Manuscript accepted on :31-10-2022
Published online on: 13-01-2023
Plagiarism Check: Yes
Reviewed by: Dr. Dewi Syahidah
Second Review by: Dr. Dhruv Desai
Final Approval by: Dr. H Fai Poon
Ashfaq Ahmad Shah1 , Amit Gupta1*
, Amit Gupta1* , Aqueel-Ur-Rehman1
, Aqueel-Ur-Rehman1 , Sanchita Kapoor1
, Sanchita Kapoor1 , Harmanpreet Kaur1
, Harmanpreet Kaur1 , Bharat Rohilla1
, Bharat Rohilla1 , Kumari Rashmi1 and AB Bajpai2
, Kumari Rashmi1 and AB Bajpai2
1Department of Microbiology and Biotechnology, Graphic Era (Deemed to be) University, Dehradun, India
2Department of Botany, D.B.S. (P. G.). College, Dehradun, Uttarakhand, India.
Corresponding Author E-mail:dr.amitgupta.bt@geu.ac.in
DOI : https://dx.doi.org/10.13005/bpj/2589
Abstract
The current study investigated the therapeutic potential of Citrullus lanatus seeds which are commonly discarded after eating the fruit. In this day and age, plant secondary metabolites are preferred therapeutic agents to manage a variety of diseases and disorders. The present study aimed to investigate the bioactive secondary metabolite profile of Citrullus lanatus seeds by investigating total phenolic and flavonoid content, antioxidant potential, and Gas Chromatography-Mass Spectrometry (GC-MS) analysis of bioactive compounds and anti-bacterial properties of four different crude extracts. Alkaloids, flavonoids, phenols, steroids, tannins, saponins, phytosterols, terpenoids, and glycosides were revealed in the seeds after qualitative phytochemical examination utilizing several solvents of varying polarity and established techniques of analysis. DPPH radical scavenging assay was used to assess the antioxidant potential and the total flavonoid and phenolic contents in seed extracts were determined using the spectrophotometric method. Methanolic extract revealed higher extractive yield, antioxidant potential, a higher total phenolic content (132.68 ± 0.861 mg of GAg), and higher total flavonoid content (48.13 ± 0.451 mg of Qg) as compared to other extracts. Gas Chromatography-Mass Spectrometry (GC-MS) analysis of all four seed extracts revealed the presence of 27 high and low molecular weight chemical entities in toto with varying amounts. These bioactive chemical substances have been revealed to be physiologically significant and essential from a pharmaceutical standpoint. This research demonstrates that the Citrullus lanatus seeds are high in bioactive secondary metabolites that are beneficial to human health, have a high antioxidant capacity, and antibacterial action against certain bacterial strains, indicating that these seeds have a lot of therapeutic value.
Keywords
Antioxidants; Antibacterial activity; Citrullus lanatus; 1,1-diphenyl-2-picrylhydrazyl (DPPH); GC-MS; Secondary metabolites
Download this article as:| Copy the following to cite this article: Shah A. A, Rehman A. U, Kapoor S, Kaur H, Rohilla B, Bajpai A. B, Gupta A. GC-MS Analysis of Phytoactive Compounds, Antioxidant and Antibacterial Activity of Citrullus lanatus Seeds. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Shah A. A, Rehman A. U, Kapoor S, Kaur H, Rohilla B, Bajpai A. B, Gupta A. GC-MS Analysis of Phytoactive Compounds, Antioxidant and Antibacterial Activity of Citrullus lanatus Seeds. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3GCqYIk |
Introduction
The major objective of introducing plant-based bioactive compounds into healthcare is to create a healthy interaction with the body’s chemistry while avoiding the unwanted and off-target side effects that medications are known for 1. Pharmacognostical researchers are being forced to seek out novel plant-based bioactive compounds for use in the treatment of a wide range of diseases and disorders due to an increase in the human population, insufficient drug supply, unmanageable treatment costs, and an increase in antimicrobial resistance to currently used drugs. Plants provide the majority of foods, pharmaceuticals, and nutritional supplements 1,2. Primary and secondary metabolites are the chemical compounds found in plants and are termed phytochemicals. Because they are involved in activities like cell division, proliferation, reproduction, metabolism, storage, and development, primary metabolites are vital for plant life. Secondary metabolites in plants aren’t merely waste byproducts of primary metabolism; they also have a big influence on plant defenses, ecology, and evolution 2. Flavonoids are predominant and paramount secondary metabolites found in plants. Flavonoid is a generic name used to describe a family of over 6000 compounds that have a 15-carbon skeleton with two phenyl rings A and B and a heterocyclic ring C. The oxygen with heterocyclic ring C mediates the joining of rings A and B. C6-C3-C6 is the chemical structure used to denote such a compound. Because of their structure, they are essential variable phenolic compounds with strong anti-oxidant properties. Plants resist oxidative damage because of their inherent secondary metabolites, and as a result, when ingested, they constitute a dietary supply of anti-oxidants 3. They might minimize the amount of ROS in stressed systems by acting as effective singlet oxygen quenchers. Secondary metabolites have received a lot of attention in pharmacognosy research in the last two decades because they’ve been shown to have anti-inflammatory, anti-carcinogenic, anti-tumor, anti-oxidative, anti-hypertensive, anti-viral, anti-aging, cardioprotective, and immunomodulatory properties, as well as the ability to modulate enzymatic functions, inhibit cell proliferation, induce apoptosis, and inhibit bacterial and fungal growth among others. Current research is also highlighting their role as potent along with their potential to act as substrates for biochemical reactions, cofactors for enzymatic reactions, ligands that antagonize or agonize cellular receptors, act as prebiotics, act as immunomodulators, etc. Bioinformatics and molecular docking information are being applied to forecast the possible use of secondary metabolites in human health and illness. 4.
Plant secondary metabolites (PSM) are naturally occurring physiologically active substances utilized in the traditional type of medicine and diversification of industrial uses. In the case of chronic illnesses, prevention is a better method than therapy. Plant-based foods, such as fruits, have high levels of bioactive phytochemicals, which may have health advantages beyond basic nutrition, such as lowering the risk of chronic illnesses 5. Watermelon (Citrullus lanatus), a Cucurbitaceae family fruit with over 1,000 cultivars, is a widely farmed fruit worldwide. The large edible fruit, which is a berry with a tough rind and no internal sections and is botanically referred to as a pepo, is grown at optimum conditions all over the world, from subtropical to temperate regions. Although seedless cultivars exist, the luscious, juicy flesh is generally deep crimson to pink, with abundant black seeds. The rind is edible after cooking, and the fruit can be eaten fresh or pickled. It can also be drunk as a juice or as part of a mixed drink 6. This fruit is a good choice for a healthier diet since it contains phytochemicals such as terpenoids, glycosides, alkaloids, flavonoids, flavonoids, coumarins, quinones, carotenoids, lycopene, anthocyanins, and phenols, as well as vitamins and minerals 7. Regular consumption of this fruit, which is high in health-promoting substances, may reduce the risk of a variety of deadly diseases, including diabetes, cardiovascular disease, liver problems, obesity, cancer, neurological disorders, and aging-related conditions 8. Considering the beneficial effects of plant bioactive compounds on human health, this study was organized to determine the presence of secondary metabolites (phenolic and polyphenolic compounds), free radical scavenging activity, and antibacterial activity in Citrullus lanatus seeds that are typically discarded after fruit consumption. These seeds are high in bioactive phenolic and polyphenolic components such as flavonoids, phenols, saponins, terpenoids, glycosides, steroids, alkaloids, coumarins, and quinones, according to the findings of this study. All of the extracts of the seeds were shown to have substantial antioxidant properties as well as antibacterial activity against some of the microbial strains.
Materials and Methods
Collection of Citrullus Lanatus Seeds
In May, Citrullus lanatus fruits were obtained at a local market in Dharmapuri Mandi, Dehradun, and their seeds were harvested. Only healthy-looking fruits were selected. Before analysis, seeds were shade dried and kept in a conducive environment.
Chemicals
The reagents used for the study were acetone, methanol, chloroform, Mayer’s Reagent, Ammonia Solution, ferric chloride, concentrated H2SO4, quercetin, ascorbic acid, gallic acid, lead acetate, 2, 2-diphenyl-1-picryl-hydrazyl (DPPH), sodium carbonate, Folin-Ciocalteau’s phenol reagent, aluminium chloride, potassium acetate, hydrogen peroxide, Mueller-hinton agar (MHA), nutrient broth, peptone water, procured from Hi-media, Merck, and sigma.
Preparation of Plant Extract
The removal of phytochemicals requires extraction. The solvents utilized and the chemical properties of samples are the two most critical elements that determine the extraction yield under the same time and temperature circumstances. Several studies have shown that extractive yield varies depending on the solvent used 9. Seeds were collected and cleaned thoroughly with tap water before being sterilized with distilled water. After that, the seeds were shade dried at room temperature for 7-8 days, then homogenized into a fine coarse powder with an electric blender, and lastly kept in airtight containers until needed. Methanol [M], Chloroform [C], Acetone [A], and water [AQ] were the various solvent systems of varying polarity used to extract the fine seed powder using the hot maceration technique. In the conical flasks, 20 grams of dry powder were poured in 100 mL of each solvent, plugged with cotton wool, and shaken at 120 rpm for 38 hours on a rotary shaker. After 38 hours, the extract was filtered with sterilized Whatman Filter Paper Grade No 1. While the solvent was being evaporated, the supernatant was being collected. The resulting blackish gummy exudates residues were weighed using a balance to calculate the extractive yield. The crude extract was maintained at 4°C in sealed Eppendorf tubes before being subjected to qualitative phytochemical analysis, total phenol and flavonoid content, and antioxidant and antibacterial properties testing. 10.
Phytochemical Preliminary Screening
Traditional procedures established by Trease and Evans in 2002 11 were used to test the extracts for phytochemicals (secondary). The qualitative assessments were carried out to confirm the presence or absence of Flavonoids, phenols, alkaloids, steroids, saponins, glycosides, phytosterols, terpenoids, triterpenoids, anthraquinones, and tannins. The results of all the above tests are summed up in results section.
Assessment of Total Phenol Content (Tpc)
The method of assessing the quantity of phenolic content in samples is known as TPC activity. The redox characteristics of phenolic chemicals found in plants allow them to serve as antioxidants. 6,10,11. Total phenolic content was estimated via Folin-Ciocalteau’s reagent assay as reported by McDonald et al 12. 0.2 gram of extracts was dissolved in 1 ml of their respective solvents. 1 ml of this solution and 0.1 ml (0.5 N) Folin-Ciocalteau’s reagent was combined and the reaction mixture was incubated at room temperature for about 15-20 min. After that 3 ml, saturated sodium carbonate solution was poured and again incubated for about 30 min. at room temperature. Finally, the absorbance was taken at 760 nm. Gallic acid was employed as a positive control for which a standard curve was developed beforehand.
Assessment of Total Flavonoid Content (Tfc)
The flavonoid content was measured using the colorimetric technique with aluminium chloride 13. The reaction mixture was incubated at room temperature for 30 minutes, with 1.0 ml of sample (1 mg/ml), 1.0 ml methanol, 0.5 ml of (1.2 percent) aluminium chloride, and 0.5 ml of (120 mM) potassium acetate in a final volume of 3 ml. At 415 nm, the absorbance of all the samples was measured. As a positive control, quercetin was utilized.
Antioxidant Assay
DPPH free radical scavenging activity
The free radical scavenging activity of Citrullus lanatus seed extracts was determined by using the 2, 2- Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging method 10,14. DPPH in oxidized form gives a deep violet color in methanol. An antioxidant compound donates the electron to DPPH thus causing its reduction and in reduced from its color changes from deep violet to yellow 15. All extracts were measured for hydrogen donating or radical scavenging ability. Extracts were diluted to obtain concentrations of 0.1, 0.3, 0.6, 0.8 and 1.0 mg/ml. Diluted extract solutions (1 ml each) were assorted with an ethanolic solution of DPPH (0.004%). After 30 min of incubation at room temperature, the reduction of the DPPH free radical was spanned by reading the absorbance at 517nm using UV-Visible Spectrophotometer. Initially, absorption of a blank sample containing an equal amount of ethanol and DPPH solution was prepared and measured as a control. Ascorbic acid was used as standard. The experiment was carried out in triplicate. Percentage inhibition was calculated using equation (1). The data were presented as mean values ± standard deviation (n = 3).
The GC–MS Analysis
Agilent Technologies GC system with GC-7890A/MS-5975C model (Agilent Technologies) integrated with HP-5MS column (30 m in length, 250 mm in diameter, 0.25 mm in film thickness) was used to analyze bioactive compounds from various Citrullus lanatus seeds extracts. An electron ionization device using high-energy electrons (70 eV) was used for spectroscopic evaluation by GC–MS. The gas phase was pure helium gas (99.995% purity) at a flow rate of 1 mL/min. The starting temperature was maintained at 50–1500 with a 30 C/min increase rate and a 10-minute hold duration. Subsequently, the temperature was raised to 300°C at a rate of 100°C per minute. After syringe filtration in splitless mode, one microliter of the obtained extracts diluted with appropriate solvents was loaded into the system. Based on the obtained peak area in the chromatogram, the relative quantity of bioactive compounds contained in each of the extracts was represented as a percentage. Based on GC retention time on HP-5MS column and spectral comparison with computer software data of standards (Replib and Mainlab data of GC–MS systems), bioactive chemicals extracted from various extracts of Citrullus lanatus seeds extracts were recognized.
Antibacterial Assay
The antibacterial potential of Citrullus lanatus seeds extracts in different solvents was tested against two Gram-positive bacteria Bacillus cereus 10451 (BC), S. aureus ATCC29213 (SA), and three Gram-negative bacteria Escherichia coli GIM1.708 (EC), Escherichia coli DH5-Alpha (ECα), Salmonella enteritidis10982 (SE). The Kirby-Bauer disc diffusion method was incorporated to assess the antibacterial potential of all extracts of Citrullus lanatus seeds 16. From the master plates of all the bacteria incorporated in the study, a loopful of colonies was transferred to the respective 10ml autoclaved nutrient broth tubes. All the tubes were incubated overnight at 370 C. On the next day, all the bacterial suspension tubes were standardized to 0.5 McFarland by diluting them with freshly autoclaved nutrient broth. Such a turbidity was confirmed spectrophotometrically wherein the final ‘ready to swab suspensions’ optical density comes out to be 0.102 showing that all the suspensions now contain 1.5 x 10^8 colony forming units per ml (CFU/ml). Sterile cotton swabs were used to make the lawn cultures of all the respective bacterial suspensions on MH plates. All the extracts (25mg/ml) were loaded on the sterile discs which along with positive and negative controls were placed equidistantly on swabbed plates with proper coding. Antibiotic tetracycline (TE 30) was used as a positive control and DMSO at a concentration of 15% was used as a negative control. All the plates were aseptically incubated for 24 hours at 370 C and thereafter looked for the zones of inhibition.
Statistical Data
All the experiments were performed at least three times. The results were presented as mean ± S.E.M. (standard error of the mean).
Results
Extractive Yield Result
The yield of crude extracts was determined in percentage using the following formula 9,17.
Yield (%) = (Dry weight of extract ÷ Dry weight of plant material) × 100
The extractive yield of samples in four different solvents viz., methanol, chloroform, acetone, and water are given in Table 1.
Table 1: Extractive yield of Citrullus lanatus seed extracts in different solvents
| Yield w/w (%) | ||||
| Seed Extract | Methanol[M] | Chloroform [C] | Acetone [A] | Distilled water [DW] |
| 10.42 | 2.94 | 5.10 | 7.96 | |
The extractive yield varied among the different solvent systems incorporated. Among all the extracts, methanol extract showed the highest extractive yield as compared to other extracts.
Qualitative Phytochemical Screening Result
All the seed extracts were undertaken for qualitative phytochemical analysis using different tests previously described. The results of the phytochemical analysis showed the presence of flavonoids, steroids, alkaloids, phenols, terpenoids, quinones, tannins, glycosides, and saponins in high amounts, while anthraquinones were not detected (Table 2).
Table 2: Phytochemical screening of Citrullus lanatus seeds extracts in different solvents.
| S no. | Phytochemical constituents | Aqueous Extract | Acetone Extract | Chloroform Extract | Methanol
Extract |
| 1 | Flavonoid | + | ++ | – | ++ |
| 2 | Phenols | ++ | ++ | ++ | ++ |
| 3 | Alkaloids | + | – | – | + |
| 4 | Steroids | ++ | + | – | + |
| 5 | Saponins | – | + | – | – |
| 6 | Glycosides | ++ | – | + | ++ |
| 7 | phytosterols | – | – | – | – |
| 8 | Terpenoid | ++ | + | + | ++ |
| 9 | Triterpenoid | + | – | + | ++ |
| 10 | Anthraquinone | – | – | – | – |
| 11 | Tannins | – | – | – | + |
| + = present, + + = relatively abundant, – = not detected | |||||
Total Phenolic Content Result
As a basis, phenolic content was measured using the Folin–Ciocalteu’s reagent in each extract. The results were derived from a calibration curve (y = 0.0079x -0.2866, R2 = 0.9861) of gallic acid (50–250 µg/mL) (Fig.1) and expressed in gallic acid equivalents (GAE) per gram dry extract weight. The total phenolic contents of all the samples ranged from 54.87 to 132.68 mg/g gallic acid equivalent (Table 3). The content of phenolic compounds was higher in methanol extract and lower in chloroform extract
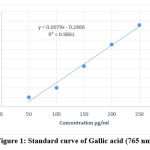 |
Figure 1: Standard curve of Gallic acid (765 nm). |
Table 3: Total phenolic contents in the extracts expressed in terms of gallic acid equivalent (mg of GA/g of extract).
| Citrullus lanatus seed extracts | Total phenolic content mg of GA/g of extract |
| Water | 94.78 ± 0.396 |
| Acetone | 65.98 ± 0.533 |
| Chloroform | 54.87 ±0.673 |
| Methanol | 132.68 ± 0.861 |
| Each value is the average of three measurements ± (standard deviation) | |
Total Flavonoid Content Result
The concentration of flavonoids in all the four Citrullus lanatus seeds extracts was analysed via the spectrophotometric method with aluminium chloride. The concentration of flavonoids was expressed in terms of Quercetin equivalents (mg of Q/ g of extracted compound) for which the standard curve equation was (y = 0.002x – 0.0076, R² = 0.9988) (Fig.2). The concentration of flavonoids in all samples ranged from 13.65 to 48.13mg/g quercetin equivalent (Table 4). The flavonoid content was higher in methanol extracts and lower in acetone & chloroform extracts.
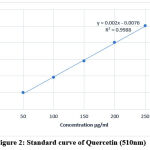 |
Figure 2: Standard curve of Quercetin (510nm) |
Table 4: Total Flavonoid contents in the extracts expressed in terms of Quercetin equivalent (mg of Q/g of extract).
| Seed extracts | Total Flavonoid content mg of Q/g of extract | |
| Water | 41.36 ± 0.396 | |
| Acetone | 16.88 ± 0.531 | |
| Chloroform | 13.65 ± 0.820 | |
| Methanol | 48.13 ± 0.451 | |
| Each value is the average of three measurements ± (standard deviation) | ||
Antioxidant Activity Result
The DPPH radical scavenging activity of Methanolic [M] extract, Chloroform [C] extract, Acetone [A], and Aqueous [AQ] extracts of Citrullus lanatus seeds was detected and juxtaposed with Ascorbic acid as standard (Fig.3). The percentage inhibition (% inhibition) at various concentrations (0.1- 1.0 mg/ml) of all 4 samples as well as standard Ascorbic acid (40 to 200 μg/ml ) were calculated and plotted in graphs using Microsoft Office Excel 2018. Our result revealed that % inhibition of methanol extract is higher among all when compared to standard L-ascorbic acid (Fig. 4 & 5).
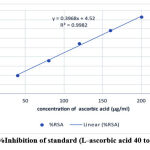 |
Figure 3: %Inhibition of standard (L-ascorbic acid 40 to 200 μg/ml). |
All the extracts in different solvents showed significant antioxidant potential when compared to the reference antioxidant ascorbic acid in a dose-dependent manner. IC50 value, representing the amount of extract which scavenged/reduced 50% of the DPPH radical, was calculated from the percent scavenging versus concentration curve. A higher concentration to reduce 50% of DPPH solution showed lower antioxidant activity. In this assay, the IC50 value of the reference standard ascorbic acid was found to be 114.62µg/ml while the IC50 value of different values varied. (Fig. 4).
Bioactive Compounds Contained in the Extracts
Tables 5-8 list the bioactive chemicals found in methanol, water, chloroform, and acetone extracts of Citrullus lanatus seeds. Their elution sequence in an HP-5MS column was used to identify and characterize them. These bioactive chemicals’ elution time, molecular formula, and quantity also are reported. Figures 6–9 show the GC chromatograms of the four extracts, which indicate the retention time in the column as well as the observed peaks that correlate to the bioactive chemicals contained in the extracts. Watermelon seeds when extracted with methanol were found to be rich in Cis-7-Dodecen-1-yl acetate (17.29%), n- Hexadecanoic Acid (15.78%), beta – Sitosterol (14.16%), and Lupeol (8.48%). Other compounds that correspond to other peaks in the methanol extract chromatogram are chatted in (Table 5). Methyl tetradecanoate (32.76%), 9 octadecenoic acid( 9.95%), and 4H-Pyran-4-one,2,3-dihydro-3,5- dihydroxy-6-methyl- (8.20%) were major abundant compounds in aqueous extract. Cyclohexene,1-pentyl-4-(4-propylcyclohexyl)- (36.87%) followed by 2- Chloroethyl linoleate (33.64%) were the major compounds in chloroform extract. 1-Azabicyclo(3,1,0) hexane (34.09%) and 1-Cyclohexyl-1-propyne(17.86%) were identified as major bioactive compounds in acetone extract.
 |
Figure 4: Graphical representation of comparative percent inhibition of DPPH free radical by Citrullus lanatus seed extracts in four different solvents. |
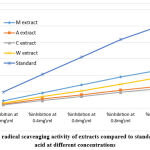 |
Figure 5: Free radical scavenging activity of extracts compared to standard L-ascorbic acid at different concentrations. |
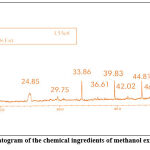 |
Figure 6: A typical gas chromatogram of the chemical ingredients of methanol extract of Citrullus lanatus seeds. |
Table 5: Biologically active chemical compounds of methanol extract from Citrullus lanatus seeds
| S.NO. | Compound identified | CAS No. | Retention time | % Area | MW |
| 1 | Lupeol | 000545- 47-1 | 24.85 | 8.48 | 426.70 |
| 2 | 11-Dodecen-1-yl acetate | 35153-10-7
|
29.75 | 0.98 | 226.40 |
| 3 | n- Hexadecanoic Acid | 000057- 10-3 | 33.86 | 15.78 | 256.42 |
| 4 | Cyclononasiloxane, octadecamethyl | 000556- 71-8 | 36.61 | 0.29 | 667.39 |
| 5 | Cis-7-Dodecen-1-yl acetate | 014959- 86-5 | 39.88 | 17.29 | 226.36 |
| 6 | Octadecanoic Acid | 000057- 11-4 | 42.02 | 0.41 | 282.50 |
| 7 | 8-Nonenoic acid | 31642-67-8 | 44.81 | 14.63 | 156.22 |
| 8 | ᵧ – Sitosterol | 000083- 47-6 | 46.93 | 0.89 | 432.70 |
| 9 | 9, 12- Octadecadienoic acid (Z, Z), methyl ester | 000112- 63-0 | 48.84 | 13.29 | 294.47 |
| 10 | ᵦ -Sitosterol | 000083- 46-5 | 52.94 | 14.16 | 412.70 |
| 11 | 11-Dodecenol | 35289-31-7 | 55.62 | 15.64 | 184.32 |
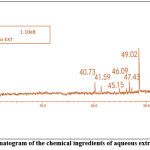 |
Figure 7: A typical gas chromatogram of the chemical ingredients of aqueous extract of Citrullus lanatus seeds |
Table 6: Biologically active chemical compounds of aqueous extract from Citrullus lanatus seeds
| S.NO. | Compound identified | CAS No. | Retention time | % Area | MW |
| 1 | (9,12- octadecadienoyl chloride) | 7459-33-8 | 16.85 | 7.98 | 298.9 |
| 2 | Oleic acid (9 octadecenoic acid ) | 112-79-8 | 40.73 | 9.75 | 282.5 |
| 3 | Palmitic acid | 57-10-3 | 41.59 | 5.31 | 256.42 |
| 4 | Dioctyl ester | – | 45.15 | 1.21 | – |
| 5 | Phenol, 2,2-methylenebis [6-(1,1-dimethylethyl)-4-ethyl] | 88-24-4 | 46.09 | 1.83 | 368.55 |
| 6 | 4H-Pyran-4-one,2,3-dihydro-3,5- dihydroxy-6-methyl- | 28564-83-2 | 47.43 | 8.20 | 144.12 |
| 7 | Hexadecanoic acid, methyl ester | 112-39-0 | 47.87 | 1.30 | 270.45 |
| 8 | Methyl tetradecanoate | 124-10-7 | 49.02 | 32.76 | 242.40 |
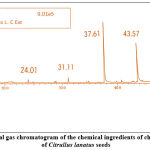 |
Figure 8: A typical gas chromatogram of the chemical ingredients of chloroform extract of Citrullus lanatus seeds |
Table 7: Biologically active chemical compounds of chloroform extract from Citrullus lanatus seeds
| S.NO. | Compound identified | CAS No. | Retention time | % Area | MW |
| 1 | Bis(2-ethylhexyl) phthalate | 0017-81-7 | 24.01 | 0.92 | 390.60 |
| 2 | Nonivamide | 002444- 46-4 | 31.11 | 1.87 | 293.4 |
| 3 | Cyclohexene, 1-pentyl-4-(4-propylcyclohexyl)-
|
108067- 17-0 | 37.61 | 36.87 | 276.5 |
| 4 | 2- Chloroethyl linoleate | 025525- 76-2 – | 43.57 | 33.64 | 342.90 |
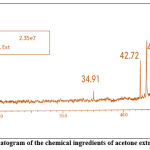 |
Figure 9: A typical gas chromatogram of the chemical ingredients of acetone extracts of Citrullus lanatus seeds. |
Table 8: Biologically active chemical compounds of acetone extract from Citrullus lanatus seeds
| S.NO. | Compound identified | CAS No. | Retention time | % Area | MW |
| 1 | Cyclopropanecarboxylic acid | 1759-53-1 | 39.91 | 1.76 | 86.09 |
| 2 | 1-Cyclohexyl-1-propyne | 17715-00-3 | 42.72 | 17.86 | 122.21 |
| 3 | 1-Azabicyclo(3,1,0) hexane | 73799-64-1
|
43.91 | 34.09 | 83.13 |
| 4 | Oxazole | 288-42-6 | 58.70 | 2.19 | 69.06 |
Antibacterial Activity Result
After the incubation time of 24 hours at 370 C, antibacterial activity of Citrullus lanatus seed crude extracts was assessed by the development and measurement of the inhibition zone around the discs that were loaded with the respective extracts (25mg/ml) on the MH agar plates swab cultured with bacterial suspensions of 0.5 McFarland turbidity. The interpretation of the results is shown in Table 9 and Figure 10.
Table 9 : Antibacterial potential of Citrullus lanatus seed extracts against five MTCC bacterial strains
| Zone of inhibition (mm) | |||||
| Test strain | Methanol extract | Water extract | Chloroform extract | Acetone extract | Positive control |
| Bacillus cereus 10451 | [9.5 ± 0.7] | [ 10.7 ± 1.1] | [N] | [N] | [22.4 ± 0.5] |
| S. aureus ATCC29213 | [8.3 ± 1.2] | [N] | [N] | [6.9 ± 0.9] | [21.5 ± 0.3] |
| Escherichia coli GIM1.708 | [12.1 ± 1.2] | [9.2 ± 0.8] | [ 9.4 ± 1.3] | [N] | [25.2 ± 0.4] |
| Escherichia coli DH5-Alpha | [N] | [8.3 ± 1.2] | [N] | [8.6 ± 0.9]
|
[24.7 ± 0.3] |
| Salmonella enteritidis10982 (SE).
|
[7.3 ± 1.2] | [N] | [N] | [7.1 ± 1.3] | [19.4 ± 0.5] |
| Values in triplicate determination (n=3) ± standard deviations, N- no zone of inhibition, S- Sample 1, S2- Sample 2 | |||||
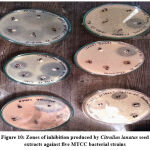 |
Figure 10: Zones of inhibition produced by Citrullus lanatus seed extracts against five MTCC bacterial strains. |
Discussion
In May 2021, Citrullus lanatus seeds were gathered and analyzed. The presence or absence of secondary metabolites, antioxidant, and antibacterial properties of Citrullus lanatus seeds were investigated in this study. Phytochemicals exhibit biological features such as antioxidant activity, antibacterial activity, detoxification enzyme modulation, immune system modulation, and general hormonal activity regulation 3,9,11. The extraction of phytochemical content is affected by the kind of solvent used and the procedure used to prepare the extract. Methanolic extract resulted in the highest extraction yield and a more complex phenolic content 17,18. Methanolic and water extracts showed the greatest extraction yield, high secondary metabolite extraction, high flavonoid content, high antioxidant potential, and effective antibacterial activity, according to the findings of this study. Terpenoids, glycosides, steroids, alkaloids, flavonoids, coumarins, and quinones were found in high concentrations in this research, however, phytosterols and anthraquinones were not. This is consistent with Ali et al., 2012 19, who found that alkaloids and terpenes are extensively dispersed throughout the Citrullus genus. For methanol extract of Citrullus lanatus seeds, Omoboyowa et al. 2015 20 reported flavonoids (2.310mg/100g), phenol (1.371mg/100g), saponins (1.553mg/100g), alkaloid (33.795mg/100g), and tannins (0.536mg/100g). Gwana, et al. 2014 21 also found flavonoids 0.01 percent, phenol (GAE) 0.01 percent, saponins 0.09 percent, alkaloid 0.91 percent, and tannins 0.04 percent in the phytochemical percentage composition of the Rosmas type of watermelon seeds. The bioactive chemicals found in the sample have been shown to have pharmacological and physiological properties. These seeds contain significant free radical scavenging activity and consequently antioxidant activity, according to our research. The amount and kind of bioactive compound produced by medicinal plants determine its anti-ailment action and different physiological impacts on the human body system. The range of this work, however, did not include an exploration into the precise functions of the extracted phytochemicals from watermelon seed; studies have revealed that these secondary metabolites are subject to a range of pharmacological effects in fruits and vegetables 22,23. Because of the companionship of alkaloids, watermelon seeds can be handed-down as basic therapeutic agents for analgesic, antispasmodic, and antibacterial effects. 24. According to research, alkaloids affect the central nervous system and can act as pain relievers in some cases like morphine. The presence of alkaloids has been discovered in phytochemical screens of most plants traditionally used to treat malaria 14,25. Saponins are found in plants and indicate that they have the ability to precipitate and coagulate red blood cells 11,20. Phytosterols are one of several nutrients that are said to be beneficial to the heart. Low-density lipoprotein (LDL) cholesterol can be reduced by roughly 10% by taking 2–3 grams of phytosterols each day for 3–4 weeks 26. According to human studies, people who consumed the most phytosterols had a lower incidence of stomach, lung, breast, and ovarian cancer 27. Phenolic compounds have been shown to act as antioxidants with a wide range of therapeutic effects, including anticancer, anti-inflammatory, and diabetic activities. Some molecular targets of pro-inflammatory mediators in inflammatory reactions are known to be inhibited by phenolic substances such as gallotannins, condensed tannins, and flavonoids. The phytochemicals also function as antioxidants, scavenging free radicals and therefore reducing inflammation 28. The GC-MS results showed that the seeds contain many phytochemicals with Lupeol, 9,12- octadecadienoyl chloride, and Bis(2-ethylhexyl) phthalate having the least retention times and 11-Dodecenol, Methyl tetradecanoate, and Oxazole having the highest retention times. Phytochemical analysis of the seeds showed many compounds, apparently with diverse pharmacological and biological significance. However, Phenol, 2,2-methylenebis [6-(1,1-dimethylethyl)-4-ethyl], n- Hexadecanoic Acid, Cyclopropanecarboxylic acid like phytochemicals present in the seeds are known for antioxidant with anti-bacterial properties29. The phytochemical profile of the watermelon seed indicated the presence of various bioactive compounds which can be utilized for medicinal purposes. The identified compounds have several biological properties with a potential pharmacological activity that play a central role in traditional medicines and also as cosmetics commercial commodities in the markets 30. Literature indicated that plants are the backbone of traditional medicine, and the medicinal activity of plant extract is due to different bioactive compounds in the extract with potential bioactive compounds. The activities of watermelon seed constituents such as Dodecenol, 11-Dodecenol, ᵦ -Sitosterol, 1-Azabicyclo (3,1,0) hexane, Cyclopropanecarboxylic acid, Nonivamide, 8-Nonenoic acid, ᵧ – Sitosterol have been revealed to possess antimicrobial, antioxidant, anti-inflammatory, hypocholesterolemic, cancer preventive, hepatoprotective, antiarthritic, antihistaminic, antieczemic, immunomodulatory and cardioprotective activities. Oxazole has anti-cancerous, anti-viral, anti-diabetic, and antibiotic activity 31. V.H.A. Enemor et al., 2019 32 revealed the presence of many other benign chemical compounds in Citrullus lanatus seeds beyond the retention time of 60 mins. Some noteworthy such compounds are Pyrrole, Isoxazole, Methyl Guanidine, 2-Propenenitrile, Thiirane, Methanesulfonyl fluoride, Silamine, Oxirane carboxaldehyde, 1,6-dichloropyruvic acid, 3-Methyl-1,3-pentadiene, Propiolamide, N-Ethylformamide, Fluoramine, and Fomepizole. These are also pharmacologically active compounds found in Citrullus lanatus. Proximate components, vitamins, amino acids, and phytochemicals readily alter depending upon the type of fruit cultivar, geographical location, climatic conditions, etc. so, results cannot be accurately compared. Because the risk of infection by antibiotic-resistant microorganisms is increasing dramatically, the identification and search for chemicals with antimicrobial action has become more important in recent years. Citrullus lanatus seeds show antibacterial action against many strains, according to our research, which might be attributable to terpenoids and phenols. Citrullus lanatus seeds, it may be established, have great therapeutic potential.
Conclusion
In this study, these seeds, which are typically thought of as a waste product of the fruit, were found to be an excellent source of physiologically important phytochemicals. Alkaloids, flavonoids, phenols, steroids, tannins, saponins, phytosterols, terpenoids, and glycosides were found in a qualitative phytochemical examination of these seeds utilizing several solvents of varying polarity and established techniques of analysis. Bioactive compounds revealed quantitively in GC-MS analysis have been revealed to be physiologically significant and essential from a pharmaceutical standpoint by an ample amount of research. Seed extracts showed substantial antioxidant and anti-bacterial action due to these phenolic and polyphenolic components. As a result, it is recommended that these compounds be analyzed as potential therapeutic agents in the management of oxidative stress-related disorders, infectious diseases, and other ailments that have taken a toll on human health.
Acknowledgement
We acknowledge the support of the Department of Life Sciences of our institution Graphic Era (Deemed to be) University for providing requisites and all the help in conducting and submitting this research article.
Conflict of Interest
There were no commercial or financial links that may be deemed a potential conflict of interest during the research.
Funding Sources
The author(s) received no financial support for the research.
References
- Mawalagedera SM, Symonds MR, Callahan DL, Gaskett AC, Rønsted N, 2019. Combining evolutionary inference and metabolomics to identify plants with medicinal potential. Frontiers in Ecology and Evolution 7, 267.
CrossRef - Mans DR, 2013. From forest to pharmacy: Plant-based traditional medicines as sources for novel therapeutic compounds. Academia Journal of Medicinal Plants, 1(1), 101-10.
- Wadood, A., Ghufran, M., Jamal, S.B., Naeem, M., Khan, A., Ghaffar, R., and Asnad, K., (2013). Phytochemical analysis of medicinal plants occurring in local area of Mardan. Biochemistry and Analytical Biochemistry. 2(4):1-4.
CrossRef - Ashfaq A Shah, Vijay Kumar, Amit Gupta, 2021. Immunomodulation via phyto active compounds a promising therapy for future medical system. Jour. of Med. P’ceutical & Allied. Sci. V 10 – S 2, 2036, P- 120-125.
- Kabera JN, Semana E, Mussa AR, He X, 2014. Plant secondary metabolites: Biosynthesis, classification, function, and pharmacological properties. Journal of Pharmacy and Pharmacology 2, 377–392.
- Tabiri, T., Agbenorhevi, J.K., Wireko-Manu, F.D., and Ompouma, E.I., (2016). Watermelon seeds as food: Nutrient composition, phytochemicals, and antioxidant activity. International Journal of Nutrition and Food Sciences. 5(2):139–144.
CrossRef - Choudhary, B.R., Haldhar, S.M., Maheshwari, S.K., Bhargava, R., and Sharma, S.K., (2015). Phytochemicals and antioxidants in watermelon (Citrullus lanatus) genotypesunder hot arid region. Indian Journal of Agricultural Sciences. 85(3):414- 417.
- Shah AA, Gupta A, 2021. Antioxidants in Health and Disease with Their Capability to Defend Pathogens that Attack Apple Species of Kashmir. In: Plant Antioxidants and Health. Reference Series in Phytochemistry. Springer, Cham.
CrossRef - U Złotek, S Mikulska, M Nagajek, and M Świeca, 2016. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts; Saudi Journal of Biological Sciences, vol. 23, no. 5, pp. 628–633
CrossRef - Jana, S. and Shekhawat, G.S., (2010). Phytochemical analysis and antibacterial screening of in vivo and in vitro extracts of Indian medicinal herb: Anethum graveolens. Research Journal of Medicinal Plants. 4:206–212.
CrossRef - Evans, William Charles., Daphne Evans, and George Edward Trease, 2009. Trease and Evans Pharmacognosy. 16th ed. Edinburgh; New York: Saunders/Elsevier
- Rajurkar NS, Hande SM, 2011. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian Journal of Pharmaceutical Sciences; 73(2):146-51
CrossRef - Chang C, Yang M, Wen H, Chern J, 2002. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. Journal of Food and Drug Analysis 10: 178-182.
CrossRef - Eleazu, C.O., Okafor, P.N., and Ahamefuna, I., (2010). Total antioxidant capacity, nutritional composition and inhibitory activity of unripe plantain (Musa paradisiacae) on oxidative stress in alloxan induceddiabetic rabbits. Pakistan Journal of Nutrition. 9:1052–1057.
CrossRef - Bibi Sadeer N, Montesano D, Albrizio S, Zengin G, Mahomoodally MF. 2020 The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants; 9(8):709.
CrossRef - Kumar, Sandeep, Lalita Budhwar, Amita Yadav, Manila Yadav, and Jaya Parkash Yadav, 2016. Phytochemical screening and antibacterial activity of Aloe vera collected from different climatic regions of India. The Natural Products Journal 6, 73-82.
CrossRef - Abdel-Aal EI, Haroon AM, Mofeed J, 2015. Successive solvent extraction and GC-MS analysis for the evaluation of the phytochemical constituents of the filamentous green alga Spirogyra longata. Egyptian Journal of Aquatic Research; 41(3):233-246.
CrossRef - Alam MA, Nyeem MAB, Awal MA, Mostofa M, Alam MS, Subhan N and Rahman MM (2008). Antioxidant and hepatoprotective action of the crude methanolic extract of the flowering top of Rosa damascena. Oriental Pharmacy and Experimental Medicine 8: 164-170.
CrossRef - Ali, M., Odiong, I.J. and Oranusi, S, (2012). Phytochemical and Antibacterial properties of the seed of watermelon (Citrullus lanatus). Prime Journal of Microbiology Research, 2(3): 99 -104
- Omoboyowa, A.D., Outchristian, G., Danladi, G.J., Igara, C.E., Ngobidi, C.K., Okon, M.U., and Agbo, F.A., (2015). Evaluation of chemical compositions of Citruluslanatus seed and Cocos nucifera stem bark African Journal of Food Science and Technology. 6(3):75–83.
- Gwana, A.M., Bako, M.M., Bagudu, B.Y., Sadiq, A.B., and Abdullahi, M.M., (2014). Determinations of phytochemical, vitamin, mineral and proximate compositions of varieties of watermelon seeds cultivated in Borno State, North–Eastern Nigeria. International Journal of Nutrition and Food Sciences. 3(4):238–245.
CrossRef - Shaikh S, Bin Yaacob H, Rahim, Z HA, 2014. Prospective Role in Treatment of Major Illnesses and Potential Benefits as A Safe Insecticide and Natural Food Preservative of Mint (Mentha spp.): A Review. Asian Journal of Biomedical and Pharmaceutical Sciences 2014, 4, 1–12.
CrossRef - Ivanova, D., Gerova, D., Chervenkov, T., and Yankova, T., (2005). Polyphenols and antioxidant capacity of Bulgarian medicinal plants. Journal of Ethnopharmacology. 96:145-150.
CrossRef - Okwu, D.E. and Okwu, M.E., (2004). Chemical composition of Spondiasmombin Linn. plant parts. Journal of Sustainable Agriculture and Environment. 6(2):140-147.
- Obadoni, B. and Ochuko, P., (2001). Phytochemical studies and comparative efficacy of the crude extracts of some homostatic plants in Edo and Delta States of Nigeria. Global Journal of Pure Applied Science. 8:203–208.
CrossRef - Katan, M.B., Grundy, S.M., Jones, P., Law, M., Miettinen, T., and Paoletti, R., (2003). Efficacy and safety of plant stanols and sterols in themanagement of blood cholesterol levels. Mayo Clinic Proceedings. 78(8):965-978.
CrossRef - McCann, S.E., Freudenheim, J.L., Marshall, J.R., and Graham, S., (2003). Risk of human ovarian cancer is related to dietary intake of selected nutrients, phytochemicals, and food groups. Journal of Nutrition. 133(6):1937–1942.
CrossRef - Abdelwahab, S.I., Hassan, L.E.A., and Sirat, H.M., (2011). Anti-inflammatory activities of Cucurbitacin E isolated from Citrullus lanatus Citroides: Role of reactive nitrogen species andcyclooxygenase enzyme inhibition. . Fitoterapia. 82:1190–1197.
CrossRef - Dutta, A.C., (2003). Botany for degree students, 6th Edition. Oxford University Press, UK.140-143.
- Tiwari, P., Kumar, B., Kaur, M., Kaur, G., and Kaur, H., (2011). Phytochemical Screening and Extraction Review. Internationale Pharmaceutica Sciencia. 1(1):98-106.
- Cox-Georgian, D., Ramadoss, N., Dona, C., & Basu, C. (2019). Therapeutic and Medicinal Uses of Terpenes. Medicinal Plants: From Farm to Pharmacy, 333–359.
CrossRef - H.A. Enemor, C.E. Oguazu, A.U. Odiakosa and S.C. Okafor, 2019. Evaluation of the medicinal properties and possible nutrient composition of Citrullus lanatus (watermelon) seed. Res. J. Med. Plants, 13: 129-135
CrossRef








