Manuscript accepted on :08-12-2022
Published online on: 14-02-2023
Plagiarism Check: Yes
Reviewed by: Dr. Yasmin Khan , Dr. Ammar Almulathanon
Second Review by: Dr. P. Shivakumar
Final Approval by: Dr. H Fai Poon
Poovizhi Bharathi R1 , Manohar V R2
, Manohar V R2 , Mohandas Rai2
, Mohandas Rai2  and Athiyamaan M S3*
and Athiyamaan M S3*
1Department of Pharmacology, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, India
2Department of Pharmacology, A.J. Institute of Medical Sciences Mangaluru, Karnataka India.
3Department of Radiation Oncology, Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, India
Corresponding Author E-mail: athiyamaan.ms@manipal.edu
DOI : https://dx.doi.org/10.13005/bpj/2611
Abstract
Many fatal diseases have inflammation and fever as clinical symptoms. NSAIDs are the drug of choice for both of these symptoms. But they cause numerous adverse drug reactions including the gastrointestinal, renal and cardiovascular systems. Herbal medicines are known for their efficacy and lack of adverse reactions. So the search for herbal remedies is always on. Terminalia bellirica fruit pulp is used to alleviate a lot of health conditions. Hence this study looked at the antipyretic and anti-inflammatory activities of aqueous extract of Terminalia bellirica fruit pulp in rodents. The carrageenan-induced paw edema model was utilized to test the plant's acute and subacute anti-inflammatory properties. Baker's yeast-induced pyrexia model was utilized to assess the plant's antipyretic activity. Three different groups were administered the extraction (9 mg/kg, 18 mg/kg, and 36 mg/kg). Positive control and negative control for the anti-inflammatory model were Indomethacin (10 mg/kg) and Gum acacia suspension (3 ml/kg of 1 percent) respectively. Positive control and negative control for the anti-pyretic model were Paracetamol 100 mg/kg and 10 ml/kg of 1 percent Gum acacia suspension, respectively. 18 and 36 mg/kg dose of extraction showed substantial minimization of edema (P≤0.01) in tests of acute anti-inflammatory action. 36 mg/kg dose showed substantial minimization of edema in tests of subacute anti-inflammatory action (P≤0.01). The rectal temperature decreased significantly (P≤0.0001) in all experimental groups in Baker's yeast-induced pyrexia tests. Results revealed that the test drug has considerable action against inflammation and pyrexia in rodents.
Keywords
Anti-inflammation; Baker’s yeast; Carrageenan; mice; pyrexia; rats
Download this article as:| Copy the following to cite this article: Bharathi R. P, Manohar V. R, Rai M, Athiyamaan M. S. Evaluation of the Antipyretic and Anti-Inflammatory Potential of Aqueous Fruit Pulp Extract of Terminalia bellirica. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Bharathi R. P, Manohar V. R, Rai M, Athiyamaan M. S. Evaluation of the Antipyretic and Anti-Inflammatory Potential of Aqueous Fruit Pulp Extract of Terminalia bellirica. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3E7rMo5 |
Introduction
The term Inflammation evolved from the Latin term “inflammo,” which refers, “to set alight, to ignite.”1 It is an adaptive reaction to potentially dangerous toxic substances including bacteria and necrotic cells to which the human body is constantly subjected to.2 The inflammation consists of vascular changes, cellular events, and systemic reactions.3 When inflammation is coupled with infection, the most common sign of the acute phase response is fever. An elevated body temperature of 1 to 4 degrees Celsius above the normal range of 36.5–37.5 degrees Celsius is considered a fever.4 The hormone prostaglandin is responsible for fever, and pyrogens promote its secretion.5, 6 This increase in body temperature aids in the resolution of infection, but if the temperature rises too high, it can cause dehydration, febrile convulsions, shock and systemic infections.7
Anti-inflammatory medicines, both steroidal and non-steroidal, can reduce inflammation and fever.8 Therapy with NSAIDs enhance the consequences including hemorrhage and perforations in the gastrointestinal (GI) tract, in which 30% of cases die.9 In the cardiovascular system, there is an increased risk of myocardial infarction and stroke. This is more common among coxib users than in nonselective NSAID users.10 Non-selective NSAIDs are known to exacerbate chronic kidney disease in a dose-dependent manner.11 NSAIDs can also lead to osteoporosis, decreased auditory capacity and lower immunity against infections.12,13 The use of acetaminophen in gestation raises the chance of developing an autism fetus.14
Herbal medicines have been used for centuries due to their efficacy and low adverse reactions, and they are a valuable source of compounds that lead to the development of new therapies.15 Medicinal herbs are inexpensive, readily available, and cost-effective. Herbal remedies have been discovered to be a therapeutic resource for drug discovery, in addition to their traditional application.16 As a result, it is critical to look for new pharmaceutical substances.17
The family Combretaceae includes about 10 genera and 530 species.18 There are roughly 250 species in the Terminalia genus. Terminalia can be found throughout Australia, South Asia, Madagascar, Indian Himalayas and Africa.19
The deciduous tree Terminalia bellirica, known as Beleric myrobalan is seen in sub-Himalayan tracks and east, south, and west regions. Leaves are shaped obovate-elliptical or broadly elliptic. The flowers are greenish-white. Fruits range in size from widely elliptical to sub globular. Because of the crowded arrangement of leaves at the tip of shoots, this tree was given the name ‘Terminalia’, which means ‘terminal’ or ‘terminus’.20
The various ancient medical systems, including Siddha, Ayurveda, Chinese medicine and Unani have each highlighted the medicinal benefits of Terminalia bellirica.21 Fruits are used to alleviate helminthic infestations, inflammation of the liver, indigestion, cough, diarrhea, eye conditions, throat hoarseness, bronchial asthma and scorpion stings. It’s also good for hair growth. Cough is treated using a preparation of the greenish fruit of Terminalia bellirica. The pulp of the fruit is used to alleviate dropsy, Mycobacterium leprae infection, and hemorrhoids. The half-ripe pulp of the fruit acts as a purgative. The triterpenoid in the fruit is responsible for its antibacterial properties. 22
Terminalia bellirica has analgesic, anti-biofilm, anti-mutagenic, and anti-salmonella properties, according to pharmacological studies. The anti-salmonella action was attributed to phenolic chemicals.23-26 Triphala is a traditional and current Ayurvedic remedy used in India.27 It is a combination made up of Terminalia bellirica, Emblica officinalis, and Terminalia chebula. Terminalia chebula has anti-inflammatory properties. Emblica officinalis was shown to have antipyretic and anti-inflammatory properties. 28-30
As a result, the researchers wanted to see if the research plant had antipyretic and anti-inflammatory characteristics. Furthermore, current research could perform to be a standard in order to conduct future studies into the molecular mechanism of action that causes the reported results.
Material and methods
Drugs and chemicals
The positive controls indomethacin and paracetamol, as well as the negative control Gum acacia, distilled water, baker’s yeast, normal saline, and Carrageenan, were obtained from AJIMS Mangaluru, Karnataka, India. All the plant materials were procured via Lakshmi Ayurvedic pharmacy, Mangaluru, Karnataka, India.
Authentication
The aqueous extract of Terminalia bellirica fruit pulp (AETBFP) was authenticated by Dr. Krishna Kumar G, Mangalore University Chairman, Applied Botany department, Karnataka, India. The fruit pulp was washed under running water and dried in the shade. Fruit pulp was thoroughly dried within the shade. In a grinder, these dried fruit pulp was then finely ground.
Preparation of aqueous fruit extract
Thousand grams of the air-dried raw granules of AETBFP were extracted along with the water in 36 hours using Soxhlet extraction. By using rotator evaporation, the powder was dehydrated and then reduced at a temperature of 40-50°C under regulated pressure. A brownish lump measuring hundred and forty-five grams emerged from the aqueous fruit pulp extract. The crude dehydrated powder extract yielded around 14.5 percent weight/weight.
Experimental Animals
The experimental rodents have been sourced out of AJIMS breeding stock house for animals in the center in Mangaluru, South Asia. About 30 healthy experimental Swiss albino mice (male, 25-30 g, aged three months) and 30 healthy experimental Wistar albino rats (male, 150-250 g, aged three months) had been kept within 22-26°C in a 12-hour light:12-hour dark rotation. Rodents received unlimited availability for chow and freshwater. Before this study, the rodents underwent an acclimatization period of ten days.
Approval
The IAEC permission was obtained for the study’s research protocol which involved two species of animals namely Swiss albino mice and Wistar albino rats (Approval No. IAEC/02/2014/CPCSEA). The registration number of the Institutional Animal Ethics Committee is 1075/ac/07/CPCSEA. This study was done according to CPCSEA guidelines. The study was performed in the animal house of the department of pharmacology, AJIMS, Mangalore, India.
Grouping and dosage of animals
30 Swiss albino mice have been randomly allocated into five groups, each with six animals, for the Carrageenan-induced paw edema model. For Baker’s yeast-induced pyrexia model, 30 Wistar albino rats have been randomly allocated into five groups, each with six animals. As a negative control, Group I received 1 percent Gum acacia (10 ml/kg intragastric gavage). The standard medicine (For the anti-inflammatory model- Indomethacin, 10mg/kg administered by oral intragastric gavage and for Baker’s yeast-induced pyrexia model – Paracetamol,100 mg/kg administered by oral intragastric gavage) was given to Group II as a positive control. while the test group III received 9 mg/kg of the test material, the test group IV received 18 mg/kg of the test material and test group V received 36 mg/kg of the test material. The dosages were chosen based on the findings of other acute toxicity studies.31 Above said medications were administered orally by intragastric gavage. For all three experiments, this grouping was used.
Carrageenan induced paw edema model
Acute and subacute administration of drugs
On day one, one hour after treatment with the extracts, standard medication, or vehicles, 1% Carrageenan at a dose of 0.1 ml with normal saline has been injected into the hind left paw’s sub-plantar site of all the Swiss albino mice to cause edematous swelling. Then it was labeled at the lateral malleolus point. The volume of the paws was measured using a plethysmograph by dipping the hind limb up to the marked level (mercury displacement method) at one, two, three, four and six hours to determine the impact of a single dose of the drug on acute inflammation. The administration of the drug was continued once a day for the next 13 days. The above procedure was repeated on the 14th day to observe the effect of the drug on subacute inflammation. The following method was used to compute the percentage inhibition of paw edema in comparison to the control groups:
The % inhibition of paw edema = [(Vc-Vt)/Vt] 100
Vt is the treatment group’s edema volume.
The volume of edema in the control group is Vc.32
Baker’s yeast induced pyrexia model
Well before this procedure, Wistar albino rats were fasted for 24 hours. The test was performed by injecting Baker’s yeast at a dose of 0.135 g/kg intraperitoneally, to bring about pyrexia. Each rat’s temperature was observed by rectal route utilizing an automated medical thermometer which was placed 1.5 cm within the rectum before and after the yeast injection. All 30 rats that experienced a temperature rise of at least 0.5 degrees Celsius in 24 hours after yeast injection was a part of the research. After that, the grouping of those rats was done. 30 Wistar albino rats were allocated into five groups, each with six animals They were administered with single dose of the following drugs. As a negative control, Group I received the vehicle, 1 percent Gum acacia (10 ml/kg intragastric gavage). The standard medicine (Paracetamol,100 mg/kg administered by oral intragastric gavage) was given to Group II as a positive control. while the test group received 9 mg/kg of the test material, the test group received 18 mg/kg of the test material and test group V received 36 mg/kg of the test material. Each animal’s rectal temperature was measured from 0 minutes, 30 minutes, 60 minutes, 90 minutes, 120 minutes, 180 minutes to 240 minutes after treatment. The antipyretic effect of the test medication was determined by its ability to avert pyrexia induced by Baker’s yeast. The percentile drops of rectal temperature in animals during all the timed measurements had been computed using control group temperatures as a reference.33
Statistical analysis
Data were reported as mean +/- standard error of the mean; p values had been computed with ANOVA software (Graphpad InStat 3) and Dunnet’s multiple comparison test. P-values of less than 0.05 were considered statistically significant.
Results and Discussion
Inflammation and pyrexia induction
Paw edema at its peak was detected in the mice which received negative (group I) control, 1 percent Gum acacia (10 ml/kg administered by intragastric gavage) after Carrageenan injection, in contrast, the least was recorded in mice that received positive (group II) control Indomethacin (10mg/kg administered by intragastric gavage) in the paw edema model. (Tables 1 and 2).
Table 1: Effect of Terminalia bellirica fruit pulp extract on Paw volume on Day 1
| Volume of mercury displaced | |||||
| Treatment | Group I | Group II | Group III | Group IV | Group V |
| 1%Gum acacia (10 ml/kg) | Indomethacin
10mg/kg |
AETBFP
9 mg/kg
|
AETBFP
18 mg/kg |
AETBFP
36 mg/kg |
|
| 0 hour | 0.13±0.00 | 0.09±0.01**
(30.8%) |
0.14±0.00
(7.7%) |
0.12±0.01
(7.7%) |
0.08±0.00**
(38.4%) |
| 1hour | 0.24±0.00 | 0.15±0.00**
(37.5%) |
0.24±0.00
(0%) |
0.18±0.00**
(25%) |
0.16±0.00**
(33.3%) |
| 2hour | 0.25±0.01 | 0.14±0.00**
(44%) |
0.24±0.01
(4%) |
0.21±0.01*
(16%) |
0.15±0.00**
(40%) |
| 3hour | 0.3±0.03 | 0.14±0.00**
(53.3%) |
0.27±0.01
(10%) |
0.19±0.01**
(36.7%) |
0.16±0.00**
(46.7%) |
| 4 hour | 0.28±0.01 | 0.14±0.01**
(50%) |
0.26±0.01
(7.1%) |
0.16±0.01**
(42.9%) |
0.15±0.00**
(46.4%) |
| 6 hour | 0.26±0.01 | 0.11±0.00**
(56%) |
0.24±0.01
(4%) |
0.16±0.00**
(36%) |
0.13±0.01**
(48%) |
The mean and standard error of the mean (SEM) is used to express the data. When compared to control, significant variables at p<0.05, p<0.01, p<0.001, and p<0.0001 are indicated by the symbols *, **, ***, and ****, accordingly.
Table 2: Effect of Terminalia bellirica fruit pulp extract on paw volume on Day 14
| The volume of mercury displaced | |||||
| Treatment | Group I | Group II | Group III | Group IV | Group V |
| 1%Gum acacia (10 ml/kg) | Indomethacin
10mg/kg |
AETBFP
9 mg/kg
|
AETBFP
18 mg/kg |
AETBFP
36 mg/kg |
|
| 0 hour | 0.15±0.01 | 0.08±0.01**
(46.6%) |
0.12±0.02
(20%) |
0.10±0.01
(33.3%) |
0.09±0.01*
(40%) |
| 1hour | 0.25±0.02 | 0.13±0.01**
(48%) |
0.19±0.01
(24%) |
0.16±0.02**
(36%) |
0.14±0.01**
(44%) |
| 2hour | 0.27±0.01 | 0.15±0.02**
(44.4%) |
0.21±0.01**
(22.2%) |
0.20±0.01**
(25.9%) |
0.14±0.01**
(48.1%) |
| 3hour | 0.31±0.02 | 0.13±0.02**
(58%) |
0.27±0.02
(12.9%) |
0.21±0.02**
(32.2%) |
0.16±0.03**
(48.4%) |
| 4 hour | 0.34±0.02 | 0.12±0.01**
(64.7%) |
0.28±0.02
(17.6%) |
0.22±0.01**
(35.3%) |
0.14±0.02**
(58.8%) |
| 6 hour | 0.28±0.03 | 0.10±0.02**
(64.2%) |
0.23±0.02
(17.9%) |
0.19±0.02*
(32.14%) |
0.11±0.02**
(60.7%) |
The mean and standard error of the mean (SEM) is used to express the data. When compared to control, significant variables at p<0.05, p<0.01, p<0.001, and p<0.0001 are indicated by the symbols *, **, ***, and ****, accordingly.
The maximum temperature was detected in the negative (Group I) control group rats compared to the positive (Group II) control plus experimental group rats (Group III, IV and V) in pyrexia induced by Baker’s yeast model, as indicated in (Table 3).
Table 3: Effect of Terminalia bellirica fruit pulp extract on rectal temperature.
| Treatment | Rectal temperature in degree Celsius at time (minutes) | ||||
| Group I | Group II | Group III | Group IV | Group V | |
| 1% Gum acacia 3ml/Kg | Standard
Paracetamol 100mg/kg |
AETBFP
9 mg/kg |
AETBFP
18 mg/kg |
AETBFP
36 mg/kg |
|
| Basal temperature | 37.08±0.06 | 36.85±0.10 | 36.85±.012 | 37.25±0.09 | 36.91±0.16 |
| O minutes | 39.96±0.05 | 39.86±0.07 | 40.06±0.08 | 39.81±0.18 | 40.08±0.07 |
| 30 minutes | 39.75±0.07 | 39.35±0.07** | 39.8±0.06 | 39.65±0.07 | 39.5±0.03* |
| 60 minutes | 39.6±0.05 | 39.01±0.04**** | 39.58±0.06 | 39.38±0.07* | 39.25±0.04*** |
| 90 minutes | 39.75±0.05 | 38.76±0.06**** | 38.76±0.06**** | 39.16±0.07*** | 38.58±0.04**** |
| 120 minutes | 40.01±0.06 | 38.0±0.05**** | 38.55±0.04**** | 38.98±0.07**** | 38.03±0.07*** |
| 180 minutes | 40.2±0.04 | 36.8±0.03**** | 38.5±0.05**** | 38.3±0.05**** | 37.2±0.05**** |
| 240 minutes | 39.9±0.05 | 36.53±0.19**** | 38.05±0.15**** | 37.23±0.29**** | 36.98±0.12**** |
The mean and standard error of the mean (SEM) is used to express the data. When compared to control, significant variables at p<0.05, p<0.01, p<0.001, and p<0.0001 are indicated by the symbols *, **, ***, and ****, accordingly.
Anti-inflammation property
Acute
The volume of the edema of the mice’s paw was observed to be minimized by the aqueous extract of Terminalia bellirica fruit pulp (AETBFP) in a dose-dependence fashion. A substantial reduction of paw edema was found in mice given Indomethacin 10 mg/kg starting at one hour and peaking at six hours (0.11±0.00). When AETBFP 9 mg/kg was given to mice, there was no significant reduction in paw edema. From two hours onwards, mice administered with AETBFP 18 mg/kg displayed a substantial minimization in the volume of paw edema, the largest reduction occurring at six hours (0.16±0.00). The paw edema of mice administered with AETBFP 36 mg/kg was significantly reduced starting at one hour and peaked at six hours. AETBFP 36 mg/kg demonstrated the highest percentage inhibition of 48%.
Subacute
AETBFP was found to diminish the volume of edema in a dose-dependent fashion. A substantial diminution in edema of the paw was seen in mice given Indomethacin 10 mg/kg starting at zero hours and peaking at six hours (0.10±0.02). From two hours, mice given AETBFP 9 mg/kg displayed a substantial diminution in edema of the paw, with the largest reduction at six hours (0.23±0.02). From one hour, mice given AETBFP 18 mg/kg showed an extensive diminution in paw edema, with the largest diminution at six hours (0.19±0.02). From one to six hours, mice given AETBFP 36 mg/kg showed a substantial reduction in paw edema, with the highest diminution at six hours (0.11±0.02). AETBFP 36 mg/kg demonstrated the highest percentage inhibition of 64.2%, which was comparable to the positive control.
Antipyretic activity
The temperature of the rectal cavity was shown to be lowered by AETBFP in a dose-dependence fashion. The rats, that were administered with 100 mg/kg paracetamol showed a temperature drop significantly after 30 minutes, with the highest temperature decline occurring at 240 minutes. Rats administered with AETBFP 9 mg/kg had a considerable decline in temperature starting at 90 minutes, with the largest temperature drop occurring at 240 minutes. The temperature of rats that were administered with AETBFP 18 mg/kg dropped significantly after 60 minutes, with the largest temperature decline occurring at 240 minutes. The temperature of rats that were administered with AETBFP 36 mg/kg dropped significantly after 30 minutes, with the largest temperature decline occurring at 240 minutes.
Discussion
Many deadly diseases are accompanied by pain, inflammation, and fever, which are frequent clinical symptoms. Inflammation, which involves vascular alterations and cellular processes, can be triggered by infections, trauma, chemical, and physical insults, hypersensitive immunological reactions, and other reasons. PGI2, PGE1, PGE2, PGD2, Leukotrienes B4 and other chemical mediators are involved in the process of inflammation. NSAIDs are globally used to alleviate inflammation. Based on the amount of selectivity for COX inhibition, anti-inflammatory drugs which are non-steroidal in nature can be classified into two namely, non-selective or COX-2-selective inhibitors. Both the class of drugs can cause gastrointestinal side effects, especially in the distal gastrointestinal tract. The deleterious function of its sustained delivery forms continues to emerge. Furthermore, previously unreported data in the sponsor’s files cast doubt on rofecoxib’s GI-sparing qualities; a COXIB was restrained from the market since it caused cardiotoxicity. GI problems, renal abnormalities, and cardiovascular events are currently the most common side effects of NSAIDs. 34 Since ancient times, herbal medicine has played a critical role in sustaining human health, Both its treatment philosophy and patient care are one of conventional medicine’s most significant assets.35 Medicinal herbs, which have been utilized for ancient herbal remedies, provide a feasible option that has a reduced risk of side effects and efficacy that is often comparable to that of conventional pharmaceuticals.36 However, no previous pharmacological examination of AETBFP’s action against inflammation and pyrexia in rodents is documented to the best of the researchers’ knowledge.
In the subcutaneous paw tissues, the suppression of paw edema was linked to the inhibition of cytokine production, including Tumor necrosis factor-beta, interleukin-1, and Interleukin-6 along with cell infiltration.37 In paw edema testing, steroidal anti-inflammatory medicines and nonsteroidal anti-inflammatory therapies that block the formation of prostaglandins are effective.38 Similarly, the test medicine, Terminalia bellirica fruit, may work alike to prevent the synthesis of prostaglandins.
The fruit of Terminalia bellirica contains phytoconstituents such as bellericanin, gallotannic acid, ellagic acid, termilignan, gallic acid, thannilignan, anolignanB, flavone, tannins, ethyl gallate, galloyl, chebulinic acid, sitosterol, glucose, phyllemblin, and fructose.39 Anti-inflammatory action is attributed to gallic acid and ellagic acid, which are present in the fruit.40,41 In light of this discovery, we conducted a study and observed that when acute administration of 9 mg/kg was given to mice, there was no significant reduction in paw edema. This demonstrates that inadequate drug dosage results in inadequate drug at the site of action. Mice that were given AETBFP 18 mg/kg demonstrated a significant reduction in edema from two hours onwards, with the highest reduction happening at six hours (0.16±0.00). The paw edema of mice that were given 36 mg/kg of AETBFP was dramatically reduced after one hour and peaked at six hours. The amount of time required for a drug to enter the bloodstream or for active metabolite formation can both be explained as a time lag for pharmacological action. The highest percentage inhibition was 48 percent for AETBFP 36 mg/kg, which was comparable to the positive control. (Figure1)
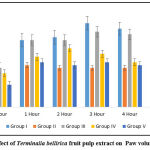 |
Figure 1: Effect of Terminalia bellirica fruit pulp extract on Paw volume on Day 1. |
AETBFP was found to diminish the volume of edema of the paw in a dose-dependence fashion. Mice subacutely treated using 9 mg/kg demonstrated a significant reduction in paw edema starting at two hours, with the greatest reduction at six hours (0.23±0.02). When compared to acute dosing of the medication, the subacute administration with 9 mg/kg was able to cause a significant reduction in paw edema volume. Mice-treated with AETBFP 18 mg/kg demonstrated a significant reduction in paw edema starting at one hour, with the greatest reduction at six hours (0.19±0.02). Mice-treated with AETBFP 36 mg/kg demonstrated a significant reduction in paw edema from one to six hours, with the greatest reduction at six hours (0.11±0.02). The highest percentage inhibition was 64.2% for AETBFP 36 mg/kg. All three doses of AETBFP on subacute administration displayed a substantial diminution in paw edema (Figure 2).
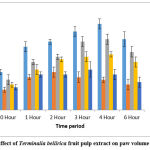 |
Figure 2: Effect of Terminalia bellirica fruit pulp extract on paw volume on Day 14. |
As soon as the chemotactic trigger zone in the brain is activated by exogenous or endogenous pyrogens, the hypothalamic “set point” rises, causing a rise in body temperature.42 Fever is part of the “acute phase reaction,” a defense response that occurs during the inflammatory response of various causes, including the Baker’s yeast-led inflammation of the peritoneal cavity that has recently been identified. Macrophages, neutrophils, dendritic cells, and lymphocytes are innate immune cells that detect foreign organism’s peculiar protein framework and expel mediators of inflammation like interleukin-1 and tumor necrosis factor. Such mediators help to interact between the immune response and microbes by enhancing blood vessel permeability combined with the translocation of immune cells towards the infectious region. Furthermore, cytokine-mediators use the vagal and humoral routes to communicate peripheral inflammation to the brain, resulting in PGE2 generation and fever.43 The thermoregulatory center of the hypothalamus is where endogenous pyrogens interact with receptors to generate heat, which is one of the body’s most significant defense mechanisms. Uncontrolled fever and febrile reactions, on the other hand, can be fatal to the person. As a result, to achieve thermoregulation, a well-functioning antipyretic mechanism is required.44
The presence of tannins and phenolic compounds similar to those found in AETBFP was found to have an antipyretic effect on Wistar albino rats in a study conducted with Emblica officinalis fruits.45 In light of this, this research had been conducted, to determine the action of an AETBFP on pyrexia in mouse models, with positive results. The temperature drop generated by two doses of 9 and 18 mg/kg was significant, but not comparable to the positive control. After 30 minutes, the temperature of rats given AETBFP 36 mg/kg decreased considerably, with the highest temperature drop occurring at 240 minutes. Thus, AETBFP 36 mg/kg displayed a substantial drop in rectal temperature while related with the positive control. (Figure 3) The AETBFP’s capacity to decrease prostaglandin synthesis may explain its dose-dependent reduction of inflammation and rise in body temperature. The current research found that an AETBFP possesses anti-inflammatory and antipyretic properties. The plant extract’s low toxicity and possible benefits in treating inflammation and pyrexia imply that it could be employed to treat inflammation and fever in the future.
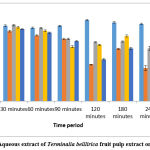 |
Figure 3: Effect of Aqueous extract of Terminalia belllirica fruit pulp extract on rectal temperature. |
Conclusion
Finally, the aqueous extract of Terminalia bellirica fruit pulp showed potential action against acute and subacute inflammation. Test extract also has antipyretic properties in the brain. A significant acute anti-inflammatory effect was observed at extraction doses of 18 and 36 mg/kg. A significant subacute anti-inflammatory effect was observed at extraction doses of 36 mg/kg. A significant antipyretic effect was observed at extraction doses of 9, 18 and 36 mg/kg. Owing to the existence of natural products like gallic acid, tannins, ellagic acid and phenolic compounds, the plant materials’ potential might be ascribed to the suppression of various inflammatory mediators as well as a temperature rise. In this view, natural chemicals are unquestionably valuable leads for the discovery of new medications, and the contemporary significance of drugs derived from nature is undisputed. More studies should be conducted to identify the bioactive constituents that are responsible for the antipyretic as well as anti-inflammatory effects. Further research on the basic structure, pharmacokinetics, and pharmacology is required.
Acknowledgments
We would like to express our gratitude to the A J Institute of Medical Sciences and Research Center for allowing us to use their facilities for this study.
Conflict of Interest
No conflicts of interest in this work
Funding Source
The authors declare that no funds, grants, or other support were received during the preparation of the manuscript.
References
- N Christian. Inflammation: Causes, Symptoms and Treatment [Internet]. UK: Medilexicon, Intl; 2012 [updated 16 Sep 2015; cited 6 Aug 2016]. Available from: http://www.medicalnewstoday.com/articles/248423.php
- Varela ML, Mogildea M, Moreno I, Lopes A. Acute Inflammation and Metabolism. Inflammation. 2018 Aug;41(4):1115-1127. doi: 10.1007/s10753-018-0739-1.
CrossRef - Diamond J. Guns, Germs and Steel: The Fates of Human Societies. New York: WW Norton; 1997.
- Mworia JK, Kibiti CM, Ngugi MP, Ngeranwa JN. Antipyretic potential of dichloromethane leaf extract of Eucalyptus globulus (Labill) and Senna didymobotrya (Fresenius) in rats models. Heliyon. 2019 Dec 7;5(12):e02924. doi: 10.1016/j.heliyon.2019.e02924.
CrossRef - Dinarello CA, Gatti S, Bartfai T. Fever: Links with an ancient receptor. Curr Biol. 1999; 9(4):147-50.
CrossRef - Dinarello CA. Cytokines as endogenous pyrogens. J Infect Dis. 1999; 179(2): 294-304.
CrossRef - Nordqvist C. Fever: Causes, Symptoms and Treatments [Internet]. UK: Medilexicon, Intl; 2012 [updated 6 Nov2015; cited 13 Aug 2016]. Available from http://www.medicalnewstoday.com/articles/168266.php.
- Sostres C, Gargallo CJ, Arroyo MT, Lanas A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Bailliere’s Best Pract. 2010; 24: 121–32.
CrossRef - Ruiz-Hurtado PA, Garduño-Siciliano L, Domínguez-Verano P, et al. Propolis and Its Gastroprotective Effects on NSAID-Induced Gastric Ulcer Disease: A Systematic Review. Nutrients. 2021; 13(9):3169. https://doi.org/10.3390/nu13093169
CrossRef - Ghosh R, Alajbegovic A, Gomes AV. NSAIDs and Cardiovascular Diseases: Role of Reactive Oxygen Species. Oxid Med Cell Longev. 2015; 2015: 536962.
CrossRef - Kuo HW, Tsai SS, Tiao MM, Liu YC, Lee IM, Yang CY. Analgesic use and the risk for progression of chronic kidney disease. Pharmacoepidemiol Drug Saf. 2010; 19(7): 745-51.
CrossRef - Whitehouse MW. Anti-inflammatory glucocorticoid drugs: Reflections after 60 years. Inflammopharmacology. 2011; 19: 1–19.
CrossRef - Ilmi H, Pamungkas IR, Tumewu L, Hafid AF, Widyawaruyanti A. Analgesic and Antipyretic Activities of Ethyl Acetate Fraction Tablet of Andrographis paniculata in Animal Models. Evid Based Complement Alternat Med. 2021 Mar 8;2021:8848797. doi: 10.1155/2021/8848797.
CrossRef - Andrade C. Use of acetaminophen (paracetamol) during pregnancy and the risk of autism spectrum disorder in the offspring. J Clin Psychiatry. 2016; 77(2): 152-4.
CrossRef - Ali A, Nasir A, Shah SWA, et al. Evaluation of antinociceptive activity of Ilex dipyrena Wall. in mice. BMC Complement Med Ther. 1 Jul 2021;21(1):184. doi: 10.1186/s12906-021-03357-4.
CrossRef - Demsie DG, Yimer EM, Berhe AH, Altaye BM, Berhe DF. Anti-nociceptive and anti-inflammatory activities of crude root extract and solvent fractions of Cucumis ficifolius in mice model. J Pain Res. 30 Apr 2019;12:1399-1409. doi: 10.2147/JPR.S193029.
CrossRef - Tesfaye R, Degu A, Abebe B, Ayalew H. Evaluation of Analgesic and Anti-inflammatory Potential of 80% Methanol Leaf Extract of Otostegia integrifolia Benth (Lamiaceae). J Inflamm Res. 23 Dec 2020;13:1175-1183. doi: 10.2147/JIR.S285932.
CrossRef - Christenhusz, M. J. M, Byng, J. W. (2016). “The number of known plants species in the world and its annual increase”. Phytotaxa. 261 (3): 201–217. doi:10.11646/phytotaxa.261.3.1.
CrossRef - Zhang XR, Kaunda JS, Zhu HT, Wang D, Yang CR, Zhang YJ. The Genus Terminalia (Combretaceae): An Ethnopharmacological, Phytochemical and Pharmacological Review. Nat Prod Bioprospect. 2019;9(6):357-392. doi:10.1007/s13659-019-00222-3
CrossRef - Deb A, Barua S, Das B. Pharmacological activities of Baheda (Terminalia bellerica): A review. JPP. 2016; 5(1): 194-197.
- Gupta A, Kumar R, Bhattacharyya P, Bishayee A, Pandey AK. Terminalia bellirica (Gaertn.) roxb. (Bahera) in health and disease: A systematic and comprehensive review. Phytomedicine. 2020 Oct;77:153278. doi: 10.1016/j.phymed.2020.153278.
CrossRef - Nadkarni KM. Indian Meteria Medica. Mumbai; Popular Prakashan Pvt.Ltd; 2002. p. 202- 1205.
- Khan AU, Gilani AH. Pharmacodynamic Evaluation of Terminalia belerica for its Anti-Hypertensive Effect. JFDA. 2008; 16: 6-14.
- Yadav S, Singh S, Sharma P, Thapliyal A, Gupta V. Antibio film Formation Activity of Terminalia bellerica Plant Extract Against Clinical Isolates of Streptococcus mutans and Streptococcus sobrinus: Implication in Oral Hygiene. Int. J of Pharmaceu & Bio Arch. 2012; 3(4): 816-21.
- Kaur S, Arora S, Kaur S, Kumar S. Bioassay-guided isolation of anti-mutagenic factors from fruits of Terminalia bellerica. J Environ Pathol Toxicol Oncol. 2003; 22(1): 69-76.
CrossRef - Madani A, Jain SK. Anti-Salmonella activity of Terminalia belerica: In vitro and in vivo studies. Indian J Exp Biol. 2008; 46(12): 817-21.
- Omran Z, Bader A, Porta A, Vandamme T, Anton N, Alehaideb Z, El-Said H, Faidah H, Essa A, Vassallo A, Halwani M. Evaluation of Antimicrobial Activity of Triphala Constituents and Nanoformulation. Evid Based Complement Alternat Med. 2020 Aug 2;2020:6976973. doi: 10.1155/2020/6976973.
CrossRef - Perianayagam JB, Sharma SK, Joseph A, Christina AJ. Evaluation of antipyretic and analgesic activity of Emblica officinalis gaertn. J Ethanopharmacol. 2004 Nov; 95(1):83-5.
CrossRef - Mahaveer Golecha et al. Anti-inflammatory effect of Emblica officinalis in rodent models of acute and chronic inflammation: Involvement of possible mechanisms. International journal of inflammation.2014: Article id 178408: 6 pages.
CrossRef - Jami SI, et al. Evaluation of analgesic and anti-inflammatory activities of ethanolic extract of Terminalia chebula fruits in experimental animal models. American journal of plant sciences. 2014; Article id: 41900:7 pages.
- Sireeratawong S, Jaijoy K, Panunto W, Nanna U, Lertprasertsuke N, Soonthornchareonnon N. Acute and chronic toxicity studies of the water extract from dried fruits of Terminalia bellerica (Gaertn.) Roxb. In Sprague-Dawley rats. Afr J Tradit Complement Altern Med. 31 Dec 2012;10(2):223-31. doi: 10.4314/ajtcam.v10i2.6.
CrossRef - Vikram P.K, Malvi R and Jain DK. Evaluation of analgesic and anti-inflammatory potential of Mimosa pudica linn. Int J Curr Pharm Res.2012; 4(4):47-50.
- C. Amaning Danquah, E. Woode, E. Boakye Gyasi, M. Duweijua, C. Ansah. Anti-Inflammatory and Anti-Pyretic Effects of an Ethanolic Extract of Capparis erythrocarposa Isert roots. Res. J. Med. Plant. 2011; 5(2):158-68.
CrossRef - Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal anti-inflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2013;16(5):821-47. doi: 10.18433/j3vw2f. PMID: 24393558.
CrossRef - Li Y, Shen Y, Yao CL, Guo DA. Quality assessment of herbal medicines based on chemical fingerprints combined with chemometrics approach: A review. J Pharm Biomed Anal. 5 Jun2020;185:113215. doi: 10.1016/j.jpba.2020.113215. Epub 2 Mar 2020. PMID: 32199327.
CrossRef - Dragos D, Gilca M, Gaman L, Vlad A, Iosif L, Stoian I, Lupescu O. Phytomedicine in Joint Disorders. Nutrients. 16 Jan 2017;9(1):70. doi: 10.3390/nu9010070. PMID: 28275210; PMCID: PMC5295114.
CrossRef - Zhang ZC, Zhang SJ, Jin B, Wu Y, Yang XF, Yu B, Xie QM. Ciclamilast ameliorates adjuvant-induced arthritis in a rat model. Biomed Res Int. 2015;2015:786104. doi: 10.1155/2015/786104. Epub 27 Apr 2015. PMID: 26000303; PMCID: PMC4426775.
CrossRef - Vogel HG. Analgesic, Anti-inflammatory and Anti-Pyretic Activity. In Drug Discovery and Evaluation: Pharmacological Assays. 3rd ed. New York: Springer; 2008.p. 725-774.
- Mot. Omari NS, Karthikeyan M, Kannan M, Rajasekar. S. Terminalia belerica Roxb-A Phytopharmacological Review. International journal of research in pharmaceutical and biomedical sciences.2012; 3(1):97-99
- Golechha M, Sarangal V, Ojha S, Bhatiya J, Arya DS. Anti-inflammatory effect of Emblica officinalis in rodent models of acute and chronic inflammation: Involvement of possible mechanisms. Int J Inflam.2014: 178408.
CrossRef - Jami SI, et al. Evaluation of analgesic and anti-inflammatory activities of ethanolic extract of Terminalia chebula fruits in experimental animal model. American journal of plant sciences. 2014; 5, 63-9.
CrossRef - Section on Clinical Pharmacology and Therapeutics; Committee on Drugs, Sullivan JE, Farrar HC. Fever and antipyretic use in children. Pediatrics. 2011 Mar;127(3):580-7. doi: 10.1542/peds.2010-3852. Epub 28 Feb 2011. PMID: 21357332.
CrossRef - Ferreira AP, Pasin JS, Saraiva AL, et al. N-Acetylcysteine prevents baker’s-yeast-induced inflammation and fever. Inflamm Res. Feb 2012;61(2):103-12. doi: 10.1007/s00011-011-0392-8. Epub 2011 Nov 8. PMID: 22057902.
CrossRef - N P, Ss A, Pv M. Comprehensive biology of antipyretic pathways. Cytokine. Apr 2019;116:120-127. doi: 10.1016/j.cyto.2019.01.008. Epub 1 Feb 2019. PMID: 30711851.
CrossRef - Perianayagam JB, Sharma SK, Joseph A, Christina AJ. Evaluation of antipyretic and analgesic activity of Emblica officinalis gaertn. J Ethnopharmacol. 2004; 95(1): 83-5.
CrossRef







