Manuscript accepted on :05-10-2022
Published online on: 13-02-2023
Plagiarism Check: Yes
Reviewed by: Dr. Tukaram Dudhamal
Second Review by: Dr. kiranmayee
Final Approval by: Dr. H Fai Poon
Lali Lingfa and Srinivas Ankanagari*
and Srinivas Ankanagari*
Department of Genetics and Biotechnology, Osmania University, Hyderabad – 500 007 (T.S), India.
Corresponding Author E-mail:asrinivas@osmania.ac.in
DOI : https://dx.doi.org/10.13005/bpj/2601
Abstract
Background: Withanias omnifera also known as Indian ginseng is commonly found in India and other Southeast Asian countries. Various parts of this plant have been used as herbal medicine to treat a variety of diseases. However, there is a lacuna in the profiling of phytochemical constituents present in the different parts of the plant at reproductive stage. Objective: To identify phytochemicals present in the methanolic extracts of leaf, root, and stem parts of W. somnifera at reproductive stage using GC-MS analysis. Methods: The airdried parts of plant (leaf, stem and root) were extracted with methanol and concentrated under reduced pressure at 40°C using a rotary evaporator. The GCMSQP2010, Shimadzu, Kyoto, Japan with headspace sampler (AOC-20s) and autoinjector (AOC-20i), was used for sample analysis. The phytochemicals were identified with the database provided by National Institute Standard and Technology (NIST11LIB). Results: The GC-MS analysis of leaf, root, and stem methanolic extracts of W. somnifera, revealed a total of eighty-two unique phytochemical peaks in the reproductive stage of the plant. Phytochemicals with antimicrobial and anticancer properties were identified in all the parts. In leaf, 2-pentanone, 5-chloro- was found to be most abundant and 2,5-dimethoxy-4-propoxy-.beta.-methyl-.beta.-nitrostyrene least abundant with antimicrobial nature, whereas, benzene, 1,1'-(1,2-ethenediyl)bis[2-methyl- was found to be most abundant and dibenzo[a,e]cyclooctene, 5,6,11,12-tetrahydro- least with anticancer property. In roots, the most abundant was benzoic acid, 3-methyl-2-trimethylsilyloxy-, trimethylsilyl ester and tris(trimethylsilyl)hydroxylamine the least abundant were identified to be antimicrobial, whereas high abundance uleine and low abundance 2-{4-[2-(4-methoxymethylphenyl)vinyl]phenyl}propan-2-olwere identified to be anticancer. In stem, acetohydroxamic acid was found to be most abundant and trans-2,3,6-trimethoxy-b-methyl-b-nitrostyrene least abundant for antimicrobial nature, whereas 3-acetoxy-2,3'-bibenzo[b]thiophene was found to be anticancer phytochemical. Conclusion: In this study, phytochemicals with antimicrobial and anticancer properties were identified in leaf, root and stem parts of W. somnifera at reproductive stage.
Keywords
Anticancer; Antimicrobial; GC-MS; Herbal medicine; Phytochemicals; Withania somnifera
Download this article as:| Copy the following to cite this article: Lingfa L; Ankanagari S. GC-MS Profiling of Reproductive Stage Withania somnifera for Antimicrobial and Anticancer Phytochemicals. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Lingfa L; Ankanagari S. GC-MS Profiling of Reproductive Stage Withania somnifera for Antimicrobial and Anticancer Phytochemicals. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3K2k5TS |
Introduction
Human civilization has been in an intimate relationship with plants since time immemorial.1 They rely on plants and other natural sources for their survival and well-being.2 Herbal medicines have become increasingly popular in recent years due to their therapeutic properties, minimal side effects, and cost efficiency.3,4 Phytochemical constituents in herbal plants are playing a central role in the development of herbal medicine that is critical for ensuring a healthy society.5 The data collected on phytochemicals helps in the discovery of new therapeutic prospects.6 However, the therapeutic potential of various plants and their parts that are available in nature has yet to be explored.7
Withania somnifera (L.) Dunal belongs to Solanaceae family is a delicate perennial shrub that grows 14-30 inches tall and grows out radial tomentose branches from a central stem.8 The leaves are dull green and elliptic, with a length of 3.9 – 4.7 inches and the flowers are green, small, and bell-shaped, the fruit is orange-red when fully ripe.9 In Latin, the term “somnifera” means sleep-inducing.10 The name “ashwagandha” is derived from the Sanskrit words “ashva” (horse) and “gandha” (smell), indicating that the root has a strong horse-like scent.11 Indian ginseng, Ashwagandha, winter cherry and poison gooseberry, are some of the many names for it. This plant can be found in India, parts of Africa and in the Middle East.12
W. somnifera is used in over 100 formulations in Indian traditional medicine, including Ayurveda, Unani, and Siddha, and is therapeutically equivalent to ginseng.13W. somnifera leaf extract has been shown to be effective against Staphylococcus aureus and Enterococcus spp.14 Other health benefits of W. somnifera have been recommended for use as a liver tonic, aphrodisiac, astringent, and anti-inflammatory agent, and more recently for the treatment of insomnia, asthma, bronchitis, ulcers, senile dementia, and emaciation, among others.15 The medicinal use of ashwagandha for cognitive and neurological diseases, such as anxiety, Parkinson’s disease, and inflammation, is also backed by clinical trials and animal research.16 Furthermore, Ashwagandha’s chemopreventive properties make it a potentially effective adjuvant for radiation and chemotherapy patients.17 Ashwagandha is also used as an immune stimulant in patients with low white blood cell counts in the blood and as an adaptogen for patients with nervous exhaustion and debility related to stress.18 The major phytoconstituents of ashwagandha root are withanolides, which include steroidal alkaloids and steroidal lactones.19
The selection of different plant parts roots, stem and leaf of W. somnifera could provide a biological and biochemical basis for identifying new pharmacologically important phytochemicals of therapeutic value.20 Extraction and characterization of bioactive compounds from W. somnifera have given birth to various phytochemicals with therapeutic importance like anaferine, anahygrine and isopelletierine etc., belonging to alkaloids, withaferins as well as withanolides belonging to steroidal lactone compounds, and saponins.21 A number of different solvent systems like chloroform, ethanol, ethyl acetate, methanol, petroleum ether and water, etc. have been reported to play important role for extraction of secondary metabolites. However, methanol is considered as an optimal solvent to obtain high variety phytochemical constituents in plant extracts.22
However, there is a lacuna in comparative profiling of phytochemicalsin W. somnifera leaf, stem, and root qualitatively and quantitatively.23 Moreover, W. somnifera is harvested at reproductive stage for the optimum dry root yield24. Thus, the chemical profiling can be established for a plant extract to identify, provide quality assurance and quantitative molecular description of plant secondary metabolites using chemical analytical methods such as Gas chromatography–mass spectrometry (GC-MS).25 The GC-MS technique has the highest sensitivity and specificity to detect the presence of phytochemical constituents.26 GC-MS analysis has long been the method of choice for determining steroid levels in clinical samples.27 Moreover, GC-MS allows effective chromatographic separation, quantification, and identification of sample constituents by using mass spectral libraries.28 Hence, in this study, GC-MS analysis was chosen as a standard approach for phytochemical profiling of leaf, stem and root at reproductive stage in W. sominfera.
Materials and Methods
Preparation of plant extract
W. sominifera were air dried and the plant parts (leaf, stem and roots each10 g) were coarsely pulverized and extracted with methanol for 24 hours in a Soxhlet (100 ml). The extract was filtered and concentrated under reduced pressure at 40°C using a rotary evaporator to get a viscous semi solid mass.
GC‑MS analysis
The GCMSQP2010, Shimadzu, which includes the headspace sampler (AOC-20s) and autoinjector, was used for GC-MS analysis (AOC-20i). The system included a mass selective detector and an ion source with a temperature of 230°C and a temperature of 250°C at the interface. The capillary column used for MS analysis was an Rt 5ms capillary column having a length of 30 m, a diameter of 0.32 mm, and a film thickness of 0.25 µm. The injector’s temperature was set to 250°C, and it had a split injection mode. The initial temperature was set at 80°C for 3 minutes, then the temperature was steadily increased to 280°C at a rate of 10°C/min. With a linear velocity of 47.1 cm/sec, helium (>99.9%) was used as the carrier gas. A total flow of 90.0 ml/min was programmed, with a column flow of 1.71 ml/min.
Identification of phytochemicals
Components were identified based on retention time (RT) for GC and interpretation of mass spectrum was done by comparing spectral fragments obtained, to the database provided by National Institute Standard and Technology (NIST11LIB). The components of the test materials were identified by their name, molecular weight, and structure.
Results
GC-MS analysis of reproductive stage Leaf
As shown in Figure 1, a total of 16 phytochemicals were exclusively identified in the methanolic leaf extracts viz. 2-pentanone, 5-chloro-; 3-butoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane; benzaldehyde, 3-methoxy-4-[(trimethylsilyl)oxy]-, O-methyloxime; 2-(7-methoxymethylphenanthren-3-yl)propan-2-ol; cyclopropanecarbonyl chloride, 1-fluoro-2,2-diphenyl-; benzene, 1,2,3-trimethoxy-5-(2-propenyl)-; 4′,6-dimethoxyaurone; dibenzo[a,e]cyclooctene, 5,6,11,12-tetrahydro-; acetic acid, 2,3-dibromo-4-methoxymethoxy-1-methyl-pent-2-enyl ester; 2,5-dimethoxy-4-propoxy-.beta.-methyl-.beta.-nitrostyrene; 1,3-dihydroxy-2,4,5-trifluoro-6-nitrobenzene; cobalt, allyl-(pentamethylcyclopentadienyl; 1-phenazinecarboxylic acid, 6-(1-methoxyethyl)-, methyl ester; cis,syn,cis-perhydrophenanthrene; benzene, 1,1′-(1,2-ethenediyl)bis[2-methyl- and pentasiloxane, 1,1,3,3,5,5,7,7,9,9-decamethyl-.
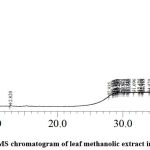 |
Figure 1: GC-MS chromatogram of leaf methanolic extract in W. somnifera |
The phytochemicals identified with antimicrobial properties in the methanolic leaf extracts is given in Table 1. The phytochemicals included2-pentanone, 5-chloro- at RT1.115with peak area 3.29%; 3-butoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane at RT 12.820 with peak area 1.66%; benzaldehyde, 3-methoxy-4-[(trimethylsilyl)oxy]-, O-methyloxime at RT 28.405 with peak area 1.12%; 2-(7-methoxymethylphenanthren-3-yl)propan-2-ol at RT 29.560 with peak area 1.15%; 4′,6-dimethoxyaurone at RT 30.960 with peak area 1.78%;2,5-dimethoxy-4-propoxy-.beta.-methyl-.beta.-nitrostyrene at RT 34.020 with peak area 1.08%;1,3-dihydroxy-2,4,5-trifluoro-6-nitrobenzene at RT 35.250 with peak area 1.24%;1-phenazinecarboxylic acid, 6-(1-methoxyethyl)-, methyl ester at RT 36.980 with peak area 1.23%; benzene, 1,1′-(1,2-ethenediyl)bis[2-methyl- at 42.525 with peak area 1.41%; and benzene, 1,2,3-trimethoxy-5-(2-propenyl)- at RT 30.740 with peak area 2.77%.
The phytochemicals identified with anticancer properties in the methanolic leaf extracts is given in Table 1. The phytochemicals included cyclopropanecarbonyl chloride, 1-fluoro-2,2-diphenyl- at RT 29.975 with peak area 1.12%; dibenzo[a,e]cyclooctene, 5,6,11,12-tetrahydro- at RT 32.630 with peak area 1.08%; cis,syn,cis-perhydrophenanthrene at RT 39.066 with peak area 1.40%; and some identified phytochemicals with both antimicrobial and anticancer properties were 2-(7-methoxymethylphenanthren-3-yl)propan-2-ol at RT 29.560 with peak area 1.15%and benzene, 1,1′-(1,2-ethenediyl)bis[2-methyl- at RT 42.525 with peak area 1.41%.
Table 1: Phytochemicals identified for antimicrobial and anticancer properties in the methanolic leaf extracts of W. somnifera.
| Sl. No. | Peak | RT | Name of the compound | Molecular formula | M.W | Peak area (%) | Therapeutic Activity |
| 1 | 2 | 1.115 | 2-Pentanone, 5-chloro- | C5H9ClO | 120 | 3.29 | Antibacterial29 |
| 2 | 6 | 12.820 | 3-Butoxy-1,1,1,7,7,7-hexamethyl-3,5,5-tris(trimethylsiloxy)tetrasiloxane | C19H54O7Si7 | 590 | 1.66 | Antibacterial30 |
| 3 | 8 | 28.405 | Benzaldehyde, 3-methoxy-4-[(trimethylsilyl)oxy]-, O-methyloxime | C12H19NO3Si | 253 | 1.12 | Antibacterial31 |
| 4 | 16 | 29.560 | 2-(7-Methoxymethylphenanthren-3-yl)propan-2-ol | C19H20O2 | 280 | 1.15 | Anticancer and Antibacterial32 |
| 5 | 17 | 29.975 | Cyclopropanecarbonyl chloride, 1-fluoro-2,2-diphenyl- | C16H12ClFO | 274 | 1.12 | Anticancer33 |
| 6 | 22 | 30.960 | 4′,6-Dimethoxyaurone | C17H14O4 | 282 | 1.78 | Antifungal34 |
| 7 | 25 | 32.630 | Dibenzo[a,e]cyclooctene, 5,6,11,12-tetrahydro- | C16H16 | 208 | 1.08 | Anticancer35 |
| 8 | 29 | 34.020 | 2,5-Dimethoxy-4-propoxy-.beta.-methyl-.beta.-nitrostyrene | C14H19NO5 | 281 | 1.08 | Antibacterial36 |
| 9 | 31 | 35.250 | 1,3-Dihydroxy-2,4,5-trifluoro-6-nitrobenzene | C6H2F3NO4 | 209 | 1.24 | Antibacterial37 |
| 10 | 37 | 36.980 | 1-Phenazinecarboxylic acid, 6-(1-methoxyethyl)-, methyl ester | C17H16N2O3 | 296 | 1.23 | Antibacterial38 |
| 11 | 40 | 39.066 | cis,syn,cis-Perhydrophenanthrene | C14H24 | 192 | 1.40 | Anticancer39 |
| 12 | 47 | 42.525 | Benzene, 1,1′-(1,2-ethenediyl)bis[2-methyl- | C16H16 | 208 | 1.41 | Antibacterial and Anticancer40 |
| 13 | 21 | 30.740 | Benzene, 1,2,3-trimethoxy-5-(2-propenyl)- | C12H16O3 | 208 | 2.77 | Antifungal41 |
GC-MS analysis of reproductive stage root:
As shown in Figure 2, a total of 19 phytochemicals were exclusively identifiedin the methanolic root extracts. The phytochemicals identified included sulfurous acid, bis(1-methylethyl) ester; 1,1′-(ethanediylidenediamino)bis(5-amino-1H-tetrazole); tris(trimethylsilyl)hydroxylamine; 1H-indole-2,3-dione, 1-(tert-butyldimethylsilyl)-5-chloro-, 3-(O-ethyloxime); N-(2-hydroxy-3,5-dimethylbenzyl)-.beta.-aminobutanoic acid; 4-(2,6,6-trimethylcyclohexa-1,3-dienyl)pent-3-en-2-ol; stannane, 1,3-dithian-2-ylidenebis[trimethyl-; S-[2-aminoethyl]-.beta.-phenyl-.alpha.-mercaptoacrylic acid; 1.alpha.-(hydroxymethyl)-7.alpha.,8.alpha.-dimethyl-7-(2-(3-furyl)ethyl)bicyclo[4.4.0]dec-2-; silane, methyltripropoxy-; 2-{4-[2-(4-methoxymethylphenyl)vinyl]phenyl}propan-2-ol; cyclohexanecarboxylic acid, 4-[[(tert-butyldimethylsilyl)amino]methyl]-, tert-butyldimethylsilyl; nickel, pentamethylcyclopentadienyl-(N,N,N’-trimethyl)-o-phenylenediamine-N’-o-; benzenepropanoic acid, 4-benzoyl-, methyl ester; silane, [[3,3-dimethyl-4-methylene-2-(trimethylsilyl)-1-cyclopenten-1-yl]methoxy]trimethyl-; uleine; benzene, dichlorodimethoxy-; 3,4,5-tris(trimethylsiloxy)-1-cyclohexene-1-carboxylic acid, trimethylsilyl ester; andbenzoic acid, 3-methyl-2-trimethylsilyloxy-, trimethylsilyl ester.
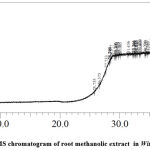 |
Figure 2: GC-MS chromatogram of root methanolic extract in Withania somnifera. |
The phytochemicals identified with antimicrobial properties in the methanolic root extracts is given in Table 2. The phytochemicals included tris(trimethylsilyl)hydroxylamine with at RT 28.290 and peak area 1.18%; 4-(2,6,6-trimethylcyclohexa-1,3-dienyl)pent-3-en-2-ol at RT 29.531 with peak area 2.36%: stannane, 1,3-dithian-2-ylidenebis[trimethyl- at RT 29.765 with peak area 1.57%; benzenepropanoic acid, 4-benzoyl-, methyl ester at RT 38.505 with peak area 1.75%; and benzoic acid, 3-methyl-2-trimethylsilyloxy-, trimethylsilyl ester. Phytochemicals identified for anticancer properties in the methanolic root extracts included 2-{4-[2-(4-methoxymethylphenyl)vinyl]phenyl}propan-2-ol at RT 34.230 with peak area 1.23 and uleine at TR 39.505 with peak area 1.90%.
Table 2: Phytochemicals identified for antimicrobial and anticancer activity in methanolic root extracts of W. somnifera.
| Sl.
No. |
Peak | RT | Name of the compound | Molecular formula | MW | Peak area (%) | Therapeutic Activity |
| 1 | 10 | 28.290 | Tris(trimethylsilyl)hydroxylamine | C9H27NOSi3 | 249 | 1.18 | Antibacterial28 |
| 2 | 15 | 29.531
|
4-(2,6,6-Trimethylcyclohexa-1,3-dienyl)pent-3-en-2-ol | C14H22O | 206 | 2.36 | Antibacterial42 |
| 3 | 16 | 29.765 | Stannane, 1,3-dithian-2-ylidenebis[trimethyl- | C10H24S2Sn2 | 448 | 1.57 | Antibacterial43 |
| 4 | 27 | 34.230 | 2-{4-[2-(4-Methoxymethylphenyl)vinyl]phenyl}propan-2-ol | C19H22O2 | 282 | 1.23 | Anticancer and Antimicrobial32 |
| 5 | 40 | 38.505 | Benzenepropanoic acid, 4-benzoyl-, methyl ester | C17H16O3 | 268 | 1.75 | Antibacterial44 |
| 6 | 44 | 39.580 | Uleine | C18H22N2 | 266 | 1.90 | Anticancer and Antibacterial45 |
| 7 | 34 | 36.850 | Benzoic acid, 3-methyl-2-trimethylsilyloxy-, trimethylsilyl ester | C14H24O3Si2 | 296 | 3.53 | Antibacterial46 |
GC-MS analysis of reproductive stage stem
As shown in Figure 3, a total of 18 phytochemicals were exclusively identified in the methanolic stem extracts. The phytochemicals identified included viz. acetohydroxamic acid; pentasiloxane, dodecamethyl-; 1,4-dibromo-2,3-butanediol; trans-2,3,6-trimethoxy-b-methyl-b-nitrostyrene; benzylamine, 2-hydroxy-N,N-di-[2-aminoethyl]-; androst-9(11)-en-17-one, 3-[(trimethylsilyl)oxy]-, O-methyloxime; 3-Acetoxy-2,3′-bibenzo[b]thiophene; 1,3,5,7-tetraethyl-1-oxycyclotetrasiloxane; 1,3-methylene-d-arabitol; 3.alpha.,4.alpha.,9.beta.,11-diepoxymuurolan-10-ol; benzenamine, N-(3,4,5,6-tetraethyl-1-phenyl-2(1H)-pyridinylidene; 3-(3-ethoxy-4-hydroxyphenyl)-2-isothiocyanatopropionic acid, ethyl; benzeneacetic acid, .alpha.,3,4-tris[(trimethylsilyl)oxy]-, trimethylsilyl; silanamine, N-[(4-methoxyphenyl)methyl]-1,1,1-trimethyl-; 4-Chloro-2-iodobenzoic acid; chlorotris(p-tolyl)methane; and cyclotetrasiloxane, octamethyl- and pentasiloxane, 1,1,3,3,5,5,7,7,9,9-decamethyl-.
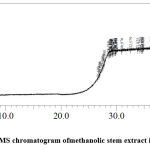 |
Figure 3: GC-MS chromatogram ofmethanolic stem extract in W.somnifera. |
The phytochemicals identified with antimicrobial properties in the methanolic stem extracts is given in Table 3.The phytochemicals with antimicrobial properties were identified in the methanolic stem extracts. These included acetohydroxamic acid at RT 1.170 with peak area 3.12%; pentasiloxane, dodecamethyl- at RT 27.030 with peak area 1.12%; 1,4-dibromo-2,3-butanediol at 27.800 with peak area 2.01%; trans-2,3,6-trimethoxy-b-methyl-b-nitrostyrene at RT 28.510 with peak area 1.06%; benzylamine, 2-hydroxy-N,N-di-[2-aminoethyl]- at RT 29.395 with peak area 1.43%; androst-9(11)-en-17-one, 3-[(trimethylsilyl)oxy]-, O-methyloxime at RT 29.804 with peak area 1.21%;1,3,5,7-tetraethyl-1-oxycyclotetrasiloxane at RT 32.579 with peak area 1.51%; 1,3-methylene-d-arabitol at RT 38.825 with peak area 1.13%: 3.alpha.,4.alpha.,9.beta.,11-diepoxymuurolan-10-ol at RT 36.255 with peak area 1.74%; benzenamine, N-(3,4,5,6-tetraethyl-1-phenyl-2(1H)-pyridinylidene at RT 40.350 with peak area 1.15%; 3-(3-ethoxy-4-hydroxyphenyl)-2-isothiocyanatopropionic acid, ethyl at 41.310 with peak area 1.15%; benzeneacetic acid, .alpha,3,4-tris[(trimethylsilyl)oxy]-, trimethylsilyl at RT 41.619 with peak area 1.18%; silanamine, N-[(4-methoxyphenyl)methyl]-1,1,1-trimethyl- at RT 44.074 with peak area 1.92%; and abcyclotetrasiloxane, octamethyl- at RT 27.480 with peak area 1.33%.The identified 3-acetoxy-2,3′-bibenzo[b]thiophene at RT 30.970 and peak area 1.11% has both antimicrobial and anticancer properties.
Table 3: Phytochemicals identified for antimicrobial and anticancer in reproductive stage stem extracts of W.somnifera.
| Sl. No | Peak | RT | Name of the compound | Molecular formula | Molecular weight | Peak area (%) | Therapeutic Activity |
| 1 | 4 | 1.170 | Acetohydroxamic Acid | C2H5NO2 | 75 | 3.12 | Antibacterial5 |
| 2 | 9 | 27.030 | Pentasiloxane, dodecamethyl- | C12H36O4Si5 | 384 | 1.12 | Antibacterial47 |
| 3 | 12 | 27.800 | 1,4-Dibromo-2,3-butanediol | C4H8Br2O2 | 246 | 2.01 | Antileishmanial48 |
| 4 | 13 | 28.510 | trans-2,3,6-Trimethoxy-b-methyl-b-nitrostyrene | C12H15NO5 | 253 | 1.06 | Antibacterial36 |
| 5 | 20 | 29.395 | Benzylamine, 2-hydroxy-N,N-di-[2-aminoethyl]- | C11H19N3O | 209 | 1.43 | Antibacterial49 |
| 6 | 22 | 29.804 | Androst-9(11)-en-17-one, 3-[(trimethylsilyl)oxy]-, O-methyloxime | C23H39NO2Si | 389 | 1.21 | Antibacterial50 |
| 7 | 25 | 30.970 | 3-Acetoxy-2,3′-bibenzo[b]thiophene | C18H12O2S2 | 324 | 1.11 | Anticancer and Antibacterial47,51 |
| 8 | 26 | 32.579 | 1,3,5,7-Tetraethyl-1-oxycyclotetrasiloxane | C8H24O5Si4 | 312 | 1.51 | Antibacterial52 |
| 9 | 27 | 33.825 | 1,3-Methylene-d-arabitol | C6H12O5 | 164 | 1.13 | Antibacterial53 |
| 10 | 32 | 36.255 | 3.alpha.,4.alpha.,9.beta.,11-Diepoxymuurolan-10-ol |
C15H24O3 |
252 | 1.74 | Antibacterial54 |
| 11 | 37 | 40.350 | Benzenamine, N-(3,4,5,6-tetraethyl-1-phenyl-2(1H)-pyridinylidene | C25H30N2 | 358 | 1.15 | Antibacterial55 |
| 12 | 41 | 41.310 | 3-(3-Ethoxy-4-hydroxyphenyl)-2-isothiocyanatopropionic acid, ethyl | C17H25NO4SSi | 367 | 1.15 | Antibacterial56 |
| 13 | 43 | 41.619 | Benzeneacetic acid, .alpha.,3,4-tris[(trimethylsilyl)oxy]-, trimethylsilyl | C20H40O5Si4 | 472 | 1.18 | Antibacterial57 |
| 14 | 46 | 44.074 | Silanamine, N-[(4-methoxyphenyl)methyl]-1,1,1-trimethyl- | C11H19NOSi | 209 | 1.92 | Antibacterial58 |
| 15 | 10 | 27.480 | Cyclotetrasiloxane, octamethyl- | C8H24O4Si4 | 296 | 1.33 | Antibacterial47 |
As shown in the Figure 4 (a-e), some unique phytochemicals of steroid structures have been identified in the reproductive stage methanolic root extracts of W. somnifera. These include cucurbitacin b, 25-desacetoxy- identified at RT 29.205, 19-norpregn-5(10)-en-20-yn-3-one, 17-[(trimethylsilyl)oxy]-, (17.alpha.)- identified at RT 32.380 (also known as Trans-3, 5, 4 -trimethoxystilbene (TMS) derivatives), carbromal identified at RT 32.260, spirost-8-en-11-one identified at RT 32.980, alpha.-D-glucopyranoside, methyl 2-(acetylamino)-2-deoxy-3-O-(trimethylsilyl)-, cyclic methylboronate identified at RT 32.490 and morphinan identified at RT. 35.215.
 |
Figure 4: (a-e). Phytochemicals with steroid structures identified in the W. sominifera methanolic root extracts. |
Discussion
In the plants, phytochemicals greatly vary from organ to organ.59 It has been reported that all the parts of Withania somnifera have been used for treatment of various human illnesses.60 W. somnifera root and leaf extracts of both aqueous and alcoholic, have previously been reported to be antimicrobial against a wide range of microorganisms.61 The roots of W. somnifera are mostly preferred for various therapeutic purposes.62 Moreover, methanolic extracts of various parts of W. somnifera especially roots have also been reported to be an effective against various kinds of cancers.63
GC-MS analysis is a rapid and cost-efficient method as it is effective in chromatographic separation, quantification, and identification of sample constituents for assessing herbal products.64 Based on this, we performed GC-MS analysis to profile phytochemicals in methanolic extracts of leaves, stems, and roots at the reproductive stage of W. somnifera. In the methanolic leaf extracts, the identified phenolic compounds 1,2-bis(trimethylsilyl)benzene have previously been reported for antibacterial and anticancer activities,65 ester compound 1,2-cinnolinedicarboxylic acid, 1,2,3,5,6,7,8,8a-octahydro-4-trimethylsilyloxy-, diethyl ester have previously been reported to be antibacterial and antifungal activities,66 and phenolic compound 1,3-Dihydroxy-2,4,5-trifluoro-6-nitrobenzene is a nitrobenzene derivative shown to have antitumor and antibacterial properties.67, 41 The identified methyl ketone1-[2,4-bis(trimethylsiloxy)phenyl]-2-[(4-trimethylsiloxy)phenyl]propan-1-one has been reported to be antibacterial in property,68 Phenolic stilbenes1-Methyl-1,2,2-triphenylindan has been reported to be antibacterial in property,69 silyl ethers 1,3,5,7-tetraethyl-1-butoxycyclotetrasiloxane has been reported to be antibacterial properties,70 for the aldehyde compound cyclopropanecarbonyl chloride, 1-fluoro-2,2-diphenyl- there is no specific available reports, however its derivatives viz. cyclopropanecarbonyl chloride have been reported to be anticancer in properties,33 and phenolic compounds 2-(7-methoxymethylphenanthren-3-yl)propan-2-ol has been reported to be both anticancer and antimicrobial in properties.71
In the root methanolic extracts, the identified esterbenzoic acid, 3-methyl-2-trimethylsilyloxy-, trimethylsilyl ester, has previously been reported to be antimicrobial in properties.46 Amines compounds tris(trimethylsilyl)hydroxylamine a hydroxylamine derivative, has been reported to be antibacterial properties.28 Ester compound boric acid, trimethyl ester has been reported to be anticancer properties69 and dextroamphetamine compound was reported to be brain stimulant and antibiotic.72 Steroid compound morphinan found in the Opium poppy (Papaver somniferum),73 has been identified in this study in the root extracts of W. somnigfera which was found to have antibacterial properties.74 Somephytochemicals identified in the root and stem methanolic extracts alkanes like ethane, 1,2-dichloro-1-ethoxy which is antimicrobial,75 steroid amines like dextroamphetamine, acids like boric acid, trimethyl which is anticancer,76 ketones like tartronic acid, 4-(dimethylethylsilyl)phenyl-, dimethyl ester, which is antibacterial,77 and aromatic acid like 3,5-dichloro-4-hydroxybenzoic acid which is antimicrobial in properties.78
In the methanolic stem extract phenolic compound identified 3,5-dichloro-4-hydroxybenzoic; 3-acetoxy-2,3′-bibenzo[b]thiophene which is a benzo[b]thiophene derivatives has previously been reported to be anticancer and antibacterial.47, 52 The identified terpenoid phytoalexins such as acetohydroxamic acid has been reported to be antibacterial in the previous study.5 Identified alkanes such as pentasiloxane, dodecamethyl- has previously been reported to be anticancer,79 and aromatic nitroalkene such as trans-2,3,6-trimethoxy-b-methyl-b-nitrostyrene which is β-nitrostyrene derivatives compounds has been reported to be antibacterial.36
Some steroids compounds identified in this study which include α-D-glucopyranoside, methyl 2-(acetylamino)-2-deoxy-3-O-(trimethylsilyl)-, cyclic methylboronate were reported to be found in the flowers of Jacaranda mimosifolia, that poses antibacterial properties80 and its derivatives like methyl-α-D-glucopyranoside in Tulbghia violacea,70 and α-D-glucopyranoside,O-α-D-glucopyranosyl- (1.fwdarw.3)-ß-D-fructo in Foeniculum vulgare81 were reported to be anticancer in properties. Similarly, carbromal is found in Decalepis hamiltonii,82 has been used to treat mild insomnia.83 Derivatives of novel steroids 19-norpregn-5(10)-en-20-yn-3-one84 i.e., 19-norpregn-4-en-20-yn-3-one is antitumor in properties and has been reported to be found in the Curcuma aeruginosa.85 Cucurbitacin b is an effective anticancer and antibacterial agent,57 found in the Cucurbitaceae plant families.87
This study agrees with previous researchers of W. somnifera to be antimicrobial and anticancer in properties.88,89 Moreover, this GC-MS investigation rationally evaluated and identified the phytochemicals that attributes the antimicrobial and anticancer properties of W. somnifera. Further research is needed to isolate and purify the identified phytochemicals for bioactivities in order to develop W. somnifera herbal based products for applications in the antimicrobial and anticancer therapies.
Conclusion
The present study describes comparative GC-MS analysis of methanol extract of W. sominfera phytochemicals distribution in the leaf, stem, and root. The GC-MS analysis revealed the distribution variation of phytochemicals contributing towards antimicrobial and anticancer properties. The highest number of unique phytochemicals was identified in the root extracts and least number of unique phytochemicals were identified in the leaf. Moreover, root and stem shared the highest number of common phytochemicals. In this study, the GC-MS analysis identified the antibacterial and anticancer phytochemicals that were not previously been reported in the W. somnifera, which included steroidal compounds like cucurbitacin b, 25-desacetoxy-; spirost-8-en-11-one, 3-hydroxy-, (3.beta.,5.alpha.,14.beta.,20.beta.,22.beta.,25R)-; alpha.-D-glucopyranoside, methyl 2-(acetylamino)-2-deoxy-3-O-(trimethylsilyl)-, cyclic methylboronate and 19-norpregn-5(10)-en-20-yn-3-one, 17-[(trimethylsilyl)oxy]-, (17.alpha.).This information can be utilized further to develop W. somnifera based traditional herbal medicines that are playing an important role in healthcare system.
Acknowledgement
LL acknowledges National Fellowship for Higher Education of ST Students (NFST), Ministry of Tribal Affairs – Government of India, for fellowship. AS acknowledges Rashtriya Uchchatar Shikhsha Abhiyan (RUSA) 2.0 Programme, under Ministry of Huma Resource Development, Government of India for funding the research. AS also acknowledge funding by CAS-II, DST-PURSE II, UPE-FAR and DBT-BUILDER.
Conflicts of Interest
The authors declare no conflicts of interest
References
- Sofowora A, Ogunbodede E and Onayade A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. African Journal of Traditional, Complementary and Alternative Medicines; 10(5): 210-229 (2013).
CrossRef - Atanasova G. A, Waltenberger B, Pferschy-Wenzig E, Linder T, Wawrosch C, Uhrin P, Temml V, Wang L, Schwaiger S, Heiss H. E, Rollinger M. J, Schuster D, Breusse M. J, Bochkov V, Mihovilovic D. M, Kopp B, Rudolf B,Dirsch M. V andStuppner H. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnology Advances; 33(8): 1582-1614 (2015).
CrossRef - Salehi B,Capanoglu E, Adrar N, Catalkaya G, Shaheen S, Jaffer M, Giri L, Suyal R, Jugran A. K, Calina D, Docea A. O, Kamiloglu S, Kregiel D, Antolak H, Pawlikowska E, Sen S, Acharya K, Selamoglu Z, Sharifi-Rad J, Martorell M, Rodrigues C. F, Sharopov F, Martins N andCapasso R. Cucurbits Plants: A Key Emphasis to Its Pharmacological Potential. Molecules; 24(10): 1-23 (2019).
CrossRef - Karunamoorthi K, Jegajeevanram K, and Vijayalakshmi J. Traditional Medicinal Plants: A Source of Phytotherapeutic Modality in Resource-Constrained Health Care Settings. Journal of Evidence-Based Complementary & Alternative Medicine; 18(1) 67-74 (2012).
CrossRef - Musher D. M, Saenz C and Griffith D. P. Interaction Between Acetohydroxamic Acid and 12 Antibiotics Against 14 Gram-Negative Pathogenic Bacteria. Antimicrobial Agents and Chemotherapy; 5(2): 106-110 (2021).
CrossRef - Mishra D and Patnaik S. GC-MS Analysed Phyto-Chemicals and Antibacterial Activity of Withania somnifera (L.) Dunal Extract in the Context of Treatment to Liver Cirrhosis. Biomedical and Pharmacology Journal; 13(1): 71-78 (2020).
CrossRef - Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in Pharmacology; 4(177): 1-10 (2014).
CrossRef - Elsharkawy E. R. Allelopathic Effects of Alkaloid Contents of Hyoscyamus muticus and Withania somnifera on the Germination of Cichorium intybus Seeds. Bioscience Biotechnology Research Communications; 12(4): 953-960 (2019).
CrossRef - Huss E, Yosef K. B and Zaccai M. Humans’ Relationship to Flowers as an Example of the Multiple Components of Embodied Aesthetics. Behavioral Sciences; 8(3): 1-10 (2018).
CrossRef - El-Dahiyat F, Rashrash M, Abuhamdah S, Farha R. andBabar Z. Herbal medicines: a cross-sectional study to evaluate theprevalence and predictors of use among Jordanian adults. Journal of Pharmaceutical Policy and Practice; 13(2): 1-9 (2020).
CrossRef - Caputi F. F, Acquas E, Kasture S, Ruiu S, Candeletti S and Romualdi P. The standardized Withania somnifera Dunal root extract alters basal, and morphine induced opioid receptor gene expression changes in neuroblastoma cells. BMC Complementary and Alternative Medicine; 18(1): 1-9 (2018).
CrossRef - Speers A. B, Cabey K. A, Soumyanath A, and Wright K. M. Effects of Withania somnifera (Ashwagandha) on Stress and the Stress-Related Neuropsychiatric Disorders Anxiety, Depression, and Insomnia. Current Neuropharmacology; 19(9): 1468-1495 (2021).
CrossRef - Gaurav N. Pharmacological and Medicinal Activity of Active Phytochemicals of Withania somnifera. Journal of Engineering and Technology; 3(2):1-6 2013).
- Gaurav N, Kumar A, Tyagi M, Kumar D and Chauhan A. P. Morphology of Withania somnifera (Distribution, Morphology, Phytosociology of Withaniasomnifera Dunal). International Journal of Current Science Research; 1(7): 164-173 (2015).
- Shaheen G, Akram M, Jabeen F, Shah S. M, Munir N, Daniyal M, Riaz M, Tahir I. M, Ghauri A.O, Sultana S, Zainab R and Khan M. Therapeutic potential of medicinal plants for the management of urinary tract infection: A systematic review. Clinical and Experimental Pharmacology and Physiology; 46(7): 613-624 (2019).
CrossRef - Aguirre-Becerra H, Pineda-Nieto S. A, García-Trejo J. F, Guevara-Gonzáleza R. G,Feregrino-Pérez A. A, Álvarez-Mayorga B. L and Pastrana D. M. Jacaranda flower (Jacaranda mimosifolia) as an alternative for antioxidant and antimicrobial use. Helion; 6(12): 1-9 (2020).
CrossRef - Hussein J. H, Hadi M. Y and Hameed I. H. Study of chemical composition of Foeniculum vulgare using Fourier transform infrared spectrophotometer and gas chromatography – mass spectrometry. Journal of Pharmacognosy and Phytotherapy; 8(3): 60-89 (2016).
CrossRef - Mohammed I. A, Shahabuddin S, Khanam R and Saidur R. Synthesis, characterization and antibacterial activity of novel poly(silyl ether)s based on palm and soy oils. Polímeros; 28 (5): 406-412 (2018).
CrossRef - Jaithliya T. Ashwagandha-The Nature’s Gift to Mankind. Journal of Pharmaceutical and Pharmacological Sciences; 2017(05): 1-4 (2017).
- Mishra L.C, Singh B.B and Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (Ashwagandha): A review. Alternative Medicine Reviews; 5(4): 334-346 (2000).
- Singh N, Bhalla M, Jager P, and Gilca M. An Overview on Ashwagandha: A Rasayana (Rejuvenator) Of Ayurveda. African Journal of Traditional, Complementary and Alternative Medicines; 8(5): 208–213 (2011).
CrossRef - Sonia K, Shree B, Lakshmi K. HPTLC Method Development and Validation: An Overview. Journal of Pharmaceutical Sciences and Research; 9(5): 652-657 (2017).
CrossRef - McDonald J. G, Matthew S and Auchus R. J. Steroid Profiling by Gas Chromatography–Mass Spectrometry and High Performance Liquid Chromatography–Mass Spectrometry for Adrenal Diseases. Discover Oncology; 2(6): 324-332 (2012).
- Kubsad V. S, Palled Y. B, Mansur C. P, Channal H. T, Basavaraj N and Koti R. V. Performance of ashwagandha (Withania somnifera Dunal) as influenced by dates of sowing and stages of harvesting. Karnataka Journal of Agricultural Sciences; 22(5):1001-1005 (2009).
CrossRef - Dai J and Mumper R. J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules; 15(10): 7313-7352 (2010).
CrossRef - Halket J. M, Waterman D, Przyborowska A. M, Patel R. K, Fraser P. D and Bramley P. M. Chemical derivatization and mass spectral libraries in metabolic profiling by GC/MS and LC/MS/MS. Journal of Experimental Botany; 56(410): 219–243 (2004).
- Joshi K, Tavhare S. D, Panara K and Nishteswar K. Studies of Ashwagandha (Withania somnifera Dunal). International Journal of Pharmaceutical & Biological Archives; 7(1): 1-11 (2016).
CrossRef - Miret-Casals L,Baelo A, Julián E, Astola J, Lobo-Ruiz A, Albericio F and Torrents E. Hydroxylamine Derivatives as a New Paradigm in the Search of Antibacterial Agents. ACS Omega; 3(12): 17057-17069 (2018).
- Duanyong Z, Jianbo N, Wei X, Hao Z, Jun Z, Zengli Z, Xiaolin W; Jiangsu Qingquan Chemical Co., LTD. 5-chloro-2-pentanone preparation method. China Patent CN103694094A, 11 September 2013.
- Wadkar S, Shete C, Inamdar F. R, Gurav R. V, Patil K. S and Ghosh J. S. Phytochemical Screening and Antibacterial Activity of Cryptocoryne spiralis var. spiralis and Cryptocoryneret rospiralis (Roxb) Kunth. Medicinal & Aromatic Plants; 6(2): 1-7 (2017).
CrossRef - Alamri A, El-Newehy M. H and Al-Deyab S. S. Biocidal polymers: synthesis and antimicrobial properties of benzaldehyde derivatives immobilized onto amine-terminated polyacrylonitrile. Chemistry Central Journal; 6(1): 1-13 (2012).
CrossRef - Şenkardeş S, Kulabaş N, Ozakpinar O. B, Kalayci S, Şahin F, KüçükgüzeI and Küçükgüzel S. G. Synthesis and Anticancer and Antimicrobial Evaluation of Novel Ether-linked Derivatives of Ornidazole. Turkish Journal of Pharmaceutical Sciences; 17(1): 81- 93 (2020).
CrossRef - Prasad T. N, Eeda K. R, Gudise V. B, Basha S. F, and Anwar S. Design, synthesis and biological evaluation of substituted 2-amino-1,3-thiazine derivatives as antituberculosis and anti-cancer agents. An International Journal for Rapid Communication of Synthetic Organic Chemistry, 49(10): 1-9 (2019).
CrossRef - Boumendjel A, Beney C, Deka N, Mariotte A. M, Lawson M. A, DorianeTrompier, Helene BaubichonCortay, and Pietro A.D. 4-Hydroxy-6-methoxyaurones with high-affinity binding to cytosolic domain of P-glycoprotein. Chemical and Pharmaceutical Bulletin; 50(6): 854-856 (2002).
CrossRef - Huang M, Jin J, Sun H and Liu G. T. Reversal of P-glycoprotein-mediated multidrug resistance of cancer cells by five schizandrins isolated from the Chinese herb Fructus schizandrae. Cancer Chemotherapy and Pharmacology; 62(6):1015-1026 (2008).
CrossRef - Milhazes N, Calheiros R, Marques M. P, Garrido J, Cordeiro N. S, Rodrigues C, Quinteira S, Novais C, Peixe L and Borges F. β-Nitrostyrene derivatives as potential antibacterial agents: A structure–property–activity relationship study. Bioorganic & Medicinal Chemistry; 14(12): 4078-4088 (2006).
CrossRef - Taheri-Ledari R, Mirmohammadi S. S, Valadi K, Maleki A and Shalan A. E. Convenient conversion of hazardous nitrobenzene derivatives to aniline analogues by Ag nanoparticles, stabilized on a naturally magnetic pumice/chitosan substrate. RSC Advances; 10(2020): 43670 – 43681 (2020).
- Fasola T. R, Oloyede G. K and Aponjolosun B. S. Chemical composition, toxicity and antioxidant activities of essential oils of stem bark of Nigerian species of guava (Psidium guajava). EXCLI Journal; 10(2011): 34 – 43 (2011).
CrossRef - Guedouara H, Alouia F, Beltifab A, Mansourb H. B and Hassine B. B. Synthesis and characterization of phenanthrene derivatives with anticancer property against human colon and epithelial cancer cell lines. Comptes Rendus Chimie; 20(8): 841-849 (2017).
CrossRef - Flamini R and Rosso M. D. Chapter 5 – High-Resolution Mass Spectrometry and Biological Properties of Grapevine and Wine Stilbenoids. Studies in Natural Products Chemistry; 61(2019): 175-210 (2019).
CrossRef - Prabha S and Kumar J. Gas Chromatographic and Mass Spectroscopic (GC-MS) Analysis of Rhizome of Acorus Calamus for Identification of Potent Antimicrobial Bio-active Compounds. Journal of Scientific Research; 13(1): 263-273 (2021).
CrossRef - Bach S. M and Fortuna M. A, Attarian R, Trimarco J. T, Catalán C. N, Av-Gay Y and Bach H. Antibacterial and cytotoxic activities of the sesquiterpene lactones cnicin and onopordopicrin. Natural Product Communications; 6(2):163-166 (2011).
- Chabake V, Chaubal S and Patil T. Studies on GC-MS profiling of some seaweeds of Mahim beach dist. Palghar, Maharashtra using various solvents. International Journal of Pharma Sciences and Research; 13(4): 1644-1650 (2022).
CrossRef - Hameed R. H, Mohammad G. J, Hameed I. H. Characterization of Antimicrobial Metabolites Produced by Salvadora persica and Analysis of Its Chemical Compounds Using GC-MS and FTIR. Indian Journal of Public Health Research and Development; 9(3): 241-246 (2018).
CrossRef - Layne T. H, Roach J. S and Tinto W. F. Review of β-carboline Alkaloids from the Genus Aspidosperma. Natural Product Communications; 10(1): 183-186 (2015).
- Beulah G. P, Soris P. T and Mohan V. R. GC-MS Determination of Bioactive Compounds of Dendrophthoe falcata (L.F) Ettingsh: An Epiphytic Plant. International Journal of Health Sciences and Research; 8(11): 261-269 (2018).
CrossRef - Keri R. S, Chand K, Budagumpi S, Somappa S. B, Patil S. A, Nagaraja B M. An overview of benzo[b]thiophene-based medicinal chemistry. European Journal of Medicinal Chemistry; 2017(138): 1002-1033 (2017).
CrossRef - Tsivgoulis G. M,Lala M. A and Ioannou P. V. Preparation of DL-2,3,4-trihydroxybutylarsonic acid and dl-2,3-dihydroxybutane-1,4-bis(arsonic acid): starting compounds for novel arsonolipids. Chemistry and Physics of Lipids; 148(2): 97-104 (2007).
CrossRef - Belz T,Ihmaid S, Al-Rawi J andPetrovski S. Synthesis Characterization and Antibacterial, Antifungal Activity of N-(Benzyl Carbamoyl or Carbamothioyl)-2-hydroxy Substituted Benzamide and 2-Benzyl Amino-Substituted Benzoxazines. International Journal of Medicinal Chemistry; 2013(436397): 1-20 (2013).
CrossRef - Luo Y, Zhang L, Hu Y, Zhang S, Fu J, Wang X and Zhu H. Synthesis and antimicrobial activities of oximes derived from O-benzylhydroxylamine as FabH inhibitors. ChemMedChem; 7(9):1587-1593 (2012).
- Keskin D, GuvensenA N. C. Uǧur A and Dbeys A. D. Antimicrobial activity and chemical constitutions of West Anatolian olive (Olea europaea L.) leaves. Journal of Food Agriculture and Environment; 10(2): 99-102 (2012).
CrossRef - Haerdi-Landerer M. C, Suter M. M, Steiner A, Wittenbrink M. M, Pick A and Gander B. A. In vitro cell compatibility and antibacterial activity of microencapsulated doxycycline designed for improved localized therapy of septic arthritis. Journal of Antimicrobial Chemotherapy; 61(2):332-340 (2008).
CrossRef - Ghosh G, Panda P, Rath M, Pal A, Sharma T, Das D. GC-MS analysis of bioactive compounds in the methanol extract of Clerodendrum viscosum Pharmacognosy Research; 7(1):110-113 (2015).
CrossRef - Ochwoto M, Muita L, Talaam K, Wanjala C, Ogeto F, Wachira F, Osman S, Kimotho J, Ndegwa L. Anti-bacterial efficacy of alcoholic hand rubs in the Kenyan market, 2015. Antimicrobial Resistance and Infection Control; 6(17): 1-6 (2017).
CrossRef - Dixit A, Pathak D and Sharma G.K. Synthesis, antibacterial and free radical scavengingactivity of some newer N-((10-nitro-1H-indolo[1, 2-c]quinazolin-12-yl)methylene)benzenamines. European Pharmaceutical Journal; 67(1): 7-16 (2020).
- Kumar P, Chandbala V, Nimesh S, Bajpai A and Singh S. Aryl Propionic Acid Derivatives: A Recent Advancement in Pharmacological Activities. International Journal of Pharmacy and Pharmaceutical Research; 17(4): 540-555 (2020).
CrossRef - Abdelkader M. A, Rateb M. E, Mohamed G. A and Jaspars M. Harpulliasides A and B: Two new benzeneacetic acid derivatives from Harpullia pendula. Phytochemistry Letters. 15(2016): 131-135(2016).
CrossRef - Moleyar V and Narasimham P. Antibacterial activity of essential oil components. International Journal of Food Microbiology; 16(4):337-342 (1992).
CrossRef - Oehmichen M, Ochs U and Meissner C. Regional potassium distribution in the brain in forensic relevant types of intoxication preliminary morphometric evaluation using a histochemical method. Neurotoxicology; 22(1); 99-107 (2001).
CrossRef - Ziauddin M, Phansalkar N, Patki P, Diwanay S and Patwardhan B. Studies on the immunomodulatory effects of Ashwagandha. Journal of Ethnopharmacology; 50(2): 69-76 (1996).
CrossRef - Afewerky H. K, Ayodeji A. E, Tiamiyu B. B,Orege J. I, Okeke E. S, Oyejobi A. O, Bate , Petuel N and Adeyemi S. B. Critical review of the Withania somnifera (L.) Dunal: ethnobotany, pharmacological efficacy, and commercialization significance in Africa. Nature Public Health Emergency Collection; 45(1): 1-16 (2021).
CrossRef - Owais M, Sharad K. S, Shehbaz A and Saleemuddin M. Antibacterial efficacy of Withania somnifera (ashwagandha) an indigenous medicinal plant against experimental murine salmonellosis. Phytomedicine; 12(3): 229-235 (2005).
CrossRef - Rai M, Jogee P. S, Agarkar G and Santos C. A. Anticancer activities of Withaniasomnifera: Current research, formulations, and future perspectives. Pharmaceutical Biology; 54(2): 189-197(2015).
CrossRef - Sofuoglu M, Mooney M, Kosten T, Waters A, and Hashimoto K. Minocycline attenuates subjective-rewarding effects of dextroamphetamine in humans. Psychopharmacology; 213(1): 61-68 (2012).
CrossRef - Salem M. Z, Elansary H. O, Elkelish A. A, Zeidler A, Ali H. M, EL-Hefny M and Yessoufou K. In vitro Bioactivity and Antimicrobial Activity of Piceaabies and Larix decidua Wood and Bark Extracts. BioResources; 11(4): 9421-9437 (2016).
CrossRef - Mirjalili M. H, Moyano E, Bonfill M, Cusido R. M and Palazón J. Steroidal Lactones from Withania somnifera, an Ancient Plant for Novel Medicine. Molecules; 14(7): 2373-2393 (2009).
CrossRef - Konappa N, Udayashankar A. C,Krishnamurthy S, Pradeep C. K, Chowdappa S and Jogaiah S. GC–MS analysis of phytoconstituents from Amomum nilgiricum and molecular docking interactions of bioactive serverogenin acetate with target proteins. Scientific Reports; 10(16438): (2020).
CrossRef - ZekryAttia E, El-Baky R. M, Desoukey S. Y, Mohamed M. H, Mohame M and Kamel M. S. Chemical composition and antimicrobial activities of essential oils of Ruta graveolens plants treated with salicylic acid under drought stress conditions. Future Journal of Pharmaceutical Sciences: 4(2); 254-264 (2018).
CrossRef - Albert S, Horbach R, Deising H. B, Siewert B and Csuk R. Synthesis and antimicrobial activity of (E) stilbene derivatives. Bioorganic & Medicinal Chemistry; 19(17): 5155-5166 (2011).
- Thirugnanasambantham P. K. Comparative Chemometric Profiles Between Leaf Tissues of Withania somnifera Cultured In Vitro And Field. International Journal of Pharmacy and Pharmaceutical Sciences; 7(11): 66-71 (2015).
CrossRef - Larkin P. J, Miller J. A, Allen R. S, Chitty J. A, Gerlach W. L, Frick S, Kutchan T. M and Fist A. J. Increasing morphinan alkaloid production by over-expressing codeinone reductase in transgenic Papaver somniferum. Plant Biotechnology Journal; 5(1): 26-37 (2007).
CrossRef - Sofuoglu M, Mooney M, Kosten T, Waters A and Hashimoto K. Minocycline attenuates subjective rewarding effects of dextroamphetamine in humans. Psychopharmacology; 213(1): 61-68 (2011).
CrossRef - Guo L, Winzer T, Yang X, Li Y, Ning Z, He Z, Teodor R, Lu Y, Bowser T. A, Graham I. A and Ye K. The opium poppy genome and morphinan production. Science; 362(6412): 343-347 (2018).
CrossRef - Orrù A, Marchese G, Casu G, Casu M. A,Kasture S, Cottiglia F, Acquas E, Mascia M. P, Anzani N and Ruiu S. Withania somnifera root extract prolongs analgesia and suppresses hyperalgesia in mice treated with morphine. Phytomedicine; 21(2014):745-752 (2014).
CrossRef - Tsukamoto S, Kato H, Hirota H and Fusetani N. Antibacterial and antifungal sulfated alkane and alkenes from the hepatopancreas of the ascidian Halocynthia roretzi. Journal of Natural Products; 57(11): 1606-1609 (1994).
CrossRef - Balachandar R, Kumar K. A and Karmegam N. GC-MS analysis of ethyl acetate extract of Sterptomyces species isolated from vermicast. International Journal of Pharma and Bio Sciences; 7(4): 416-419 (2016).
CrossRef - Shankar R, Shahi V and Sahoo U. Comparative Study of Linear Poly(alkylarylsilane)s as Reducing Agents toward Ag(I) and Pd(II) Ions−Synthesis of Polymer−Metal Nanocomposites with Variable Size Domains of Metal Nanoparticles. ACS Publication; 22(4): 1367-1375 (2010).
- Das S, Saraf A, Sharma D, Raja W and Sohal J. K. Study of Antibacterial Activity of Withania somnifera Plant Extract against Some Human Pathogenic Bacteria. International Journal of Science and Research (IJSR); 9(6): 1506 -1509 (2020).
CrossRef - Rahman S and Jahan N. Anti-inflammatory activity of crude and detoxified leaves of Daphne oleoides on carrageenan-induced paw edema in wistar rats. Journal of Ayurveda and Integrative Medicine; 12(3): 500-505 (2021).
- Saleem S, Muhammad G, Hussain M. A, Altaf M and Bukhari S. A. Withania somnifera: Insights into the phytochemical profile, therapeutic potential, clinical trials, and future prospective. Iranian Journal of Basic Medical Sciences; 23(12): 1501-1526 (2020).
CrossRef - Tiago F. Jorge, Ana T. Mata and Carla António. Mass spectrometry as a quantitative tool in plant metabolomics. Philosophical Transactions of the Royal Society A; 374(2079): 1-26 (2016).
- Mohan C, Bhukya M and Bogarapu R. Phytochemical screening, GC-MS analysis of Decalepis hamiltonii Wight &Arn. An endangered medicinal plant. Journal of Pharmacognosy and Phytochemistry; 5(5): 10-16 (2016).
- Kothari V, Patgiri B. J and Prajapati P. K. Ashvagandhadhya Ghrita-A Classical Formulation in A New Perspective. International Journal of Ayurveda and Pharma Research, 5(5), 58-61 (2017).
CrossRef - Pandey V, Ansari W. A and Atri P. N. Withania somnifera: Advances and Implementation of Molecular and Tissue Culture Techniques to Enhance Its Application. Frontiers in Plant Science; 8(1390): 1-11 (2019).
CrossRef - Vies J. Pharmacological studies with (7α,17α)-17-hydroxy-7-methyl-19-norpregn-5(10)-en-20-yn-3-one (Org OD 14). Maturitas; 9(1): 15-24 (1987).
CrossRef - Kaushik U, Aeri V and Mir S. R. Cucurbitacins – An insight into medicinal leads from nature. Pharmacognosy Reviews; 9(17): 12-18 (2015).
CrossRef - Garg S, Kaul S. C, Wadhwa R. Cucurbitacin B and cancer intervention: Chemistry, biology and mechanisms (Review). International Journal of Oncology; 52(1): 19-37 (2018).
CrossRef - Seymour V. The Human–Nature Relationship and Its Impact on Health: A Critical Review. Frontiers in Public Health; 4(260): 1-12 (2016).
- Verma S. K. Therapeutic uses of Withania somnifera (Ashwagandha) with a note on withanolides and its pharmacological actions. Asian Journal of Pharmaceutical and Clinical Research; 4(1): 1-4 (2011).







