferreira P K , Rosa A O ,et al. Evidence for the involvement of the monoaminergic system in the antidepressant-like effect of magnesium. Prog. Neuropsychopharmacol. Biol. Psychiatry, 2009;33:235-242.
CrossRef - Szewczyk B , Kotarska K , Siwek A , Olech L , Kuter K. Antidepressant activity of zinc: Further evidence for the involvement of the serotonergic system. Pharmacol. Rep., 2017; 69:456-461.
CrossRef - Rubino T , Zamberletti E , Parolaro D. Endocannabinoids and Mental Disorders. Handb. Exp. Pharmacol., 2015; 231:261-283.
CrossRef - Ashton C H , Moore PB. Endocannabinoid system dysfunction in mood and related disorders. Acta. Psychiatr. Scand., 2011;124:250-261.
CrossRef - Wośko S , Serefko A , Szopa A , Wlaź P , Wróbel A , Wlaź A , et al. CB 1 cannabinoid receptor ligands augment the antidepressant-like activity of biometals (magnesium and zinc) in the behavioural tests. J. Pharm. Pharmacol., 2018; 70:566-575.
CrossRef - Murri M B , Prestia D , Mondelli V , Pariante C., Patti S ,Olivieri B , et al. The HPA axis in bipolar disorder: Systematic review and meta-analysis. Psychoneuroendocrinology 2016; 63:327-342.
CrossRef - Rybakowski J K , Twardowska K. The dexamethasone/corticotropin-releasing hormone test in depression in bipolar and unipolar affective illness. J. Psychiatr. Res., 1999; 33:363-370.
CrossRef - Vieta E , Martínez-De-Osaba M J , Colom, F., Martínez-Arán A , Benabarre A , Gastó,C.Enhanced corticotropin response to corticotropin-releasing hormone as a predictor of mania in euthymic bipolar patients. Psychol. Med.,1999 ;29:971-978.
CrossRef - Aubry J M. CRF system and mood disorders. J .Chem. Neuroanat., 2013 ;54:20-24.
CrossRef - Sartori S B , Whittle N , Hetzenauer A , Singewald N. Magnesium deficiency induces anxiety and HPA axis dysregulation: modulation by therapeutic drug treatment. Neuropharmacology, 2012; 62:304-312.
CrossRef - Ghasemi M , Claunch J , Niu K. Pathologic role of nitrergic neurotransmission in mood disorders. Prog. Neurobiol., 2019 ;173:54-87.
CrossRef - Hu Y, Zhu D Y.(2014). Hippocampus and nitric oxide.Vitam. Horm., 2014; 96:127-160.
CrossRef - Dhir A , Kulkarni S K. Nitric oxide and major depression. Nitric Oxide, 2011; 24:125-131.
CrossRef - Łojko D , Stelmach-Mardas M S , Suwalska A. Is diet important in bipolar disorder?. Psychiatr. Pol., 2018;52:783-795.
CrossRef - Anjom-Shoae J, Sadeghi O, Keshteli A H, Afshar H , Esmaillzadeh A ,Adibi P.The association between dietary intake of magnesium and psychiatric disorders among Iranian adults: a cross-sectional study. Br. J . Nutr., 2018; 120:693-702.
CrossRef - Jacka F N , Overland S, Stewart R, Tell G S , Bjelland I , Mykletun A. Association between magnesium intake and depression and anxiety in community-dwelling adults: the Hordaland Health Study. Aust. N. Z .J. Psychiatry, 2009; 43:45–52.
CrossRef - Ranjbar E, Kasaei M S , Mohammad-Shirazi M , Nasrollahzadeh J,Rashidkhani B, Shams J, et al. Effects of zinc supplementation in patients with major depression: a randomized clinical trial. Iran J. Psychiatry ,2013; 8:73–79.
- Hajianfar H , Mollaghasemi N, Tavakoly R, Campbell M S , Mohtashamrad M , Arab A.The Association Between Dietary Zinc Intake and Health Status, Including Mental Health and Sleep Quality, Among Iranian Female Students. Biol. Trace. Elem., 2021;199:1754-1761.
CrossRef - Tahmasebi K , Amani R , Nazari Z., Ahmadi K , Moazzen S , Mostafavi S A.(2017).Association of Mood Disorders with Serum Zinc Concentrations in Adolescent Female Students. Biol. Trace. Elem. Res., 2017;178:180-188.
CrossRef - Jonsson BH , Orhan F, Bruno S , Oliveira A O, Sparding T , Landen M , Sellgren CM. Serum concentration of zinc is elevated in clinically stable bipolar disorder patients. Brain Behav., 2022;12:e2472.
CrossRef - Lakhan S E , Vieira K F. Nutritional therapies for mental disorders. Nutr. J., 2008; 7:2.
CrossRef - Sarris J , Mischoulon D , Schweitzer I. Adjunctive nutraceuticals with standard pharmacotherapies in bipolar disorder: a systematic review of clinical trials. Bipolar. Disord., 2011; 13:454-465.
CrossRef - Mota de Freitas D , Castro M M , Geraldes CF. Is competition between Li + and Mg 2+ the underlying theme in the proposed mechanisms for the pharmacological action of lithium salts in bipolar disorder? Acc. Res., 2006;39: 283-291.
CrossRef - Abukhdeir A M , Layden B T, Minadeo N, Bryant F B, Stubbs Jr E B , Mota de Freitas D. Effect of chronic Li+ treatment on free intracellular Mg2+ in human neuroblastoma SH-SY5Y cells. Bipolar. Disord., 2003; 5:6-13.
CrossRef - Goodnick ,P.J.The use of nimodipine in the treatment of mood disorders. Bipolar. Disord., 2000; 2(3 Pt 1):165-173.
CrossRef - Górska N, Słupski J , Szałach LP ,Włodarczyk A , Szarmach J, Jakuszkowiak-Wojten K, et al. Magnesium and ketamine in the treatment of depression. Psychiatr. Danub., 2019;31(Suppl 3):549-553.
- Zarate Jr C A, Brutsche N E, Ibrahim L, Franco-Chaves J , Diazgranados N, Cravchik A , et al.Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biological Psychiatry ,2021;71:939–946.
CrossRef - Nechifor M. Interactions between magnesium and psychotropic drugs. Res., 2008; 21:97-100.
- Imada Y , Yoshioka S I , Ueda T , Katayama S., Kuno Y., Kawahara R. Relationships between serum magnesium levels and clinical background factors in patients with mood disorders. Psychiatry. Clin . Neurosci., 2002 ;56:509-514.
CrossRef - Chouinard G , Beauclair L, Geiser R, Etienne A pilot study of magnesium aspartate hydrochloride (Magnesiocard) as a mood stabilizer for rapid cycling bipolar affective disorder patients. Prog Neuropsychopharmacol Biol Psychiatry 1990; 14:171-80.
CrossRef - Sylvia L G , Peters A T, Deckersbach T , Nierenberg A A. Nutrient-based therapies for bipolar disorder: a systematic review. Psychother. Psychosom., 2013; 82:10-19.
CrossRef - Nechifor M .Magnesium and the treatment of major depression-the relapses problem. Magnes. Res. 2014;27:200.
CrossRef - SiwekM , Styczeń K , Sowa-Kućma M , Dudek D , Reczyński W, Szewczyk B, et al.The serum concentration of magnesium as a potential state marker in patients with diagnosis of bipolar disorder. Psychiatr. , 2015; 49:1277-1287.
CrossRef








