Manuscript accepted on :12-10-2022
Published online on: 27-01-2023
Plagiarism Check: Yes
Reviewed by: Dr. Nagham Aljamali , Dr. Satyajit Mohapatra
Second Review by: Dr. Vijay Kumar
Final Approval by: Dr. Eman Refaat Youness
Aishwarya Jayan and Swati Gupta*
and Swati Gupta*
Amrita School of Pharmacy, Amrita Institute of Medical Sciences, Amrita Vishwa Vidyapeetham, AIMS Ponekkara P.O , Cochin, Kerala, India.
Corresponding Author E-mail: swatigupta@aims.amrita.edu
DOI : https://dx.doi.org/10.13005/bpj/2583
Abstract
Dermatophytes and Candida are the two most common causes of fungal infections worldwide, affecting millions of people annually. The emergence of resistance among these groups of fungi and the limited availability of effective antifungal drugs may become a real challenge in the coming era. Thus, use of a combination of resistant reversion agents along with antifungal drugs is worth investigating. One of the causes of resistance development is the overexpression of efflux pumps and associated genes. Therefore, we examined the scientific literature on antifungal combinations against resistant species of dermatophytes and Candida caused by efflux pump overexpression. A literature search on the subject performed in PubMed and Google scholar resulted from a total of sixteen relevant publications. The inclusion criteria mainly focused on dermatophyte and Candida strains resistant to azoles, as well as publications that combined antifungal medications with natural compounds or other chemicals to combat resistance. Out of sixteen, fourteen articles focused on resistant strains of Candida and two on dermatophytes. Among articles published on resistant strains of Candida, five articles were based on combining azole with other drugs, while nine were with natural compounds like essential oils, curcumin etc. Whereas with resistant strains of dermatophytes, both articles were based on combining azole with natural compounds. It can be concluded that antifungal combinations against resistant strains of Candida and dermatophytes are more effective than single drugs. Combinatorial approaches have gained considerable scientific interest over the years, with promising results. Thus, it is worthwhile to continue research in this area.
Keywords
Antifungal Combination; Candida; Efflux pumps; Dermatophytes; Dermatophyte resistance
Download this article as:| Copy the following to cite this article: Jayan A, Gupta S. Effective Combinations Against Efflux Pump Overexpressed on Azole Resistance Candida and Dermatophytes: A Systematic Review. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Jayan A, Gupta S. Effective Combinations Against Efflux Pump Overexpressed on Azole Resistance Candida and Dermatophytes: A Systematic Review. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3Dgscs4 |
Introduction
The development of resistance against antifungal therapy is the primary emerging challenge for the coming era. Many fungus species may develop resistance to antifungal drugs after prolonged exposure, prophylaxis therapy and irrational use.1 Resistance may arise from overexpression of genes encoding efflux pumps, overexpression of efflux pumps, reduced drug import into fungal cells, and overexpression/ alteration/ reduced affinity of target enzyme [14-alpha demethylase] towards antifungal drugs. Efflux pumps are commonly present on fungal cells for detoxification and extended exposure to antifungal drugs; these efflux pumps and associated genes are over-expressed. Consequently, antifungal drugs are actively flushed from fungal cells and resistance develops in susceptible fungi. Dermatophytes & yeasts, mostly Candida are the common fungi known for overexpression of efflux pumps, ABC (ATP binding cassettes) and MFS (major facilitator super family), and genes which are responsible for coding of these efflux pumps are mainly CDR1, CDR2 (Candida drug resistant gene) MDR1( Multi drug resistance) FLU1(Fluconazole resistance).2-3
Infection with resistant species is predominantly observed in immunocompetent and immunocompromised patients, where the host’s immune status plays a significant role in the outcome. Chronic conditions with considerable morbidity rates can be severe in immunocompromised patients and result in invasive infection.
Candida is an opportunistic fungal pathogen, affecting the nails, skin, and oral and genital mucosa. Certain conditions like diabetes4, genetic disorders, and circulatory diseases impact the immune system, and such patients are at high risk of getting fungal infections.
Dermatophytes are a group of related fungi belonging to the three main categories, i.e. Epidermophyton, Trichophyton, and Microsporum. Two new classes i.e. Lophophyton and Nannizia were included according to the new proposal of phylogenetic taxonomy.5 Dermatophytosis is a superficial infection of hair, skin and nail caused by dermatophytes. Most commonly, it is caused by Trichophyton genus.6 The prevalence of both these infections is high worldwide and, if not treated appropriately, could lead to invasion into the deeper layers of skin and resistance.
Treatment/therapy
Two major classes of antifungal agents active against susceptible strains of Candida and dermatophytes uniformly, have been detailed in Table 1. For cutaneous infection, topical therapy is preferred and for extensive infection, systemic treatment.7 In general, topical therapy shows fewer side effects. The side effects are dose-related, dependent on the length of treatment and route of administration.
Table 1: Details of commonly used antifungal drugs, their mechanisms and adverse effects
| SN | Antifungal agent | Mechanism of action | Adverse effects | Reference |
| 1 | Class: Imidazoles
Bifonazole, Clotrimazole, Econazole, Ketoconazole, Luliconazole, Miconazole, Sertaconazole, and Tioconazole |
Block the synthesis of ergosterol, alter the cell membrane permeability of fungi | Gastrointestinal disturbances like nausea, vomiting, abdominal pain, diarrhea and nephrotoxicity, hepatotoxicity photosensitivity, and neurotoxicity | [7] |
| 2 | Class: Triazole
Fluconazole, Isavuconazole, Itraconazole, Posaconazole, Ravuconazole, Voriconazole, Luliconazole,and Lanoconazole |
Interruption of conversion of lanosterol to ergosterol |
Among azoles, fluconazole (FLC) and itraconazole (ITZ) are the safest drugs. Both are triazoles and inhibit cytochrome P450 14α lanosterol demethylase. Compared to FLC, ITZ had a much lower MIC (Minimum Inhibitory concentration) value. On long-term azoles exposure unfortunately, there are an increasing number of resistant strains of Candida spp. and dermetophytes.8-9 The strains found to be resistant to FLC include Trichophyton tonsurans (T. tonsurans), Microsporum canis, Microsporum gyp. and Trichophyton rubrum (T. rubrum).8 The resistance was mediated by TruMDR2, TruMDR1, and ABC for FLC, and in the case of candida Cdr1p and/or CaMdr1p belonging to the ABC and MFS superfamilies. Among dermatophytes, T. rubrum prevalence increased in the past few years. Yamada et al., (2021) proposed that ITZ resistance in T. rubrum was mediated by ABC transporter TruMDR2 and, to a small extent, by TruMDR3genes.9
Studies indicate that the Azole class of drugs exhibits resistance by various mechanisms, primarily overexpression of the efflux pump. Azoles bind to cell efflux transporter proteins and are expelled from the cells. Overexpression of these efflux pumps can lead to treatment failure. Another reason might be the mutation of Erg II gene, which is responsible for drug target modification.8, 10
The major mechanisms of antifungal drug resistance and possible ways to overcome it are depicted in Figure 1.
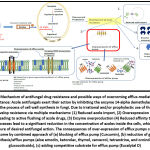 |
Figure 1: Mechanism of antifungal drug resistance and possible ways of overcoming efflux-mediated azole drug resistance: |
The development of new antifungal drugs requires more time and efforts. Researchers are trying to resolve the problem of resistance in several ways; combination therapy is one of them.11-12 For a decade, many compounds, including phytoconstituents combined with standard antifungal drugs, were found to be effective against resistant strains of fungus, but these areas are less explored. Other advantages of combination therapy include reduced toxicity, fewer side effects compared to the single drug prescribed at a high dose, and enhanced antifungal effects.
In combination therapy, one molecule may sensitize fungal species against antifungal drugs primarily by blocking efflux pumps like ABC and MFS in which ABC is most often found to be overexpressed. These are two classes of azole pumps. These proteins carry compounds across the cell membrane with the help of some energy sources. Both have distinct protein domain parts, like the nucleotide-binding domain in the case of ABC pumps and the transmembrane domain in the case of MFS pumps. The genes encode these pumps are CDR1, CDR2, FLU1, MDR1 and MDR2. In the past decade, much work have been done to resolve the issue of resistance.13
Brescini and co-workers (2021) reviewed antifungal combinations against recalcitrant and resistant dermatophytes. These combinations were mainly focused on combination of two antifungal drugs or combination of one antifungal drug with some chemical entity. In this review, the combination of antifungal drugs with plant-based molecules/phytoconstituents and the mechanism of combinatorial effect were less explored.12
In another review by Holmes et al., (2016), all the targets for efflux pump against all the species, including Candida and dermatophytes were described. It was mainly focused on reversal of resistance by targeting these efflux pumps but the combination used to overcome resistance or for targeting the efflux pumps wasn’t discussed.14
The present review mainly focuses on azoles, since these antifungal drugs play an essential role in treating dermatophytosis and candidiasis, where overexpression of drug efflux is a major cause of resistance. Different strategies like combination therapy suitable for reducing gene expression, blockage of efflux pumps and compitative substrates for efflux pumps proven effective against azole-resistant Candida and dermatophytes have been discussed in detail. The studies on the resistant of dermatophytes are less explored as compared to Candida. It needs immediate scientific intervention and attention before the disease becomes a pandemic.
Materials and Methods
This systematic review was conducted as per the latest PRISMA guidelines [Fig: 2]. The published articles were exhaustively searched on PubMed and Google scholar during 23rd December 2021 to 14th March 2022. Different search strings like “antifungal resistance,” “combination used for overcoming,” etc were used while retrieving the articles. Consensus for all the discrepancies which occurred during the process of inclusion of papers was reached through discussion. The inclusion criteria were papers that focussed mainly on antifungal resistance. Another inclusion criterion was the articles that focussed on the combination of antifungal drugs with phytoconstituent or the combination that focussed primarily in efflux pump blockage. The exclusion criteria were the papers that didn’t refer to dermatophyte or candida resistance, unreachable publications and the ones that didn’t specify the species name.
 |
Figure 2: Flowchart of the different phases of article selection of the review. |
Results and Discussion
A total of 61832 articles were initially identified (Figure 2). The articles published from 2010 to 2022 were selected through further screening. The exclusion was done based on papers that didn’t focus on resistance, which didn’t refer to dermatophytes or related species. Other exclusions were those papers that were out of the scope of this review. Total of sixteen articles were selected for the review. Out of 16 article 14 includes combinational therapy against resistant strains of Candida, only 2 articles were found to be based on combinational therapy against resistant strains of dermatophytes.
Combination against the resistant strain of Candida isolates
In recent years, a number of studies have been published on Candida as compared to other fungus species. Combination therapy has shown reversal of azole resistance as evidenced from the reduction in MIC values, reduction in gene expression etc. Most of these studies contain in vitro study data, thus lacking the studies of adverse effects associated with use of these drugs for antifungal therapies. A comprehensive account of antifungal combinations against azole resistant candida species with over expression of efflux pumps has been presented in Table 2.
Fluconazole and ketorolac
Sayed et al., (2021) conducted the sensitivity and efficacy study of fluconazole and ketarolac combination on resistance species of Candida albicans (C.albicans) obtained from acute myeloid leukemia patients and compared with monotherapy. They also confirmed the expression of efflux pump gene (CDR1, CDR2, MDR1) by real-time PCR on clinical isolates of C.albicans, which are responsible for resistance development. They reported a drastic decrease in the minimum inhibitory concentration of fluconazole and ketarolac combination as compared to fluconazole and ketarolac alone as 0.3-1.25 µg/ml, 160 µg/ml and 10 µg/ml respectively. Reported FICI (Fractional inhibitory concentration index) value (0.25) confirms synergism.15
Fluconazole and Ginko biloba
Yiman et al.,(2020) conducted a study where phytoconstituent like Ginkolide B was combined with fluconazole and its effect on fluconazole-resistant C. albicans was observed. Fluconazole alone had no effect on an Azole-resistant strain of C. albicans, as seen by its MIC value (>256 g/ml). The MIC was lowered from >512 g/ml to 0.25 g/ml in conjunction with Ginko biloba, indicating synergism. They also discussed that calcium homeostasis is the basis for the average growth and pathogenicity of C. albicans. The intracellular calcium concentration in these strains was altered, which can be the base of synergism. Yet the mechanism is not mentioned correctly. A fluorescent assay was performed, and the result indicated enhanced uptake of fluconazole when given in combination with ginkolide.16
Eugenol and methyleugenol with fluconazole
Ahmed et al., (2010) evaluated the efficacy of two bioactive compounds found in essential oil, eugenol and methyleugenol, alone and in combination with fluconazole against fluconazole resistant clinical Candida isolates. The method used to confirm the resistance has not been discussed. Even though both the bioactive compounds were effective against resistant species, methyl eugenol was much more effective than eugenol. The MIC value of eugenol and methyleugenol against resistant strain ranged from 475 – 500 µg/ml and 340-350 µg/ml, while in the combination ranged from 110–120 µg/ml and 70-90 µg/ml, respectively. The high fungicidal effect of these compounds on combination with fluconazole was confirmed using a disc diffusion assay. Proper mechanisms regarding how this combination helps to overcome resistance have not been discussed. But it was reported that the lipophilic character of eugenol and methyleugenol might enable these compounds to enter into the membrane lipid bilayer altering the fluidity and permeability, thus interfering with enzyme activity which helps in cell wall synthesis.17
Monoterpenes with fluconazole
Ahmed et al., (2012) conducted a study where the efficacy of the combination of thymol and carvacrol with fluconazole was tested against fluconazole-resistant clinical Candida isolates. MIC values of thymol, carvacrol, and fluconazole for fluconazole-sensitive and fluconazole-resistant strains of C. albicans were 90-150µg/ml, 50-100µg/ml, and 2.5-7.5µg/ml, respectively. Using combination MIC was reduced to 21-32µg/ml, 7-23µg/ml, 0.5-2µg/ml respectively. MIC values and wider inhibition zones revealed that all of the isolates were sensitive to monoterpenes and their combination. The sensitivity index was comparatively larger for the carvacrol fluconazole combination [3.2+0.05]. A reduction in the efflux activity was confirmed by using a fluorescent assay. RT PCR (Real time polymerase chain reaction) was used to confirm the decrease in the quantity of CDR and MDR genes that encode for efflux pumps and are responsible for fluconazole resistance. C. albicans resistant strains overexpress just CDR1 and not MDR1, whereas Candida glabrata and Candida krusei overexpress both CDR1 and MDR1.18
Fluoxetine with fluconazole
Oliveira et al., (2014) investigated the efficacy of fluoxetine alone and in combination with fluconazole against Candida strains isolated from patients with vulvo vaginal candidiasis. The combination resulted in a reduction in MIC values (decreased up to 64 fold). Among the tested strains, six of the candida species showed a synergistic effect, among which 4 of them were resistant to fluconazole whereas the remaining showed an indifferent effect which was evident from fractional inhibitory index values between 0.15-0.31 and 0.63-1, respectively.19
Calcineurin inhibitors with fluconazole
Kaya et al., 2021 reported the calcineurin mutants exhibited increased susceptibility towards antifungal drugs, including azoles. Calcineurin inhibitors such as cyclosporin A and tacrolimus demonstrated synergistic activity with fluconazole against various fungi, including C. albicans. The interaction of cyclosporin A with azoles such as fluconazole and itraconazole against azole susceptible and resistant C. albicans was studied using the chequerboard method. Based on a fluorescent study, this combination inhibited C. albicans growth and hyphal formation, which in turn inhibits biofilm formation. Similarly, tacrolimus in combination with fluconazole could reverse drug resistance. The synergistic activity was studied with the checkerboard method and time-killing test.20
Tetrandrine with fluconazole
Keyal et al., 2017 evaluated that Tetrandrine (ancient Chinese medicine ), a dibenzylisoquinoline alkaloid increased the sensitivity of C. albicans towards fluconazole. Both compounds had synergistic interactions. Tetrandrine could inhibit the expression of genes like MDR1, FLU1, CDR1, and CDR2 reducing the drug efflux and increasing intracellular azole accumulation. It also had antibiofilm activity and targeted sterol biosynthesis altering fluidity and permeability of cell membrane.21
Glucocorticoid with fluconazole
Wenwen et al., 2017 combined glucocorticoids like dexamethasone and budesonide along with fluconazole. They exhibited synergistic activity against Candida spp. which was evident by FICI index of less than 0.5 but had no antifungal action when used alone. RTPCR results confirmed the reduction in expressions of drug-resistant genes like CDR1, CDR2, and MDR1, which indicates synergism and extracellular phospholipase activity. A critical virulence factor was also measured using the egg yolk agar method showed a reduction in virulence.22
Gentamycin with fluconazole
Lu et al., 2017 combined gentamycin with fluconazole against resistant C. albicans species. The combination reduced the MIC and FICI values by 0.13-0.14, confirming synergism. The rhodamine efflux assay was used to verify the reduction in efflux activity dose-dependently. A decrease in phospholipase activity also showed a reduction in the pathogenicity of the strains.23
Oridonin with azole
Chen et al., 2020 used a combination of azole drugs (Itraconazole, Fluconazole) with oridonin against resistant C albicans strain isolated from cancer patients. A reduction in MIC was observed. MIC of fluconazole and itraconazole decreased from >512µg/ml and >8µg/ml to <8 and <0.2µg/ml, respectively. The resistance reversal mechanisms, namely inhibition of drug efflux and induction of apoptosis, were investigated by flow cytometry. Expression levels of the efflux pump-related genes CDR1 and CDR2 were assessed by RT-qPCR.24
Antimicrobial photodynamic therapy with aloe-emodin
Leu et al., 2019 evaluated the photodynamic effect of Aloe-emodin on drug-resistant C. albicans. Aloe-emodin could effectively inactivate C. albicans in a concentration-dependent manner in the presence of light. The uptake of aloe-emodin by cells was studied using confocal laser scanning microscopy. SEM and TEM analysis showed that therapy could induce the damage of the fungal cell wall, cytoplasm and nucleus.25
Curcumin with fluconazole
Gomez et al., 2012 evaluated the capability of curcumin to sensitize the clinical fluconazole-resistant isolate of C. albicans. The MIC was reduced from 256 µg/l to <2µg/l. Synergistic interaction was assessed using a checkerboard experiment. Efflux pump activity was evaluated using Nile red accumulation assay. 11µM of curcumin was able to restore Nile red accumulation indicating inhibition of efflux activity.26
Fluconazole with citral
In an investigation by Cadena et al., 2022, citral, a phytochemical constituent of lemongrass oil, was found effective in combating Candida infections when combined with fluconazole. Citral reduced the biofilm formation and the combination exhibited synergism against most of the strains tested with FICI 0.5. RNA analysis further revealed the efflux pump reduction encoded by MDR1. However, the results weren’t conclusive about the expression of ERG11 gene. The expression of ERG11 gene weren’t significant even though a slight upregulation was observed.27
Eucalyptal D with Fluconazole
Xu et al., (2019) reported that Eucalyptal D (ED), significantly enhance the anticandidal activity of fluconazole (FLC) in treating FLC resistant C. albicans. They performed, checkerboard microdilution assay, rhodamine 6G (R6G) efflux assay, and reverse transcription PCR analysis and reported that the combination shows a synergistic effect. It was hypothesized that ED was a substrate of efflux pump (Cdr1p and Cdr2p), actively flushed out in place of fluconazole in resistant C. Albicans.28
Combination against resistant dermatophytes
A few studies have been published on antifungal combinations against azole resistant dermetophytes with over-expression of efflux pumps.29-30 These are detailed in the Table 3. Both the studies were conducted in vitro and hence lacking in studies/data on adverse effects.
Essential oils with fluconazole
In a study Khan et al., 2011 analyzed several essential oils and their active compounds like S. Aromaticum, C. Verum, C. Martini, Geraniol, Cinnamaldehyde etc. The strains A. ompounds and T. rubrum were tested for azole drug resistance and fluconazole showed higher resistance level than itraconazole. Most of the essential oils showed intense activity against tested fungi, among which cinnamaldehyde showed the highest zone of inhibition against T.rubrum. When combined with fluconazole, all the tested oils showed a significant synergistic interaction against T. Rubrum and A.ompounds, evident from FICI values. Among all the tested ones, cinnamaldehyde showed the highest degree of synergism. Its effect on ultrastructure was investigated further using TEM, and it was discovered that hyphal specimen treatment with cinnamaldehyde resulted in cell lysis as well as cytoplasmic disorganization.29
Protocatechuates with fluconazole
Luciana et al., (2014) investigated the antifungal activity of protocatechuic acid 3,4 diacetoxybenzoic acid and its 14 alkyl derivative against clinical strains of T. rubrum and T. metagrophytes alone and in combination with fluconazole. Both these compounds are considered to have low antidermatophytic activity because of their very high MIC values (125mg/ml and above). But addition of methyl groups to these compounds showed reduction in MIC values and increase in antifungal activity. The best MIC values were shown by pentyl, hexyl, heptyl, octyl, nonyl and decyl protocatechuate compounds ranging from 1.95 to 7.8mg/ml. It was reported that carbon chains have a crucial role in antifungal and antioxidant activity, but the actual mechanism has not been discussed in the paper. FIC index of 0.49 showed additive and synergistic action of all the compounds in combination with fluconazole.30
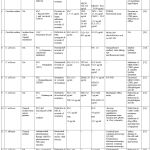 |
Table 2: Antifungal combinations against azole resistant Candida species with over expression of efflux pumps |
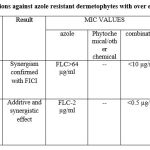 |
Table 3: Antifungal combinations against azole resistant dermetophytes with over expression of efflux pumpsConclusions |
The persistence and frequent recurrence of dermatophyte infections necessitate prolonged treatment. This leads to the risk of severe adverse effects and development of drug resistance. It has already been witnessed in systemic fungal infections caused by Candida and the emergence of drug resistant strains among T. rubrum. Most of the in vitro studies have investigated the combination of azole antifungal agents with several other chemical compounds and phytochemicals. The association between an antifungal drug and plant extract, including essential oils, seems to evoke a particular interest. The reciprocal potentiation of the molecules upon combination makes these approaches particularly appealing against resistant strains. Although the intrinsic mechanisms of antifungal activity of these natural products have not been thoroughly investigated, several cell targets are simultaneously involved, thereby chances of occurrence of resistance remains minimal. Anti–fungal studies indicate that an association of antifungal agents is influential, and it might be helpful in speeding up the microbiological healing of superficial infections. One of the major limitations with published studies is lack of sufficient evidences in support of combination therapy over treatment with single drug as a viable approach in minimizing the adverse effects associated with irrational use of individual drugs. In summary, antifungal combinations against dermatophytes and Candida have gained considerable scientific interest over the years. To establish this approach as a reliable treatment option in clinical settings, additional studies are warranted.
Conflict of Interest
There are no conflict of interest.
Reference
- Mishra L, Gupta S. Fluconazole and Curcumin Loaded Nanoemulsion Against Multiple Drug Resistance Dermatophytes. Biomed. Pharmacol. J., 14(4): 2085-2089 (2021).
- Prasad R, Rawal MK. Efflux pump proteins in antifungal resistance. Front Pharmacol. 29(5):202 (2014)
- Nair GG, Sathianarayanan S and Namboori K, Study of Using Syzygium Samarangese Against Azole Resistant Candida Species- a Computational and Pharmacogenomic Approach. Proceedings of International Conference on Drug Discovery (ICDD) (2020)
- Arun CS, Raju P, Lakshmanan V, Kumar A, Bal A, Kumar H. Emergence of Fluconazole-resistant Candida Infections in Diabetic Foot Ulcers: Implications for Public Health. Indian J. Community Med. ;44(1): S74-S76 (2019)
- Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev.;12(4):501-517 (1999).
- Gupta AK, Ryder JE, Chow M, Cooper EA. Dermatophytosis: the management of fungal infections. Skinmed., 4(5): 305-10 (2005).
- Kontoyiannis DP. Antifungal Resistance: An Emerging Reality and A Global Challenge. J. Infect. Dis. 15;216(3):S431-S435 (2017).
- Ghannoum M. Azole Resistance in Dermatophytes: Prevalence and Mechanism of Action. J. Am. Podiatr Med. Assoc., 106(1): 79-86 (2016).
- Yamada T, Yaguchi T, Tamura T, Pich C, Salamin K, Feuermann M, Monod M. Itraconazole resistance of Trichophyton rubrum mediated by the ABC transporter TruMDR2. Mycoses., 64(8): 936-946 (2021).
- Martinez-Rossi NM, Bitencourt TA, Peres NTA, Lang EAS, Gomes EV, Quaresemin NR, Martins MP, Lopes L, Rossi A. Dermatophyte Resistance to Antifungal Drugs: Mechanisms and Prospectus. Front Microbiol., 29(9): 1108 (2018).
- Khurana A, Sardana K, Chowdhary A. Antifungal resistance in dermatophytes: Recent trends and therapeutic implications. Fungal Genet. Biol., 132:103255 (2019).
- Brescini L, Fioriti S, Morroni G, Barchiesi F. Antifungal Combinations in Dermatophytes. J. Fungi (Basel)., 5;7(9): 727 (2021).
- Chowdhary A, Prakash A, Sharma C, Kordalewska M, Kumar A, Sarma S, Tarai B, Singh A, Upadhyaya G, Upadhyay S, Yadav P, Singh PK, Khillan V, Sachdeva N, Perlin DS, Meis JF. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009-17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob Chemother., 1;73(4): 891-899 (2018).
- Holmes AR, Cardno TS, Strouse JJ, Ivnitski-Steele I, Keniya MV, Lackovic K, Monk BC, Sklar LA, Cannon RD. Targeting efflux pumps to overcome antifungal drug resistance. Future Med. Chem., 8(12): 1485-501 (2016).
- Sayed SA, Hassan EAB, Abdel Hameed MR, Agban MN, Mohammed Saleh MF, Mohammed HH, Abdel-Aal AM, Elgendy SG. Ketorolac-fluconazole: A New Combination Reverting Resistance in Candida albicans from Acute Myeloid Leukemia Patients on Induction Chemotherapy: In vitro Study. J. Blood Med., 15;12: 465-474 (2021).
- Li Y, Yang J, Li X, Su S, Chen X, Sun S, Li Y. The effect of Ginkgolide B combined with fluconazole against drug-resistant Candida albicans based on common resistance mechanisms. Int. J. Antimicrob Agents., ,56(2): 106030 (2020).
- Ahmad A, Khan A, Khan LA, Manzoor N. In vitro synergy of eugenol and methyleugenol with fluconazole against clinical Candida isolates. J. Med. Microbiol., 59(10):1178-1184 (2010).
- Ahmad A, Khan A, Manzoor N. Reversal of efflux mediated antifungal resistance underlies synergistic activity of two monoterpenes with fluconazole. Eur. J. Pharm Sci. 23;48(1-2): 80-6 (2013).
- Oliveira AS, Gaspar CA, Palmeira-de-Oliveira R, Martinez-de-Oliveira J, Palmeira-de-Oliveira A. Anti-Candida activity of fluoxetine alone and combined with fluconazole: a synergistic action against fluconazole-resistant strains. Antimicrob Agents Chemother., 58(7): 4224-6 (2014).
- Şen Kaya S, Kiraz N, Bariş A, Turan D, Öz Y, Dağ İ, Aygün G. Effects of calcineurin inhibitors, cyclosporine A and tacrolimus (FK506), on the activity of antifungal drugs against Candida spp. J. Med. Microbiol., 70(4) (2021).
- Keyal U, Huang X, & Bhatta AK. Drug interaction of traditional Chinese medicines with fluconazole against fluconazole resistant strains of Candida albicans. Int. J. Clin. Exp. Med.. 2016. 22357-22362 (2016).
- Sun W, Wang D, Yu C, Huang X, Li X, Sun S. Strong synergism of dexamethasone in combination with fluconazole against resistant Candida albicans mediated by inhibiting drug efflux and reducing virulence. Int. J. Antimicrob Agents., 50(3): 399-405 (2017).
- Lu M, Yu C, Cui X, Shi J, Yuan L, Sun S. Gentamicin synergises with azoles against drug-resistant Candida albicans. Int. J. Antimicrob Agents., 51(1):107-114 (2018).
- Chen H, Li H, Duan C, Song C, Peng Z, Li H, Shi W. Reversal of azole resistance in Candida albicans by oridonin. J Glob Antimicrob Resist., 24: 296-302 (2021).
- Ma W, Liu C, Li J, Hao M, Ji Y, Zeng X. The effects of aloe emodin-mediated antimicrobial photodynamic therapy on drug-sensitive and resistant Candida albicans. Photochem Photobiol Sci. 15;19(4): 485-494 (2020).
- Garcia-Gomes AS, Curvelo JA, Soares RM, Ferreira-Pereira A. Curcumin acts synergistically with fluconazole to sensitize a clinical isolate of Candida albicans showing a MDR phenotype. Med Mycol., 50(1): 26-32 (2012).
- Miranda-Cadena K, Marcos-Arias C, Perez-Rodriguez A, Cabello-Beitia I, Mateo E, Sevillano E, Madariaga L, Quindós G, Eraso E. In vitro and in vivo anti-Candida activity of citral in combination with fluconazole. J Oral Microbiol., 2;14(1): 2045813 (2022).
- Xu J, Liu R, Sun F, An L, Shang Z, Kong L, Yang M. Eucalyptal D Enhances the Antifungal Effect of Fluconazole on Fluconazole-Resistant Candida albicans by Competitively Inhibiting Efflux Pump. Front Cell Infect. Microbiol., 20;9: 211 (2019).
- Khan MSA, and Ahmad I. “In vitro antifungal activity of oil of Cymbopogon citratus and citral alone and in combination with fluconazole against azole-resistant strains of Aspergillus fumigatus and Trichophyton rubrum.” Phcog Commn., 3.3: 29 (2013).
- Soares LA, Gullo FP, Sardi Jde C, Pitangui Nde S, Costa-Orlandi CB, Sangalli-Leite F, Scorzoni L, Regasini LO, Petrônio MS, Souza PF, Silva DH, Mendes-Giannini MJ, Fusco-Almeida AM. Anti-trichophyton activity of protocatechuates and their synergism with fluconazole. Evid Based Complement Alternat Med., 2014: 957860 (2014).







