Manuscript accepted on :25-01-2023
Published online on: 20-02-2023
Plagiarism Check: Yes
Reviewed by: Dr. Liudmila Spirina
Second Review by: Dr. B. Kirthika
Final Approval by: Dr. H Fai Poon
Nosheen Mahmood1* , Qamar Jamal2
, Qamar Jamal2 , Reem Al Kahtani1
, Reem Al Kahtani1 , Shamim Mustaq2
, Shamim Mustaq2 , Humera Akhlaq3
, Humera Akhlaq3 and Saima Aamir1
and Saima Aamir1
1Department of Basic Medical Sciences King Saud bin Abdulaziz University for Health Sciences, Riyadh, 14611, Kingdom of Saudi Arabia.
2Department of Pathology, Ziauddin University, Karachi, 75600, Pakistan.
3Department of Oral Pathology, Jinnah Sind Medical University, Karachi, 75510, Pakistan,
Corresponding author email: mahmoodn@ksau-hs.edu.sa
DOI : https://dx.doi.org/10.13005/bpj/2620
Abstract
MicroRNA-21, an oncomiR, plays a pivotal role in carcinogenesis and is upregulated in many cancers including oral squamous cell carcinoma (OSCC). Use of smokeless tobacco (ST) products and cigarettes smoking in causation of OSCC is well established. This study sought to reconnoiter miR-21 expression in relation to smoking and chewing habits among subjects with oral cancer. Methods After gaining approval from IRB of Ziauddin University, analysis of miR-21 expression was conducted in 100 biopsy proven OSCC cases and 100 controls. All participants gave informed written consent after which venous blood sample was collected. qRT-PCR (Quantitative real-time Polymerase chain reaction) was performed to check miR-21 expression. SPSS Version 24 was used for analyzing the data. Results Consumption of ST was reported by 85 % and 63% were smoking cigarettes. miR-21 expression was significantly higher among smokers and those addicted to ST products, p<0.001. Subjects addicted to gutka chewing and those using more than one chewable product showed significant upregulation of miR-21, p<0.05. Amount of ST product use and smoking cigarettes was found to be positively correlated with miR-21expression. Conclusion Our study provides the evidence that use of ST products and cigarette smoking trigger miR-21 which in turn potentiate carcinogenesis in OSCC.
Keywords
Head and neck cancer; MicroRNA-21; Oral Cancer; Smokeless tobacco
Download this article as:| Copy the following to cite this article: Mahmood N, Jamal Q, Al- Kahtani R, Mustaq S, Akhlaq H, Aamir S. Deciphering the Link Between Chewing Habits and Microrna 21 Dysregulation in Oral Squamous Cell Carcinoma: A Potential Cancerous Blend. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Mahmood N, Jamal Q, Al- Kahtani R, Mustaq S, Akhlaq H, Aamir S. Deciphering the Link Between Chewing Habits and Microrna 21 Dysregulation in Oral Squamous Cell Carcinoma: A Potential Cancerous Blend. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3I8iyJw |
Introduction
Cancers are at a rise. It is worrying to note that 1 in 5 men and 1 in 6 women will end up developing cancer in their lifetime. More than half of the global burden of cancers is contributed by Asia. It is further disturbing to note that Asian countries contribute more to global cancer related mortality than incidence. For 2018, 57.3 % of global cancer related mortality was contributed by Asia whereas it added 48.4% to global incidence.1
Asian countries including Pakistan have a remarkably high incidence of Oral squamous cell carcinoma (OSCC). A sudden shift from a less common cancer worldwide to second most common cancer after breast is reported in Pakistan. A significant rise in incidence is witnessed from 12761(8.6%) in 2012 to 18,881(12.72%) in 2018. 2 Despite maintaining a stable rank as second most common cause of cancer related death in Pakistan, proportion of people dying is increasing as evident by a rise from 7.2% (7266) in 2012 to 13.07% (13351) in 2018. Interestingly a slight decline in both incidence 16959 (9.5%) and mortality 10,617 (9.1 %) is observed in International Agency for Research on Cancer, Globocan 2020 reports. However, it may be due to under reporting of cases in the setting of lockdown imposed by COVID-19. 3
Nearly half of the patients are diagnosed in Stage III and IV throughout the world. Advanced stage linked to a poor prognosis is a well-established fact. 4 Seo et al collected data from a single institute to analyze the changes in disease pattern and treatment provided over a period of 30 years and did not observe any change in stage of presentation which remained IV throughout this period. 5 6,7
Diagnosis at an advanced age is linked to poor survival. 8 Stage at diagnosis is the key determinant of prognosis as evidenced by an 80% 5-year survival for localized disease9 which drops to 50% or less in advanced stage.10
A direct dose response association between tobacco products and OSCC has been consistently observed. Cigarette smoking accounts for 47% of oral cavity cancer related deaths. Smokeless tobacco in the form of various chewable tobacco products contributes to observed incidence and prevalence in Southeast Asia. A survey conducted in 2015 covering 121 countries found 351.9 million people were consuming ST products and around 90% consumption was reported in South Asia. 11
To evaluate the impact of smokeless tobacco in OSCC causation, Asthana et al conducted a meta-analysis based on data collected on researches conducted between 1960 to 2016 from four WHO regions. Analysis was based on 37 case control/cohort studies which met selection criteria. ST led to increases risk of OSCC which was most significant in Southeast Asia Region (SEAR) having a relative risk of 4.44, followed by Eastern Mediterranean Region (EMR) having a relative risk 1.28. Risk of various ST was also calculated showing highest risk with gutka intake, relative risk of 8.67, followed by pan tobacco/betel quid having a risk of 7.18.12
ST products is a rich source of many carcinogens including Tobacco Specific Nitrosamines (TSNAs). ST is linked to a variety of systemic and oral disorders, including head and neck malignancies. According to estimates, seventeen million people use smokeless tobacco in Pakistan. In Pakistan’s 2014 Global Adult Tobacco Survey (GATS), 12.4% of adult tobacco users reported smoking, while 7.7% reported using smokeless tobacco. The most popular ST products in Pakistan are paan which is a tobacco and betel nut product (7.4%), naswar (7.2%), and gutka (6.4%).13 Gutka is one of the most used products and its constituents include areca nut, tobacco, catechu and slaked lime. It is loaded with tons of carcinogens14,15which promote inflammation, oxidative stress,16 DNA damage, apoptosis, and cell proliferation. Genotoxic effects stem from tobacco and areca nut specific nitrosamines which are pro-carcinogenic. Due to lack of awareness, common people consider it less harmful compared to smoking tobacco leading to its higher consumption.17 In addition, low cost, easy access, artificial flavors and tempting packaging are added reasons for its higher consumption.18
The interplay between these risk exposures and various genetic and epigenetic factors is largely responsible for carcinogenesis. Mutations in protein coding genes have been considered main driver of mutagenesis until discovery of miRNAs (micro RNAs).19 miRNAs function as important players because of proximity of their genes to chromosomal break points.
miRNAs have transformed the research landscape and attained substantial research focus. Capability of miRNAs as genomic signatures for OSCC is magnifying steeply as more research is adding. Their stable detection in circulation, potential of discriminating various tumor traits, promise in predicting survival and foreseeing treatment response brands them as ideal diagnostic, prognostic, and predictive biomarkers. Based on their ability to regulate several genes, their therapeutic potential should not come as a surprise. Another tempting feature of miRNA is its capability of regulating multiple genes and oncogenic pathways which gives it an advantage over therapeutics targeting a single oncogene ending up in a suboptimal response. 20
Small size, and trivial quantity in available samples makes miRNA detection a challenging task necessitating development of advances in miRNA extraction, amplification, and detection strategy. Moreover, miRNA differ from each other by only one or two nucleotides demanding precision in developing detection assays. Currently we are relying on conventional methods for miRNA detection. The earliest method used for miRNA detection was Northern blotting which is relatively time consuming and difficult to perform in samples having low abundance of miRNA making it less sensitive, However, it has far greater specificity in identifying miRNAs. Following this qRT-PCR emerged as most reliable and effective method used for miRNA profiling. It has a higher sensitivity and specificity and allows quantification of individual assay. However, normalization of miRNA expression by using suitable reference or housekeeper gene following qRT-PCR is mandatory. Microarrays and deep sequencing are another technique which allows profiling of numerous miRNAs (~1000 miRNAs) simultaneously using lesser amounts of total sample. It involves hybridization between samples and microarrays. These microarrays are provided with probes for each identified miRNA from the Sanger miRbase public database. A major limitation of this technique is that prior sequence information is required to synthesize the probes. Moreover, it is essential to use positive and negative control probes to aid normalization and provide absolute reference.21
Present study is designed to ascertain the effect of smoking and ST products use on miRNA-21 expression in patients with OSCC.
Methodology
This case control study was conducted at Department of Oncology, Ziauddin University, Karachi, Pakistan between 2013 to 2017. Institutional Review Board of Ziauddin University approved the project ERC#0410612NMPATH. Hundred cases and controls were recruited after obtaining informed written consent. Pathologically confirmed subjects with OSCC were included as cases and healthy age and sex matched individuals who were nonsmokers and did not consume ST products were included as controls. Existence of comorbidities including hypertension, diabetes and cardiac pathologies was considered as exclusion criteria. OpenEpi software was used to calculate a sample size. At a confidence level of 99 % and power of 95 %, a sample size was of 100 cases and 100 controls.
Detailed history, physical exam and chart review was conducted to document sociodemographic profile, tumor characteristics, grade, and stage of disease.
Sociodemographic variables registered included gender, age, habits of smoking, use of various ST including consumption of pan, chalia, naswar and Gutka. A smoker was defined as a subject who smoked cigarette, huka, pipe, cigar, or bidi daily for at least one month. A subject was labeled habitual chewer if he/she used any form of chewable product like pan, chalia, gutka, or naswar daily for at least one month. Frequency and duration of smoking and ST products was recorded.
The anatomical site of the tumor was categorized according to ICD-10 22 and stages were assigned according to American Joint Committee on Cancer (AJCC) staging criteria. 23 Tumor grading was done as defined by Broders’s classification.24
For nucleic acid extraction, kit was ordered from Favorgen. Total RNA was extracted as guided by protocols provided with the kit. The purified RNA was reverse transcribed immediately to cDNA by Revert Aid First Strand cDNA kit from Thermo scientific, USA. The first strand transcribed was used for further miRNA analysis using miRNA specific stem loop primers. miR-21 expression was checked on CFX 96 Real Time PCR machine from BIORAD using SYBR green PCR kit from Thermofisher to detect fluorescence. Detailed methodology including primer sequence and thermal profiles has been explained by Mahmood et al. 25.
For normalization of data, mir16 expression was used as endogenous control (Wei et al., 2011) and cel-mir-39 spike in was used as exogenous control. cel- mir-39 is spiked in as positive control before cDNA synthesis and it gives an estimate of reverse transcription efficiency.
Using SYBR green fluorescence quantitative PCR reagent kit, each sample was analyzed in duplicate and appropriate negative controls were included. The 13 µl PCR volume for amplification included 2µl of cDNA, 6. 5 µl of SYBR Green real-time PCR Master mix, 1.5 µl of primer and H2O 3µl. The reactions were incubated at 95°C for 3 min, followed by 40 cycles of 95°C for 5 s, 62°C for 35 s in which fluorescence was acquired. Rox was used to control for background noise on PCR program. At the end of the PCR cycles, melting curve were generated.
miR-21 expression was calculated as cycle threshold (Ct) on qT-PCR. Where Ct is the cycle number when fluorescence is first detected. The fold change of miR-21 was calculated utilizing the 2–△ △ Ct method.
Statistical analysis
SPSS software package SPSS (Version 24.0; SPSS Inc. Chicago, IL, USA) was used to conduct all statistical analysis. Chi square test was applied to compare nominal and ordinal variables between groups. Mean with standard deviation was derived for all continuous variables and comparison among groups was examined using Student’s t-test. One-way analysis of variance (ANOVA) followed by post hoc Tukey test was used to compare symmetric continuous variables among groups. Significance was set at P-value of <0.05.
Results
Two hundred subjects were studied including one hundred cases and 100 controls. Mean age at presentation was not significantly different between cases (45.12± 12.42 years) and controls (44.22± 12.27 years), p=0.530.
Among cases, 36 were below 40 years of age and 64 were above 40. A higher percentage of males was recorded among cases (69% vs.31%, p = 0.003). Proportion of males was reported to be higher (4.2:1) among subjects less than 40 as compared to patients older than 40 (1.6:1).
Educational level was grouped as illiterate, primary and secondary education or above. In our participants, one was illiterate, 24 had primary level of education and 75 had secondary or above level of education. ST products use and the clinico-pathological variables of cases is described in Table 1.
Influence of various habits on studied markers.
Smoking cigarettes was reported by 63% of patients whereas 37% denied smoking. 85% subjects were habituated to use chewing preparations with a major number consuming more than one chewable product. On an average, smokers were consuming 16.6± 5.6 cigarettes a day for an average of 16.7± 6.5 years.
Subjects consuming various ST products were using an average of 9.1± 3.8 times a day for 28.7± 10.8 years. Figure 1 shows heat map generated showing frequency per day and duration of cigarette smoking and chewing formulations for all cases.
Association of miR-21 with smoking and chewing habits in OSCC
To investigate association of smoking with miR-21, mean and standard deviation was calculated and comparison made using Student’s t-test. To explore the association of various chewing habits with miR-21, mean and standard deviation for each habit was calculated and analyzed via one-way ANOVA. Our results showed a significant difference in miR-21 expression among groups, p<0.001 as shown in Table 2. Post hoc Tukey test confirmed a higher expression of miR-21 in subjects addicted to gutka chewing and those using more than one chewable product compared to other groups as shown in Figure 2.
Pearson’s correlation was calculated to compare correlation between miR-21 and frequency and duration of smoking and various chewing habits. A positive correlation between miR-21 expression and amount of smoking was witnessed, r2= 0.375, p<0. 001.Increasing amount of ST use was linked with increasing miR-21 expression, r=0.650, p<0.001.
Receiver operating characteristic (ROC) curve was generated through SPSS. Discriminating potential of miR-21 for ST use was analyzed and it gave area under the curve of 0.80, p<0.001 suggesting potential of miR-21 to discriminate ST users from non-ST users as shown in Figure 3.
Table 1: Association of ST use with clinico-pathological variables of OSCC.
|
Characteristic |
Smokeless Tobacco use | P value | ||
| Yes | No | |||
| Age (years) | 45.79 ± 11.8 | 42.82 ±11.9 | 0.202 | |
| Gender | Male | 58 | 11 | 0.475 |
| Female | 27 | 4 | ||
| Tumor size | T1 & T2 | 39 | 11 |
0.05 |
| T3 & T4 | 46 | 4 | ||
| Nodal involvement | Yes | 81 | 12 | 0.032 |
| No | 4 | 3 | ||
| Metastasis | Yes | 5 | 2 | 0.297 |
| No | 4 | 3 | ||
N: number of cases, T1&T2: tumor size ≤ 4cm, T3 &T4: tumor size> 4cm
Table 2: Association of smoking and smokeless tobacco use with miR-21 expression.
| N (%) | miR-21
Mean ±SD |
P value | |
| Smoking Habits | |||
| Smoker | 63 | 29.8 ± 4.8 | <0.001** |
| Non-Smoker | 137 | 33.1 ±4.9 | |
| Smokeless Tobacco Use | |||
| Chewers | 85 | 29.2± 5.0 | <0.001** |
| Non chewers | 115 | 34.2± 3.9 | |
N: number of cases, ** p<0.001, a low miR-21 level suggest higher expression.
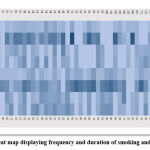 |
Figure 1: Heat map displaying frequency and duration of smoking and chewing. |
Heat map generated for frequency and duration of smoking and chewing. Color codes denotes variables presented in rows. Color scale from light to dark blue represents increasing number as the intensity of color increases.
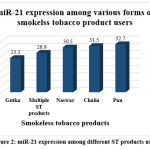 |
Figure 2: miR-21 expression among different ST products users. |
**; p<0.001, Ct: Cycle threshold, a low miR-21 level suggest higher expression
 |
Figure 3: Receiver operating characteristic (ROC) curve analysis for discriminating ST users from non-users based on miR-21 expression. |
Discussion
Consumption of ST is a common practice in south Asia and a wealth of literature can be extracted to confirm how it has contributed to high incidence and prevalence of OSCC in this region. To our knowledge no research has been conducted in Pakistan to explore the effect of smoking and ST on miR-21 expression in OSCC either on tissue biopsy or liquid biopsy. In present study we investigated upregulation of miR-21 among OSCC cases and subsequently explored its association with smoking and use of various ST products. Our results indicated miR-21 upregulation in patients as compared to normal healthy age and sex matched controls. These findings are consistent with findings of previous reports. 26,27,28
We further reported that smokers and ST products users had miR-21 upregulation. Among various chewable products, gutka consumption and use of more than one product had the most significant association with miR-21. Gutka is known to have numerous pro-carcinogens.19 Possibly such pro-carcinogenic ingredients of gutka turn on miR-21, which is an oncogenic miRNA, hence propagating the carcinogenic cascade.
Like our findings Sing et al observed an upregulation of miR-21 in OSCC patients. However, in contrast to our study they compared OSCC cases to both premalignant cancers and healthy controls and observed higher expression in OSCC compared to premalignant lesions suggesting a potential role in early diagnosis. However, a small sample size of only 20 OSCC cases, 20 premalignant lesions and 40 healthy controls limits the strength of study.26
Similar results were reported by Ren et al, who investigated circulating miR-21 in a set of 90 subjects including 58 OSCC patients and 32 healthy volunteers. They observed significant over expression in patients compared to controls. They further compared tissue expression in 10 patients having highest miR-21 expression and observed a positive correlation between tissue and circulating miR-21. This step further validated that circulating miR-21 mirrors tissue expression and can be used as less invasive alternative source of biomarker .29.
Zahran et al investigated miR-21 expression in saliva of patients with OSCC. In a cohort of 100 subjects including 20 each OSCC, premalignant lesions with dysplastic changes, premalignant lesions without dysplastic changes, recurrent aphthous ulcers and healthy controls, a significantly high expression was seen in OSCC and premalignant lesions with dysplastic changes.30An upregulation of miR-21 in graded manner clearly explains its role in oral carcinogenesis. It is further interesting to note that a recent study found a direct link between miR-21 and BCL2 anti-apoptotic gene. It was experimentally validated that miR-21 upregulation led to suppression of BCL2. Upregulation of BCL2 led to inhibition of miR-21, an effect synergistic with antagomir -21.31 This provides a new therapeutic approach towards cancer treatment and is expected to improve survival by hitting the right target.
It can be explained by the fact that Yan et al found upregulation of miR-21 in oral submucosal fibrosis tissue as compared to normal tissue. They further studied role of arecoline, found in areca nut which is most widely consumed smokeless tobacco product on miR-21 expression in Buccal mucosal fibroblasts. Exposure of buccal mucosal fibroblasts to increasing concentrations of arecoline led to a significant upregulation in miR-21. The upregulation in miR-21 was linked to TGF-β activation which was experimentally validated. To confirm their findings, they used miR-21 inhibitors and observed arecoline induced myofibroblast activation was abolished. Induction of fibrogenic response by miR-21 has already been established by many studies. It has been shown to facilitate extracellular matrix deposition. It may be concluded that miR-21 acts synergistically with arecoline to upregulate fibroblastic activity and collagen deposition.32 Myofibroblasts have been experimentally validated to promotes matrix invasion, perineural invasion, muscular invasion, and trans-endothelial migration.33 The fact that antagomir-21 was able to eliminate myofibroblast activation is very satisfying and paves a path for researchers to validate and implement this finding as a step towards targeted therapy.
Bhat et al investigated effect of cigarette smoke and smokeless tobacco extract on immortalized keratinocytes. They observed that such exposure led to increased cell scattering an important feature of tumor cells. Moreover, it was observed that these treated cells displayed differential expression of set of novel miRNAs suggesting the effect of tobacco is mediated via miRNAs dysregulation and this synergistic effect turned out to be a recipe of making cells malignant.34
We also observed that magnitude of smoking led to upregulation of miR-21. Likewise, Ali et al studied salivary miR-21 level in a group of 40 patients including 20 smokers and 20 nonsmokers and they concluded an upregulation of miR-21 among smokers.35.
Emerging miRNA therapeutics are paving the way as a new promise for cancer treatment that remain intangible yet powerful tool. Introduction of anti-miR-21 antagomir based therapeutics as personalized medicine and monitoring response is expected to step forward towards personalized treatment in OSCC.
Conclusion
In the present study we could naked a positive correlation of miR-21 expression with amount of smoking and ST. A significant up regulation of miR-21 was seen among gutka users and subjects using more than one ST product. Additional large-scale studies are expected to validate the existing data.
Limitations
Study collected data from oncology department of single center in Karachi. The type of ST products used in different regions of Pakistan is different where Pan and gutka are commonly used in Karachi whereas naswar is a popular product in Khyber Pakhtunkhwa. Hence, multi-centric data from all regions is likely to give more precise picture. Moreover, all smokers and ST product users had OSCC hence including a group of smokers and ST product users free from any premalignant or malignant disease would give a more scrupulous association between miR-21 and OSCC.
Acknowledgments
We would like to acknowledge Dr Javed Malik Consultant Oncologist Ziauddin Hospital for his support in recruiting patients and allowing access to patient files and records
Conflict of Interest
There is no conflict interest.
Funding Source
Ziauddin University funded the project, grant number is 0410612NMPATH.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394-424.
CrossRef - International Agency for Research on Cancer, World Health Organization [Internet] Available from: https://gco.iarc.fr/today/data/factsheets/ populations/586-pakistan-fact-sheets.pdf (2020), Accessed 25th May 2022
- International Agency for Research on Cancer, World Health Organization [Internet] Available from: https://gco.iarc.fr/today/data/factsheets/populations/ 586-pakistan-fact-sheets.pdf (2018), Accessed 25th May 2022
- Le Campion ACOV, Ribeiro CMB, Luiz RR, da Silva Júnior FF, Barros HCS, Dos Santos KCB, Ferreira SJ, Gonçalves LS, Ferreira SMS. Low survival rates of oral and oropharyngeal squamous cell carcinoma. Int J Dent. 2017; 2017:5815493. doi: 10.1155/2017/5815493. Epub 2017 May 30. PMID: 28638410; PMCID: PMC5468590.
CrossRef - Seo BY, Lee C O, Kim JW. Changes in the management and survival rates of patients with OSCC: a 30-year single-institution study. J Korean Assoc Oral Maxillofac Surg. 2016., 42(1): 31–37. doi: 10.5125/jkaoms.2016.42.1.31. Epub 2016 Feb 15. PMID: 26904492; PMCID: PMC4761570.
CrossRef - Stefanuto P., Doucet J.C., Robertson C. Delays in treatment of oral cancer: A review of the current literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014; 117(4):424–429. doi: 10.1016/j.oooo.2013.12.407.
CrossRef - Jitender S, Sarika G, Varada H, Omprakash Y, Mohsin K. Screening for oral cancer. J Exp Ther Oncol 2016 Nov;11(4):303–307. PMID: 27849341.
- Mahmood N, Hanif M, Ahmed A, Jamal Q, Saqib, Khan A. Impact of age at diagnosis on clinicopathological outcomes of oral squamous cell carcinoma patients. Pak J Med Sci. 2018 May-Jun;34(3):595-599. doi: 10.12669/pjms.343.14086. PMID: 30034422; PMCID: PMC6041552
CrossRef - Güneri P, Epstein JB. Late stage diagnosis of oral cancer: components and possible solutions. Oral Oncol. 2014 Dec;50(12):1131-6. doi: 10.1016/j.oraloncology.2014.09.005. Epub 2014 Sep 23. PMID: 25255960.
CrossRef - Grafton-Clarke C, Chen KW, Wilcock J. Diagnosis and referral delays in primary care for oral squamous cell cancer: a systematic review. Br J Gen Pract. 2019;69: e112–e126. doi: 10.3399/bjgp18X700205.
CrossRef - Azeem N, Sarfraz Z, Sarfraz A, Hange N, Sarfraz M, Cherrez-Ojeda I. Vaping, and smokeless tobacco control in South Asia: A policy review. Ann Med Surg (Lond). 2022 Aug 1; 81:104285. doi: 10.1016/j.amsu.2022.104285. PMID: 36147071; PMCID: PMC9486430.
CrossRef - Asthana S, Labani S, Kailash U, Sinha DN, Mehrotra R. Association of smokeless tobacco use and oral cancer: a systematic global review and meta-analysis. Nicotine Tob Res. 2019 Aug 19;21(9):1162-1171. doi: 10.1093/ntr/nty074. PMID: 29790998.
CrossRef - Ahmad F, Javaid A, Khan Z. A Smokeless tobacco control in Pakistan. J Postgrad Med Inst 2020; 34(3): 139-41.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Smokeless Tobacco and Some Tobacco-specific N-nitrosamines. Geneva, Switzerland: World Health Organization; 2007.
- Dwivedi S, Goel A, Khattri S, Mandhani A, Sharma P, Pant KK. Tobacco exposure by various modes may alter proinflammatory (IL-12) and anti-inflammatory (IL-10) levels and affects the survival of prostate carcinoma patients: An explorative study in North Indian population. Biomed Res Int. 2014; 2014:158530
CrossRef - Nair U, Bartsch H, Nair J. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: A review of agents and causative mechanisms. Mutagenesis. 2004 July;19(4):251–62. doi: 10.1093/mutage/geh036. PMID: 15215323.
CrossRef - Bhisey RA. Chemistry and toxicology of smokeless tobacco. Indian J Cancer. Indian J Cancer. 2012 Oct-Dec;49(4):364-72. doi: 10.4103/0019-509X.107735. PMID: 23442400.
CrossRef - Sankhla B, Kachhwaha K, Hussain SY, Saxena S, Sireesha SK, Bhargava A. Genotoxic and Carcinogenic Effect of Gutkha: A Fast-growing Smokeless Tobacco. Addict Health. 2018 Jan;10(1):52-63. doi: 10.22122/ahj. v10i1.537. PMID: 30627385; PMCID: PMC6312563.
- Aghiorghiesei O, Zanoaga O, Nutu A, Braicu C, Campian RS, Lucaciu O, Berindan Neagoe I. The world of oral cancer and its risk factors viewed from the aspect of microRNA expression patterns. Genes (Basel). 2022 Mar 26;13(4):594. doi: 10.3390/genes13040594. PMID: 35456400; PMCID: PMC9027895.
CrossRef - O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018 Aug 3;9:402. doi: 10.3389/fendo.2018.00402. PMID: 30123182; PMCID: PMC6085463.
CrossRef - Dave VP, Ngo TA, Pernestig AK, Tilevik D, Kant K, Nguyen T, Wolff A, Bang DD. MicroRNA amplification and detection technologies: opportunities and challenges for point of care diagnostics. Lab Invest. 2019 Apr;99(4):452-469. doi: 10.1038/s41374-018-0143-3. Epub 2018 Dec 12. PMID: 30542067.
CrossRef - ICD 10 Data.com. Available from: https://www.icd10data.com/ICD10CM/Codes/C00-D49/C00-C14. Accessed 20 May 2022
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010 Jun;17(6):1471-4. doi: 10.1245/s10434-010-0985-4. PMID: 20180029.
CrossRef - Vijayakumar G, Sharma G, Narwal A, Kamboj M. Broder versus Bryne’s histologic grading parameters on incision biopsy specimens: A comparative study with P53 and KI67 expression. J Oral Maxillofac Pathol. 2021 Jan-Apr;25(1):55-60. doi: 10.4103/jomfp.JOMFP_328_20. Epub 2021 May 14. PMID: 34349412; PMCID: PMC8272518.
CrossRef - Mahmood N, Hanif M, Ahmed A, Jamal Q, Mushtaq S, Khan A, Saqib M. Circulating miR-21 as a prognostic and predictive biomarker in oral squamous cell carcinoma. Pak J Med Sci. 2019 Sep-Oct;35(5):1408-1412. doi: 10.12669/pjms.35.5.331. PMID: 31489016; PMCID: PMC6717445.
CrossRef - Singh P, Srivastava AN, Sharma R, Mateen S, Shukla B, Singh A, Chandel S. Circulating microRNA-21 expression as a novel serum biomarker for oral sub-mucous fibrosis and oral squamous cell carcinoma. Asian Pac J Cancer Prev. 2018 Apr 27;19(4):1053-1057. doi: 10.22034/APJCP.2018.19.4.1053. PMID: 29699056; PMCID: PMC6031776.
- Arantes LM, Laus AC, Melendez ME, de Carvalho AC, Sorroche BP, De Marchi PR, Evangelista AF, Scapulatempo-Neto C, de Souza Viana L, Carvalho AL. MiR-21 as prognostic biomarker in head and neck squamous cell carcinoma patients undergoing an organ preservation protocol. Oncotarget. 2017;8(6):9911-9921. doi: 10.18632/oncotarget.14253. PMID: 28039483; PMCID: PMC5354780.
CrossRef - Hedbäck N, Jensen DH, Specht L, Fiehn AM, Therkildsen MH, Friis-Hansen L, Dabelsteen E, von Buchwald C. MiR-21 expression in the tumor stroma of oral squamous cell carcinoma: an independent biomarker of disease-free survival. PLoS one. 2014 Apr 22;9(4): e95193. doi: 10.1371/journal.pone.0095193. PMID: 24755828; PMCID: PMC3995812.
CrossRef - Ren W, Qiang C, Gao L, Li SM, Zhang LM, Wang XL, Dong JW, Chen C, Liu CY, Zhi KQ. Circulating miRNAs-21 (MIR-21) and phosphatase and tensin homolog (PTEN) are promising novel biomarkers for detection of oral squamous cell carcinoma. Biomarkers. 2014 Nov 1;19(7):590-6. doi: 10.3109/1354750X.2014.955059. Epub 2014 Sep 1. PMID: 25174622.
CrossRef - Zahran F, Ghalwash D, Shaker O, Al‐Johani K, Scully C. Salivary microRNAs in OSCC. Oral Dis. 2015 Sep;21(6):739-47. doi: 10.1111/odi.12340. Epub 2015 Apr 22. PMID: 25784212.
CrossRef - Ge J, Yao Y, Jia H, Li P, Sun W. Inhibition of miR-21 ameliorates LPS-induced acute lung injury through increasing B cell lymphoma-2 expression. Innate Immun. 2020 Nov;26(8):693-702. doi: 10.1177/1753425920942574. Epub 2020 Jul 29. PMID: 32727244; PMCID: PMC7787552.
CrossRef - Yang HW, Yu CC, Hsieh PL, Liao YW, Chu PM, Yu CH, Fang CY. Arecoline enhances miR-21 to promote buccal mucosal fibroblasts activation. J Formos Med Assoc. 2021 Apr;120(4):1108-1113. doi: 10.1016/j.jfma.2020.10.019. Epub 2020 Nov 13. PMID: 33191095.
CrossRef - Sekhon HK, Sircar K, Kaur G, Marwah M. Evaluation of Role of Myofibroblasts in Oral Cancer: A Systematic Review. Int J Clin Pediatr Dent. 2016 Jul-Sep;9(3):233-239. doi: 10.5005/jp-journals-10005-1370. Epub 2016 Sep 27. PMID: 27843256; PMCID: PMC5086012.
CrossRef - Bhat MY, Advani J, Rajagopalan P, Patel K, Nanjappa V, Solanki HS, Patil AH, Bhat FA, Mathur PP, Nair B, Prasad TSK, Califano JA, Sidransky D, Gowda H, Chatterjee A. Cigarette smoke and chewing tobacco alter expression of different sets of miRNAs in oral keratinocytes. Sci Rep. 2018 May 4;8(1):7040. doi: 10.1038/s41598-018-25498-2. PMID: 29728663; PMCID: PMC5935709.
CrossRef - Ali S, Amer E. Evaluation of the salivary level of mir-21 in cigarette smokers: case-control study. Egyptian Dental Journal. 2017 April 63(2) (Oral Medicine, X-Ray, Oral Biology & Oral Pathology):1507-1512. doi: 10.21608/edj.2017.74547
CrossRef







