Manuscript accepted on :31-01-2023
Published online on: 10-03-2023
Plagiarism Check: Yes
Reviewed by: Dr. Raja Azman Raja Awang , Dr. Loai Aljerf
Second Review by: Dr. Nagham Aljamali
Final Approval by: Dr. Jihan Seid Hussein
Windingoudi Rimwagna Christian Ouedraogo1,2* , Lazare Belemnaba1
, Lazare Belemnaba1 , Mathieu Nitiema1
, Mathieu Nitiema1 , Boukaré Kabore3
, Boukaré Kabore3 , Noufou Ouedraogo1
, Noufou Ouedraogo1 , Moumouni Koala1
, Moumouni Koala1 , Rasmané Semde2
, Rasmané Semde2 , Sylvin Ouedraogo1
, Sylvin Ouedraogo1
1Laboratoire de Recherche-Développement de Phytomédicaments et Médicaments-Département Médecine et Pharmacopée Traditionnelles - Pharmacie (MEPHATRA-PH), Institut de Recherche en Sciences de la Santé/Centre National de la Recherche Scientifique et Technologique (IRSS/CNRST), 03 BP 7047, Ouagadougou 03, Burkina Faso
2Centre de Formation, de Recherche et d’Expertises en Sciences du Médicament (CEA-CFOREM) / École Doctorale des Sciences de la Santé (ED2S), Université Joseph KI-ZERBO, 03 BP 7021, Ouagadougou 03, Burkina Faso
3Laboratoire de Chimie Organique et de Physique Appliquée (LCOPA) / École Doctorale Sciences et Technologie, Université Joseph KI-ZERBO, 03 BP 7021 Ouagadougou 03, Burkina Faso.
Corresponding Author E-mail: ouedrock@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2635
Abstract
Introduction: Oxidative stress, through the increased bioavailability of reactive oxygen species (ROS), is a major cause of hypertension. The resulting endothelial remodeling promotes the production of vasoconstrictor substances leading to an increase in blood pressure. This study aimed to evaluate the antioxidant and vasorelaxant properties of the decoction (PAD), ethyl acetate (EAP), and residual aqueous (ARP) fractions of immature Phaseolus vulgaris pods on NMRI mice thoracic aorta rings. Methods: Phytochemical screening was performed by high-performance thin-layer chromatography. Folin-Ciocalteu and aluminum trichloride colorimetric methods were used to quantify total polyphenol compounds (TPC) and total flavonoids (TFC), respectively. Antioxidant activities of the extracts were determined by 2,29-azinobis-3-ethylbenzothiazoline-6-sulfonic (ABTS●), 2,2-Diphenyl-1-picrylhydrazyl (DPPH●), and ferric ion (FRAP) radical reduction methods. The DMT 620M ADInstruments myograph technique was used to evaluate the ex-vivo vasodilatory effects of Phaseolus vulgaris extracts on aortic rings. Results: Chromatographic fingerprints showed the presence of flavonoids, coumarins, tannins, steroids, triterpenes, and saponins in the extracts studied. The TPC (61.07±0.04 mgGAE/g) and TFC (6.16±0.03 mgQE/g) of EAP were statistically significant compared to that of PAD and ARP (p<0.001). The antioxidant power of Trolox was statistically significant compared to all studied extracts (p<0.05). It should be noted that among these extracts, EAP showed the better antiradical capacities for ABTS (IC50=71.87±0.30 µg/mL) and DPPH (IC50=9.93±0.00 µg/mL). However, for FRAP activity, it was the PAD extract (T=170.68±0.11 µgAAE/g) that obtained the best score. In terms of vasorelaxant activity, all extracts induced concentration-dependent relaxation of aortic rings precontracted with U46619. Pharmacodynamic parameters were significantly in favor of EAP [EAP(E+)=(Emax=100.06±0.00%; pD2=1.24±0.01) and EAP(E-)=(Emax=101.01±0.00%; pD2=0.84±0.02)] followed by PAD and then ARP. Conclusion: Phaseolus vulgaris immature pod extracts possess concentration-dependent vasorelaxant effects on isolated mouse aorta. These preliminaries results were scientific evidence to support the use of this plant in traditional and complementary medicine for the treatment of hypertension.
Keywords
Antioxydant; Hypertension; Myography; Phaseolus vulgaris; Phytochemical screening; Vasorelaxation
Download this article as:| Copy the following to cite this article: Ouedraogo W. R. C, Belemnaba L, Nitiema M, Kabore B, Ouedraogo N, Koala M, Semde R, Ouedraogo S. Antioxidant and Vasorelaxant Properties of Phaseolus vulgaris Linn (Fabaceae) Immature Pods Extract on the Thoracic Aorta of NMRI Mice. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Ouedraogo W. R. C, Belemnaba L, Nitiema M, Kabore B, Ouedraogo N, Koala M, Semde R, Ouedraogo S. Antioxidant and Vasorelaxant Properties of Phaseolus vulgaris Linn (Fabaceae) Immature Pods Extract on the Thoracic Aorta of NMRI Mice. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3l09izH |
Introduction
Cardiovascular diseases are responsible for nearly one-third of the world’s mortality and complications of high blood pressure alone accounting for 9.4 million deaths per year 1. This pathology is a serious medical problem that significantly increases the risk of cardiovascular and cerebral events, renal failure, and other related organ dysfunctions 1,2. Numerous investigations into the pathophysiology of hypertension have revealed that it is strongly correlated with oxidative stress 3,4. Indeed, reactive oxygen species (ROS) generated during oxidative stress are a major factor in the development of vascular endothelial dysfunction 5,6. Beyond being a simple barrier, the vascular endothelium plays an important endocrine role in maintaining the homeostasis of vasodilator and vasoconstrictor substances 7. It regulates vascular tone through the production of chemical mediators, the most abundant of which is nitric oxide (NO•) 8. Among antihypertensive drugs, there are vasodilators, diuretics, angiotensin II-converting enzyme inhibitors/angiotensin AT1 receptor blockers and calcium channel blockers , etc9–11. Vasodilators are of great interest because of their high selectivity and long-term efficacy even in the presence of coronary and/or cardiac failure. The pharmacological properties mentioned above give vasodilators an essential role in the treatment of hypertension and its complications9,11,12. Despite the plethora of existing therapeutic means, the adverse effects and inaccessibility of antihypertensive drugs are significantly notable 13,14. In front of these therapeutic limitations, the exploration of new therapeutic agents using medicinal plants is topical in the management of this pathology 15,16. In fact, many studies have shown the vasodilating properties of plant extracts, notably Anogeissus leiocarpa, Lannea microcarpa, Moringa oleifera, and Odontonema strictum 16–19. Thus, this alternative medicine is meant to be integrative and complementary. Medicinal herbs, long used for culinary and medicinal purposes, can be considered potential drug for the prevention or treatment of some pathologies, including hypertension. Phytochemical antioxidant molecules derived from Moringa oleifera have shown protective clinical effects against heart damage and vascular endothelial dysfunction20. This represents a therapeutic opportunity like vitamin C or ascorbic acid has been widely prescribed to improve the condition of patients with COVID19 and as a supplement for the prevention of comorbidities21. Previous studies have shown the benefits of beans on human and animal health through the nutritional, anti-inflammatory, and antioxidant properties of Phaseolus vulgaris seeds but none reported on the vasodilatory properties of the extracts studied 22–25. In addition, this article investigated the antiradical capacities and the phytochemical profile of the studied extracts. In Burkina Faso, ethnobotanical data have shown that immature pods commonly called “green beans” are used to decrease blood pressure in hypertensive patients 26. Thus, this study aimed to evaluate the vasorelaxant effects of immature Phaseolus vulgaris pods on the aortas of NMRI mice.
Material and Methods
Collection of plant material
Immature pods of Phaseolus vulgaris (P. vulgaris) were collected in 2020 at Loumbila in Burkina Faso (North 12°52’88.1’’ and West 14°35’07.1’’). An herbarium was made and authenticated by a botanist and deposited at the Departement of plants biology of the Université Joseph KI-ZERBO under the number 18018. The harvested plant material was treated, dried in a suitable room, and protected from sunlight and dust. Once dried, the leafy stems were ground with a mechanical grinder to obtain a dry powder (fig.1).
 |
Figure 1: Immatures pods and dry powder of Phaseolus vulgaris. |
Experimental animals
Male and female mice with average weights of 23.10±2.05 g and 21.22±3.12 g, respectively, from the pet Shopof the Institut de Recherche en Sciences de la Santé/Centre National de la Recherche Scientifique et Technologique (IRSS/CNRST) were used. The animals were housed in plastic cages with free access to water and standard laboratory pellet enriched with proteins (29%). The animals were placed in an enclosure at a temperature of 25±2°C with a relative humidity of 50-70% and subjected to a cycle of 12 h of light/darkness according to the rearing conditions of this species27. All the experiments were carried out following the procedures of the Guide of Good Practices in Animal Experimentation under the Declaration of Helsinki28.
Chemical reagents
The following reagents were used : Neu’s reagent, ferric chloride, Liberman-Buchard, sulfuric anisaldehyde, potassium hydroxide, Folin Ciocalteu Reagent (FCR), sodium carbonate, gallic acid, aluminum trichloride, quercetin, 2,29-azinobis-3-ethylbenzothiazoline-6-sulfonic, Potassium persulfate, Trolox, ascorbic acid, 2,2-Diphenyl-1-picrylhydrazyl, potassium hexacyanoferrate, trichloroacetic acid, ferric chloride, Krebs-Henseleit, ketamine, 9,11-dideoxy-11α,9α epoxymethanoprostaglandin F2α (U46619) and potassium chloride. All these reagents were from Sigma Aldrich, France.
Organoleptic characteristics
The organoleptic characteristics of the used P. vulgaris powder were determined from the sense organs eye, nose, and tongue according to the European Pharmacopoeia 9.0 recommandations.
Residual moisture content
A thermogravimetric method’s validated by IRSS was used to determine the residual moisture content of the plant material. Briefly, three test samples of the powder of 1 g (initial weight) each were placed in different watch glasses and then heated to 105 °C for 3 hours in an oven (MEMMERT brand). After cooling, the final weight of the plant powder was recorded. The residual moisture content (RMC) was determined according to the formula :
RMC (%) = [Pi – (Pf /Pi)] *100; with Pi = sample initial weight and Pf= sample final weight
Preparation of the extracts
Aqueous decoction
500 mL of distilled water was added to a flask containing 50 g of the dry powder of P. vulgaris pods. Then, the mixture was boiled for 30 min according to the traditional preparation method. A centrifugation step (10 000 rpm for 5 min) was carried out after filtration of the mixture. The clear aqueous decoction was freeze-dried with a CHRIST® Type ALPHA 1-2 freeze-dryer (BIOBLOCK SCIENTIFIC) equipped with a pump (ROTARY VANE VACUUM PUMP Type RZ2 series 21525419). Labeled as PAD, the resulting lyophilized was stored in an anti-adsorbent packaging against humidity.
Fractionation
To a volume of 250 mL of the obtained aqueous decoction, dichloromethane (3×150 mL) was added successively in an ampoule and left decanted. The organic phase (dichloromethane fraction) was recovered, oven-dried, and stored for further studies. The residual aqueous phase was then taken in ethyl acetate (3×150 mL). After decantation, the organic phase was concentrated and then dried to give the ethyl acetate fraction of P. vulgaris (EAP). The second residual aqueous phase was recovered, concentrated in Rotavapor, and then dried understudy to give the aqueous residual fraction of P. vulgaris (ARP).
Phytochemical screening
High-performance thin-layer chromatography
The High-performance thin-layer chromatography (HPTLC) was performed as previously described 29.
Applications: A concentration of 10 mg/mL of each extract (w/v) was prepared in distilled water/methanol (50:50; v/v). After filtration with a 0.2 µm millipore membrane, a quantity of 20 µL was deposited on silica gel plates type HPTLC 60F254 (glass holder 20 cm x 10 cm from Merck, Darmstadt, Germany). The semi-automatic Linomat 5 applicator (CAMAG® Muttenz, Switzerland) controlled by the visionCATS essential software (CAMAG® HPTLC) was used for the strip deposition. The quercetin, coumarin, and tannic acid were used as reference for flavonoids, coumarins, and tannins respectively.
Migration: After deposition and drying of extracts on 60F254 plates, a CAMAG-type vessel was used as a migration chamber. This tank was previously saturated with a specific eluent according to the secondary metabolite to be investigated. Afterward, the plates were placed in the said tank. Chromatograms were developed on a path of 8 cm. A mobile phase ethyl acetate (100)-formic acid (11)-acetic acid (11) and water (26) (v/v/v) was used for the migration of flavonoids, tannins, coumarins, and saponins. A mixture of toluene/ethyl acetate (93:7; v/v) was used for the migration of sterols and triterpenes. After migration, all plates were dried at 110 °C on a hot plate (ThermoFischer®) for 2 min. Chromatographic profiles were observed under visible light and at UV wavelengths of 254 nm and 366 nm in a photographic CAMAG chamber.
Revelation of secondary metabolites : Neu’s reagent and ferric chloride (5%) were used as developers for flavonoids and tannins respectively. The Liberman-Buchard reagent was used for the revelation of sterols and triterpenes. In addition, sulfuric anisaldehyde was used for the revelation of steroidal and triterpene saponins. Potassium hydroxide (KOH at 2%) was used to highlight coumarins under UV 366 nm light.
Estimation of total phenolics compound
Quantification of total phenolics compound (TPC) was performed according to the colorimetric method of Folin-Ciocalteu 30 with slight modifications. Typically, 1 g/mL primary solution was first prepared for each extract. Then, a first mixture, consisting to 1 mL of the primary solution and 1 mL of the Folin Ciocalteu reagent, was made up and incubated for 10 min at a temperature of 105 °C. After that, a volume of 2 mL of sodium carbonate (7.5%) was added to obtain the second mixture which was incubated at room temperature for 30 min. At this time end, absorbances were read using a UV visible spectrophotometer at 760 nm (model-UV-1800, UV- spectrophotometer, SHIMADZU-Japan). A cascade dilution of the primary solution was used as a blank control. The phenolic compounds content was calculated using the gallic acid equation used as a reference (Y=10.46X+0.03; r2=0.99). Thus:
TPC = (A-b) x FD x V / (a x m) (1) ;
with: A = absorbance; FD = diluation factor; b = origin ordered; a= pente; V = extract volume (liter); m = Extract weight (gramm).
Total flavonoids compound estimation
The estimation of total flavonoids coumpounds (TFC) was performed following the aluminum trichloride colorimetric method reported previously 31. Briefly, 1 g/mL primary solution was prepared for each extract, and quercetin was used as a reference. Indeed, a hemolysis tube containing 1 mL of the primary solution and 1 mL of a 2% AlCl3 solution was incubated for 10 min in the dark, and then the absorbance was read at 415 nm. A blank was prepared under similar conditions using methanol as the sample. The flavonoid content was calculated by reporting the absorbance in the quercetin equation: Y=10.43X-0.11 with r2=0.98 by applying the formula (1).
Antioxidant activities
ABTS (2,29-azinobis-3-ethylbenzothiazoline-6-sulfonic) radical scavenging activity assay
The test was carried out according to the principle that a hydrogen-donating antioxidant can reduce the 2,29-azinobis-3-ethylbenzothiazoline-6-sulfonic radical (ABTS●) generated by the oxidation of ABTS with potassium persulfate 32. The reagent consisted of ABTS stock (7 mM) and 2.45 mM potassium persulfate allowed to stand for 12 h at room temperature in the dark. Trolox (Hoffman-La Roche) (6-hydroxy-2,5,7,8-tetramethychroman-2-carboxylic acid; Aldrich Chemical Co., Gillingham, Dorset, UK) and ascorbic acid (Sigma Aldrich, St. Louis, MO, USA) were used as positive controls. The reaction mixture was made up of 0.2 mL of each diluted extract solution and 02 mL of ABTS stock diluted in ethanol. A negative control (reagent blank) was made up using ethanol. After an incubation phase of 30 min in the dark, the absorbance was read at 734 nm with a spectrophotometer (model-UV-1800, UV-SPECTROPHOTOMETER, SHIMADZU-JAPAN). The percentage of inhibition was calculated as follows:
Inhibition (%) = [(Ablank – Asample) /Ablank]x100 (2) with A = Absorbance
Successive dilutions (1/2;1/4;1/8;1/16;1/32 and 1/64) were performed with the extract, Trolox, and ascorbic acid (01 mg/mL) to progressively follow the reduction of ABTS by the extract. This operation allowed the calculation of the inhibitory concentration of 50% (IC50) of the extracts and positive controls. The antioxidant contents of the extracts were calculated using the equations of the calibration line of Trolox (Y = -13.91X+1.31 with r2=0.99) and ascorbic acid (Y = -10.29X+1.35 with r2=0.98), respectively, by applying the formula (1).
DPPH (2,2-Diphenyl-1-picrylhydrazyl) radical scavenging activity assay
The DPPH scavenging activity was performed according to the method described in the literature with minor modifications (Brand-Williams, Cuvelier, and Berset 1995; Nenadis and Tsimidou 2017). The DPPH● radical is a stable, methanol-soluble, dark purple molecule with maximum absorption at 515 nm33. For this purpose, solutions were prepared in methanol, one of the extracts (01 mg/mL) and the other of DPPH (100 µg/mL). Successive increasing dilutions (1/2;1/4;1/8;1/16;1/32 and 1/64) were performed. The reaction mixture was made up of 0.5 mL of the extract and 02 mL of DPPH. Methanol was considered as the negative control (blank). Trolox and ascorbic acid were used as positive controls. Absorbances were measured at 517 nm with a spectrophotometer (model UV-1800 240V, UV-SPECTROPHOTOMETER, SHIMADZU-JAPAN), after incubation for 30 min in the dark. The percentage of inhibition was obtained by applying the formula (2). The inhibitory concentration of 50% (IC50) of each extract as well as the references were determined. In addition, the antioxidant content of each extract was calculated by applying the previously established formula (1).
Ferric reducing assay power
The ferric ion reducing the power (FRAP) of the extracts was assessed following the previously established protocol with slight modifications 34. Briefly, volumes of 1.25 mL of phosphate buffer (pH=6.6) and potassium hexacyanoferrate were introduced into 0.5 mL of the initial solution (1 mg/mL). After incubation for 30 min at 50°C in a water bath, 1.25 mL of trichloroacetic acid (10%) was added and then centrifuged at 2000 rpm for 10 min. The reaction mixture was made up of 0.625 mL of the supernatant, 0.625 mL of distilled water, and 0.125 mL of freshly prepared ferric chloride (0.1%). The absorbance was measured at 700 nm by spectrophotometer (model UV-1800 240V, UV SPECTROPHOTOMETER, SHIMADZU-JAPAN) against the ascorbic acid curve. The reducing power of the extract was expressed as microgramm equivalent of ascorbic acid per grams of extract (µgAAE/g dry extract) according to the preset formula (1).
Vasodilation activity
The evaluation of the vasorelaxant effect of the different extracts was performed using 2 steps.
Step 1: Organ mounting
Healthy NMRI mice with a mean weight of 26±2 g was used after their euthanized with 100 mg/kg bw ketamine intraperitoneally. Mice thoracic aortas were removed by microdissection equipment and pinned into a petri dish containing physiological Krebs-Henseleit solution (mM: 130 NaCl; 14.9 NaHCO3; 3.7 KCl; 1.2 MgSO4-7H2O; 1.6 CaCl2-H2O; 1.2 KH2PO4 and 11 D-C6H12O6). The isolated thoracic aorta was then gently cleared of tissue adhesions under a stereo microscope (OPTICA Brand at x20 objective) and then sectioned into rings 1.8 to 2 mm long. Each ring was mounted in myograph tank (Danish Myo Technology 620M, Aarhus, Denmark) containing a physiological solution at 37°C and oxygenated via a pneumatic pump. This study protocol was approved by the local ethics committee of the University Joseph KI-ZERBO (Protocol number: CE-UOI/2019-04).
Step 2: Experimental process
The vasorelaxant activity was conducted following the method described by Nitiema et al. (2019) with some modifications 19. Briefly, previously mounted aorta rings were subjected to a baseline voltage of 5 mN and then held at equilibrium for one hour with a turnover of Krebs-Henseleit solution every 20 min. After this time, 80 mM potassium chloride (KCl) solution was introduced into each tank to sensitize the organs. Following this, a rinse was performed. After a 20 min rest phase, a cumulative of increasing concentrations (10-9 M to 3×10-7 M) of 9,11-Dideoxy-11α,9α epoxymethanoprostaglandin F2α (U46619) were applied to achieve maximal ring contraction. This cumulative allowed the determination of the effective concentration of 80% (EC80) of U46619 to be used for further experimentation. The presence of vascular endothelium was then verified by cumulative increasing concentration of Acetylcholine chloride (10-9 M to 10-5 M) on the rings precontracted with EC80 of U46619. When acetylcholine chloride (ACh) relaxation of the rings of at least 80% was considered to be endothelium-intact (E+) and if less than 10%, rings were endothelium-denuded (E-). After a new rest period (20 min), cumulative increasing concentrations (3×10-3 to 1 mg/mL) of the different extracts were performed on the precontracted aortic rings with U46619 EC80 value. Apolar extracts were not soluble in water and were dissolved in 2% dimethyl sulfoxide (DMSO), which was also used as a negative control.
Statistical analysis
The data collected during the different tests were classified and processed with Microsoft Excel software. Results were expressed as mean ± standard deviation. Similarly, the raw data were processed with the Microsoft EXCEL Software Package and analyzed with the Grapad Prism 8.0.1 software. Statistical comparisons were performed using one-way ANOVA or two-way ANOVA. Post hoc test was performed using Bonferroni’s test analysis to compare all the groups.
Results
Organoleptic characteristics
The dry powder of the pods of P. vulgaris is of beige color, a smell and a taste characteristic of the bean.
Residual moisture content
The residual moisture of the dry powder of P. vulgaris pods is of 7.81±0.06%.
Extraction yield
The yields of the different extracts in the study were of 14.77%, 9.56% and 0.92% respectively for PAD, ARP and EAP.
Phytochemical screening
Flavonoids
Figure 2 shows the chromatographic fingerprinting of flavonoids from P. vulgaris extracts (PAD, ARP, and EAP) at the wavelength of 366 nm. Quercetin (Q), highlighted by Neu’s reagent, appears in yellow with a frontal ratio of 0.96. The PAD fingerprint showed 5 majors spots of yellow color (Rf=0.02; 0.34; 0.48; 0.54; 0.71) and 03 blue spots (0.14; 0.28; 0.95). In the ARP fraction, two spots were observed; one is yellow (Rf=0.02) and the second is blue (Rf=0.35). At the level of the EAP fraction, it is noted the presence of 02 major’s yellow spots (Rf=0.50; 0.64) and 01 blue spot (Rf=0.95) with a solvent front materialized by blue color.
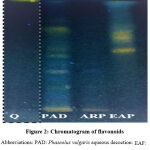 |
Figure 2: Chromatogram of flavonoids. |
Coumarins
Figures 3a and 3b represents the chromatographic profile of coumarins of the extracts in the study visualized at wavelengths of 254 nm and 366 nm. The analysis of these figures shows that KOH at 2% allows the detection of coumarin which appears blue-green at ʎ=254 nm (Rf=0.99) and blue-purple at 366 nm (Rf=0.99). The chromatogram analysis of the PAD decoction showed 04 majors blue-green bands at the wavelength of 254 nm (Rf=0.07; 0.75; 0.94; 0.99) and 01 majors’ purple band (Rf=0.02), then 02 majors blue-purple bands (Rf=0.38; 0.99, ʎ=366 nm). In the chromatographic profile of the ARP fraction, 01 major of blue-green spot were observed at 254 nm (Rf=0.07). At a wavelength of 366 nm, 01 purple spot (Rf=0.02) and 01 blue-purple spot (Rf=0.38) were observed. Concerning the chromatographic fingerprint of the EAP fraction, 03 majors blue-green spots were detected at 254 nm (Rf= 0.76; 0.85; 0.99). In addition, this fraction showed 04 blue-purple bands, detected at 366 nm (Rf= 0.68; 0.78; 0.90; 0.99).
 |
Figure 3: Chromatogram of coumarins at 254 nm (a) and 366 nm (b). |
Tannins
Figure 4 shows the chromatographic profile of tannins of the extracts of the study. Ferric chloride (2%) highlights the tannic acid (TA) which appears as black spots for the majority at the frontal references of 0.74 and 0.85. For the PAD and ARP extracts, the majority-black spots were observed at the frontal references of 0.67 and 0.82. In the case of EAP extract, it is noticed the presence of 02 majors -black spots (Rf=0.71; 0.85).
 |
Figure 4: Chromatogram of tannins |
Steroids and triterpenes
Figure 5 represents the chromatographic fingerprint of extracts at ʎ=366 nm. On the one hand, the Libermann-Buchard reagent revealed sterols and triterpenes which appeared mainly in blue-green (Rf=0.07; 0.1) and purple-purple (Rf=0.28) bands in the EAP extract. On the other hand, no spots indicating the presence of sterols and triterpenes were observed on the chromatographic profiles of the PAD and ARP extracts.
 |
Figure 5: Chromatogram of sterols and triterpenes. |
Saponins
Figure 6 shows the chromatographic profile of the saponins of the extracts of the visible study. The analysis of this figure shows that the sulphuric anisaldehyde highlights the saponin (S) which appears in black (Rf=0.21; 0.28; 0.57) and yellow (Rf=0.24; 0.40; 0.42; 0.50) spots with an eluent front materialized by a black band. For the study extracts, the PAD chromatogram showed majority black (Rf=0.08; 0.21) and purple (Rf=0.40; 0.48) spots. For the ARP extract, 02 majors black (Rf=0.08; 0.21) and a purple (Rf=0.40; 0.48; 0.71) spots were also observed. For the EAP chromatographic fingerprint, it showed a major-black spot at Rf=0.95.
 |
Figure 6: Chromatogram of saponins. |
Total phenolic and flavonoid compound contents
The results of the TPC of extracts were recorded in table 1. The EAP extract had the highest content (61.07±0.04 mgGAE/g) while the lowest value was those of ARP extract (17.94±0.03 mgGAE/g). Statistically significant differences were found between EAP versus ARP (p<0.001) and PAD versus ARP (p<0.001). Similarly, TFC showed a similar trend. The EAP fraction (6.16±0.03 mgQE/g) was richer than the PAD extract (4.34±0.18 mgQE/g) which in turn had a higher content than that of ARP (2.30±0.01 mgQE/g) with significant difference between EAP and ARP (p<0.001) and between PAD and ARP (p<0.01).
| Pad | EAP | ARP | |
| TPC(mgGAE/g) | 55.20±0.07*** | 61.07±0.04### | 17.94±0.03 |
| TFC(mgQE/g) | 4.34±0.18** | 6.16±0.03### | 2.30±0.01 |
Note: (**p<0.01 and ***p<0.001: PAD vs ARP; ###p<0.001: EAP vs ARP)
Abbreviations: PAD: P. vulgaris aqueous decoction; EAP: Ethyl acetate fraction of P. vulgaris decoction; ARP: Aqueous residual fraction of P. vulgaris decoction
In vitro antioxidant activities of extracts
Table 2 summarizes the 50% inhibitory concentrations of ABTS, DPPH, and FRAP antioxidant activities of the extracts in the study.
| ABTS IC50(μg/mL) |
DPPH IC50(μg/mL) |
FRAP (μgAAE/g dw) |
|
| PAD | 415.7±1.01*** | 4745.93±0.01*** | 170.68±0.011## |
| EAP | 71.87±0.30* | 252.97±0.01** | 167.52±0.04# |
| ARP | 505.30±1.75*** | 579.53±0.02*** | 157.26±0.09 |
| Trolox | 26.89±1.05 | 9.93±0.00 | – |
| AA | 33.56±0.63 | 15.62±1.00 | – |
IC50 value is defined as the inhibitory concentration of extract necessary to decrease the radical concentration by 50%. FRAP values are explain as microgram acid ascorbic equivalent per g dry extract. EAP extract showed significantly potent ABTS and DPPH antiradical capacity compared to PAD and ARP. However, these antioxidant activities are lower than those obtained with Trolox and ascorbic acid. (*p<0.05; **p<0.01; ***p<0.001: Trolox vs (PAD, EAP and ARP)). Furthermore, all the extracts were able to reduce the ferric ion (#p<0.05: EAP vs ARP and ##p<0.01: PAD vs ARP). Abbreviations: PAD: P. vulgaris aqueous decoction; EAP: Ethyl acetate fraction of P. vulgaris decoction; ARP: Aqueous residual fraction of P. vulgaris decoction; AA: Ascorbic acid.
ABTS
The inhibition percentages calculated in this test were used to determine the 50% inhibitory concentrations (IC50) of the references and the study extracts. Trolox showed high antioxidant capacity compared to ascorbic acid without statistical significance (IC50=26.89±1.05 µg/mL and IC50=33.56±0.63 µg/mL for Trolox and ascorbic acid respectively). It should be noted that the antioxidant power of Trolox was statistically significant compared with all extracts in the study (p<0.05). The EAP extract represents the most active among extracts with a IC50=71.87±0.30 µg/mL followed by PAD (IC50=415.7±1.01 µg/mL) and, then ARP (IC50=505.30±1.75 µg/mL).
DPPH
For DPPH free radical scavenging activity, Trolox (IC50=9.93±0.00 µg/mL with a percentage of inhibition of 99.95±0.0.05% (n=3)) showed relatively higher inhibitory activity compared to ascorbic acid (IC50=15.62±1.00 µg/mL with a percentage of inhibition of 99.02±0.08% (n=3)) without significant difference (p>0.05). The IC50 values comparison showed that Trolox has significantly reduced the DPPH radical scavenger compared to all extracts. Among investigated extracts, EAP showed a strong antiradical capacity followed by PAD and then ARP with IC50 of 252.97±0.01 µg/mL, 474.93±0.01 µg/mL and 579.53±0.02 µg/mL respectively. The percentages of DPPH radical inhibition (n=3) were 92.52±0.41%, 74.68±0.46% and 75.47±0.19% for EAP, PAD and ARP respectively.
FRAP
Results showed that all extracts were able to reduce ferric ions. Analysis of the values showed that PAD extract witch content 170.68±0.11 µg AAE/g dw significantly reduced ferric ions compared to ARP (157.26±0.09 µg AAE/g dw) with p<0.01. Similarly, a statistical difference was observed between EAP (167.52±0.04 µg AAE/g dw) and ARP extract (p<0.05).
Correlation between phenolic compounds and flavonoids and antioxidants
Table 3 summarizes the correlation coefficients between the contents in phenolic compounds (TPC), flavonoids (TFC), and antioxidants activities of extracts. The results analysis showed a strong statistically significant correlation between phenolic compounds and flavonoids in the PAD extract (R2=0.99 with p=0.007). Moreover, a non significant correlation was found between ABTS antiradical activity and the different phenolic compounds and flavonoids contents (R2>0.80 and p>0.05). Also, no possible correlation was found between the DPPH antiradical power and the TPC and TFC of the PAD extract. In the same way, the reducing power of ferric ions of this extract is not directly related to the TPC and TFC of this one (R2=0.01 for DPPH and R2=0.02 for FRAP). For the EAP extract, analysis did not show a significant correlation between TPC and TFC (R2=0.12 with p>0.05). Equally, it should be noted that the DPPH, ABTS, and FRAP radical inhibitory activity were correlated with the extracts TPC but without significant difference for ABTS (R2=0.79 and p=0.30); DPPH (R2=0.85 and p=0.25) and for FRAP (R2=0.97 and p=0.10). ABTS and DPPH activities were very weakly correlated with the TFC of the EAP extract (R2=0.54 for ABTS and R2=0.46 for DPPH). No correlation was reported between TFC and FRAP activation (R2=0.03).
Regarding the ARP extract, analysis did not report a correlation between TPC and TFC nor between the antioxidant activities ABTS, DPPH, FRAP, and TPC (R2<0.5). Also, a strong significant correlation was reported between DPPH antiradical activity and TFC (R2 =0.99 and p=0.02). Moreover, a weak correlation between TFC and ABTS and FRAP activities was reported (R2>0.5) without any significance difference (p>0.05).
Vasorelaxation activity
Concentration-effect curve of extracts
Results showed that P. vulgaris extracts (3×10-3 to 1 mg/mL) has induced a concentration-dependent relaxation of mices aortic rings in the presence as well as in the absence of endothelium (Figure 7) precontracted with U46619. In low concentrations, the EAP extract showed better relaxation of rings with endothelium compared with those without endothelium, but remained identical when high concentrations. This is shown in Figure 7A indicating a leftward deviation of the concentration-effect curve in endothelium-intact [EAP(E+)] compared to those in endothelium-denided [EAP(E-)]. After endothelium destruction, EAP extract has induced a concentration-dependent vasorelaxation effect of the aorta rings. However, it is remarkable that the difference in maximum relaxation induced by EAP(E+) and EAP(E-) was not statistically significant (p>0.05). The Emax values were of 100.06±0.00% and 101.01±0.00% for EAP(E+) and EAP(E-), respectively. It should be noted that for the PAD extract, a concentration-dependent relaxation of the precontracted aortic rings was also observed on rings in intact and denuded endothelium. Figure 7B shows a slight rightward deviation of the relaxation curve in the presence of endothelium [PAD(E+)] compared to those in the absence of endothelium [PAD(E+)]. Although the maximum relaxation get with this extract was not total in both the presence and absence of endothelium, it should be noted that it was greater than 50%. The maximum effects recorded were 88.32±3.49% for PAD(E+) versus a value of 95.39±5.65% for PAD(E-). A comparison of the effects from PAD(E+) and PAD(E-) revealed that there was no significant difference.
In addition, the vasorelaxant effect induced by ARP were concentration-dependent. The concentration-effect curves of this extract, both in the presence and absence of endothelium, showed similar trends of a superimposable nature. However, it should be noted that ARP was unable to induce total relaxation of U46619-induced contraction in aortic rings both in the presence and absence of endothelium (Figure 7C). Therefore, the values of maximum efficiencies were of 72.23±6.97% and of 84.45±5.34% for ARP(E+) and ARP(E-) extracts, respectively. Nevertheless, a significant difference should be noted when analyzing the two concerned curves (p<0.01). The present results also shows that the vehicle consisting in DMSO (2%) had no significant effect on the relaxation of the aortic rings (Data not showed).
Relaxation power of extracts
The power values (pD2) of the extracts from the aortic ring study are materialized by the histograms (Figure 8). Analysis of fifty per cent effective concentrations (EC50) indicates that the EAP extract produced significantly greater relaxation in the presence of endothelium than those in its absence [EAP(E+)=0.05±0.00 mg/mL; EAP(E-)=0.14±0.02 mg/mL) with p=0.009]. The pD2 power intensities were of 1.24±0.01 and of 0.84±0.02 for EAP(E+) and EAP(E-), respectively. On the other hand, PAD and ARP extracts showed better relaxation in the absence of endothelium than in the presence. The resulting 50% effective concentrations were, on the one hand, 0.30±0.03 mg/mL and 0.38±0.02 mg/mL, respectively, for PAD(E-) and ARP(E-) on rings with denuded endothelium. On the other hand, these EC50 values were estimated to be 0.43±0.03 mg/mL and 0.64±0.01 mg/mL respectively for PAD(E+) and ARP(E+) on rings with intact endothelium. The pD2 values obtained were of 0.30±0.02 and 0.16±0.02 for PAD(E+) and ARP(E+), respectively, versus 0.50±0.02 and 0.41±0.01 for PAD(E-) and ARP(E-), respectively. Analysis of pD2 values showed significant differences in both PAD(E+) versus PAD(E-) (p=0.049) and ARP(E+) versus ARP(E-) (p<0.01).
 |
Figure 8: Histogram of relaxation powers of Phaseolus vulgaris aqueous extract and its fractions from the study of mouse aortic rings pre-contracted with U46619 |
Moreover, EAP extract showed significantly a potent vasodilation activity compared to both PAD and ARP extracts when comparing the effects on rings with endothelium with those without endothelium. Furthermore, there was no significant difference between the effects of PAD and ARP extracts regardless of the type of ring (p>0.05). However, the destruction of the endothelium did not affect the intensity of the effects produced by PAD and ARP.
Discussion
The use of herbal medicine as a therapeutic and complementary alternative has reportedly quadrupled in the last three decades 35. This medicine using herbal preparations has proven to be very effective in treating diseases due to a process of synergistic action of the chemical components involved 36. Commonly known as green beans, P. vulgaris is a globally cultivated plant whose pods and seeds are consumed for their high protein content 25,37. In addition, the decoction of immature P. vulgaris pods is used in herbal medicine to lower blood pressure in hypertensive patients 26. The anti-diabetic, antioxidant, and angiotensin-converting enzyme inhibitory properties of the seeds have been widely documented in recent decades 38,39. This study reports for the first time the vasorelaxant effects of P. vulgaris immature pod decoction (PAD) and its ethyl acetate (EAP) and aqueous residual fractions (ARP) on the aorta of NMRI mice model. Exploration of the vasculature through myography is a favorable asset in understanding the vascular pathophysiology of hypertension. This method allows the comparison of agonist- and antagonist-induced contractions and relaxations of vessels to obtain evidence of vascular smooth muscle cell (VSMC) receptor function 19,40,41. Furthermore, the discovery of natural vasoactive molecules and their pharmacological mechanism of action is one of the main research areas for the prevention and management of cardiovascular and respiratory diseases. The vasoconstrictor activity of the thromboxane (TXA-2)-mimetic analogue U46619 on vascular smooth muscle has been widely documented 42. The U46619 was chosen to induce the contraction of myograph-mounted aortic rings. Indeed, U46619, through its ability to bind to G protein-coupled receptors (GPCRs), causes a progressive and sustained contraction of vascular smooth muscle. This contraction involves the increase of cytosolic calcium ([Ca2+]i) either through the release of stored calcium in the sarcoplasmic reticulum (SR) or through the modulation of transmembrane calcium channels leading to the entry of extracellular calcium 42. Myographs for the activity of P. vulgaris PAD, EAP, and ARP extracts showed an ability of these extracts to significantly relax, in a concentration-dependent manner, vessels pre-contracted with U46619. The compelling explanation for this observation is thought to be related to the ability of the extracts to orchestrate a decrease in [Ca2+]i concentration in VSMCs via transmembrane efflux 43,44. The performance of PAD and ARP extracts on aortic rings would suggest probable endothelium-independent activity. Especially since, the comparison of pD2 and maximum effects of these extracts showed statistically significant differences in favor of vasorelaxant effects in absence of endothelium. These results would imply that the extracts would have the power to regulate the membrane potential of VSMCs via modulation of chloride and potassium channels or either by induction of Ca2+ reuptake or sequestration by the SR 45. Hyperpolarization of the VSMC membrane resulting from the opening of K+ channels contributes to vasodilation by altering the resting membrane potential 46. The EAP extract showed 100% total relaxation of aortic rings with intact or denuded endothelium. In addition, the strength of the EAP induced relaxation was statistically significant on rings with intact endothelium. These results would suggest that EAP-induced vasorelaxation could be mediated by different signaling pathways. It has been widely demonstrated that in the vasorelaxation process, calcium influx inhibition, channel blockade, and GPCR inactivation are the most important steps 45,47. The presence of the endothelium resulted in rapid relaxation of the rings by the extract. In contrast, the destruction of the endothelium contributed to an increase in the effective concentration of 50% so the potency of the extract decreased significantly. Despite the absence of endothelium, the EAP extract induced 100% total relaxation of the aortic rings and this observation would suggest an involvement of VSMC in the onset of the effect. From the above, the endothelium would be considered a catalyst for vascular relaxation 47,48. Thus, EAP extract would be able to interfere with the production of vasorelaxant mediators by the endothelium including NO. Besides, this endothelium-independent vasorelaxant effect also involves prostaglandins, in particular prostacyclin, which by diffusion activates protein kinase A after a cascade reaction. This process leads to the opening of hyperpolarizing K+ channels and the closure of voltage-gated calcium channels27,49. Moreover, elimination of the endothelium did not significantly affect the maximum relaxation of the extract, which was similar in both cases. Although the potency of the effect was significantly decreased in the absence of endothelium, EAP would show a pattern suggestive of a partially endothelium-dependent mechanism. Several studies have reported the richness of P. vulgaris seeds in phenolic compounds and flavonoids 39. Chromatographic fingerprints affixed by P. vulgaris pods through HPTLC also showed the presence of phenolics, flavonoids, coumarins, steroids and triterpenes, tannins, and saponins. The plurality of phytochemicals found in the extracts of the present study is a considerable asset to support the vaso-active muscle relaxant and antioxidant properties of P. vulgaris extracts. In addition, coumarin glycosides have known be able to block calcium channels by inhibiting K+ channels, thereby lowering the intracellular calcium concentration of vascular smooth muscle 5,50. The weak correlation between the flavonoids of the EAP extract and the antioxidant activities does not exclude them from activity but would suggest the existence of a synergy of action of the phenolic compounds present in high contents. The phenolic compounds constitute a wide group of bioactive compounds, most the complementary 51. In addition, this extract has shown a significant vasorelaxant activity on mouse aorta rings. The high content of TPC and TFC correlated with the antioxidant activities would have contributed heavily to increasing the relaxation power of aortic rings. There is ample evidence that antioxidant-mediated ROS inhibition would enhance NO-induced vasorelaxation 5. These assertions corroborate those of other authors who showed endothelium-independent vasorelaxant effects of extracts of medicinal plants such as Lannea microcarpa, Anogeissus leiocarpa, and Dyospiros kaki through NO production 19,48. The EAP extract also showed a significantly higher ROS scavenging capacity than that of PAD and ARP which were otherwise almost similar. Therefore, these free radical scavenging capabilities may help explain the potency of the vasorelaxant effects of P. vulgaris.
Conclusion
The phytochemical study of P. vulgaris pod extracts showed the presence of flavonoids, coumarins, sterols and triterpenes, tannins, and saponins. The richness of these extracts in secondary metabolites could be at the origin of their antioxidant and vasorelaxant effects. These preliminary results will contribute to scientifically validating the traditional therapeutic uses of this plant for the management of hypertension. After this study, it is necessary to explore the mechanisms of the pharmacological action of P. vulgaris decoction and its ethyl acetate fraction.
Acknowledgment
Not applicable
Funding Sources
Not applicable
Conflict of Interest
The authors declare that there are no conflicts of interest in the publication of this article.
References
- Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021; 398: 957–980.
CrossRef - Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens. Res. 2019; 42: 1235–1481.
CrossRef - Rodrigo R, González J, Paoletto F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens Res 2011 344 2011; 34: 431–440.
CrossRef - Rodrigo R, Prat H, Passalacqua W, Araya J, Guichard C, Bächler JP. Relationship between Oxidative Stress and Essential Hypertension. Hypertens Res 2007 3012 2007; 30: 1159–1167.
CrossRef - Touyz RM, Rios FJ, Alves-Lopes R, Neves KB, Camargo LL, Montezano AC. Oxidative Stress: A Unifying Paradigm in Hypertension. Can J Cardiol 2020; 36: 659–670.
CrossRef - Sharifi-Rad M, Anil Kumar N V., Zucca P, Varoni EM, Dini L, Panzarini E et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front Physiol 2020; 11: 694.
CrossRef - Pearson PJ, Vanhoutte PM. Vasodilator and vasoconstrictor substances produced by the endothelium. Rev Physiol Biochem Pharmacol 1993; 122: 1–67.
CrossRef - Krüger-Genge A, Blocki A, Franke RP, Jung F. Vascular Endothelial Cell Biology: An Update. Int J Mol Sci 2019; 20. doi:10.3390/IJMS20184411.
CrossRef - Chrysant SG, Chrysant GS. New and emerging cardiovascular and antihypertensive drugs. https://doi.org/101080/1474033820201810232 2020; 19: 1315–1327.
CrossRef - Stewart MH, Lavie CJ, Ventura HO. Emerging Therapy in Hypertension. Curr Hypertens Rep 2019; 21: 1–9.
CrossRef - Cameron AC, Lang NN, Touyz RM. Drug Treatment of Hypertension: Focus on Vascular Health. Drugs 2016; 76: 1529–1550.
CrossRef - Waller JR, Waller DG. Drugs for systemic hypertension and angina. Medicine (Baltimore) 2018; 46: 566–572.
CrossRef - Makker V, Taylor MH, Oaknin A, Casado Herraez A, Orlowski R, Dutta L et al. Characterization and Management of Adverse Reactions in Patients with Advanced Endometrial Carcinoma Treated with Lenvatinib Plus Pembrolizumab. Oncologist 2021; 26: e1599–e1608.
CrossRef - Hofferer A, Dolladille C, Chretien B, Sassier M, Laugier D, Atzenhoffer M et al. Antidepressive agents and hypertension: A case/no-case study in French pharmacovigilance database. Encephale doi:10.1016/J.ENCEP.2021.04.009.
CrossRef - Atanasov AG, Zotchev SB, Dirsch VM, Orhan IE, Banach M, Rollinger JM et al. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov 2021; 20: 200–216.
CrossRef - Tang F, Yan HL, Wang LX, Xu JF, Peng C, Ao H et al. Review of Natural Resources With Vasodilation: Traditional Medicinal Plants, Natural Products, and Their Mechanism and Clinical Efficacy. Front Pharmacol 2021; 12: 257.
CrossRef - Aekthammarat D, Tangsucharit P, Pannangpetch P, Sriwantana T, Sibmooh N. Moringa oleifera leaf extract enhances endothelial nitric oxide production leading to relaxation of resistance artery and lowering of arterial blood pressure. Biomed Pharmacother 2020; 130. doi:10.1016/J.BIOPHA.2020.110605.
CrossRef - Nitiéma M, Koala M, Belemnaba L, Claude J, Ouédraogo W, Ouédraogo S et al. Endothelium-Independent Vasorelaxant Effects of Anthocyanins-Enriched Extract from Odontonema strictum (Nees) Kuntze (Acanthaceae) Flowers: Ca2+ Channels Involvement. European J Med Plants 2019; 29: 1–11.
CrossRef - Nitiéma M, Soleti R, Koffi C, Belemnaba L, Mallegol P, Ouédraogo N et al. Ethyl Acetate Fraction of Lannea microcarpa Engl. and K. Krause (Anacardiaceae) Trunk Barks Corrects Angiotensin II-Induced Hypertension and Endothelial Dysfunction in Mice. Oxid Med Cell Longev 2019; 2019: 1–13.
CrossRef - Alia F, Putri M, Anggraeni N, Syamsunarno MRAA. The Potency of Moringa oleifera Lam. as Protective Agent in Cardiac Damage and Vascular Dysfunction. Front Pharmacol 2022; 12: 3911.
CrossRef - Padhani ZA, Moazzam Z, Ashraf A, Bilal H, Salam RA, Das JK et al. Vitamin C supplementation for prevention and treatment of pneumonia. Cochrane Database Syst Rev 2021; 2021. doi:10.1002/14651858.CD013134.PUB3/FULL/FR.
CrossRef - Wang S, Guo C, Xing Z, Li M, Yang H, Zhang Y et al. Dietary Intervention With α-Amylase Inhibitor in White Kidney Beans Added Yogurt Modulated Gut Microbiota to Adjust Blood Glucose in Mice. Front Nutr 2021; 8: 664976.
CrossRef - Kasali FM, Kadima JN, Peter EL, Mtewa AG, Ajayi CO, Tusiimire J et al. Antidiabetic Medicinal Plants Used in Democratic Republic of Congo: A Critical Review of Ethnopharmacology and Bioactivity Data. Front Pharmacol 2021; 12: 757090.
CrossRef - Sharma A, Kaur M, Katnoria JK, Nagpal AK. Polyphenols in Food: Cancer Prevention and Apoptosis Induction. Curr Med Chem 2017; 25: 4740–4757.
CrossRef - Hayat I, Ahmad A, Masud T, Ahmed A, Bashir S. Nutritional and Health Perspectives of Beans (Phaseolus vulgaris L.): An Overview. Crit Rev Food Sci Nutr 2014; 54: 580–592.
CrossRef - Thiombiano A, Schmidt M, Dressler S, Ouédraogo A, Hahn K, Zizka G. Catalogue des plantes vasculaires du Burkina Faso. Geneva, 2012.
- Aljerf L, Williams M, Ajong AB, Onydinma UP, Dehmchi F, Pham VT et al. Comparative Study of the Biochemical Response Behavior of Some Highly Toxic Minerals on Selenosis in Rats. Rev Chim 2021; 72: 9–18.
CrossRef - Salas SP, Russo N M. [Analysis of the main ethical conflicts in the 2008 declaration of Helsinki and the proposed changes in the new version]. Rev médica Chile 2014; 142: 475–480.
CrossRef - Koala M, Ramde-Tiendrebeogo A, Ouedraogo N, Ilboudo S, Kaboré B, Kini FB et al. HPTLC Phytochemical Screening and Hydrophilic Antioxidant Activities of Apium graveolensL., Cleome gynandra L., and Hibiscus sabdariffaL. Used for Diabetes Management. Am J Anal Chem 2021; 12: 15–28.
CrossRef - Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 1999; 299: 152–178.
CrossRef - Koala M, Ramde-Tiendrebeogo A, Ouedraogo N, Ilboudo S, Kaboré B, Kini FB et al. HPTLC Phytochemical Screening and Hydrophilic Antioxidant Activities of Apium graveolens, Cleome gynandra L., and Hibiscus sabdariffa L. Used for Diabetes Management. Am J Anal Chem 2021; 12: 15–28.
CrossRef - Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 1999; 26: 1231–1237.
CrossRef - Zheng J, Ding C, Wang L, Li G, Shi J, Li H et al. Anthocyanins composition and antioxidant activity of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Food Chem 2011; 126: 859–865.
CrossRef - Oyaizu M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Japanese J Nutr Diet 1986; 44: 307–315.
CrossRef - Organization WH. WHO global report on traditional and complementary medicine 2019. World Health Organization: Geneva, 2019https://apps.who.int/iris/handle/10665/312342.
CrossRef - Singh B. Medicinal plants and phytomedicines. In: Herbal Insecticides, Repellents and Biomedicines: Effectiveness and Commercialization. Springer India, 2016, pp 127–145.
CrossRef - Reverri E, Randolph J, Steinberg F, Kappagoda C, Edirisinghe I, Burton-Freeman B. Black Beans, Fiber, and Antioxidant Capacity Pilot Study: Examination of Whole Foods vs. Functional Components on Postprandial Metabolic, Oxidative Stress, and Inflammation in Adults with Metabolic Syndrome. Nutrients 2015; 7: 6139–6154.
CrossRef - Pradeepkumar MR; JSD; KVH; CS. Phytochemical screening and evaluation of analgesic and antiinflammatory activities of Phaseolus vulgaris Linn., seeds in rodents. J Appl Pharm Sci 2015; 5: 66–69.
CrossRef - Ganesan K, Xu B. Polyphenol-rich dry common beans (Phaseolus vulgaris L.) and their health benefits. Int. J. Mol. Sci. 2017; 18: 2331.
CrossRef - Bridges LE, Williams CL, Pointer MA, Awumey EM. Mesenteric Artery Contraction and Relaxation Studies Using Automated Wire Myography. J Vis Exp 2011; : 3119.
CrossRef - Belemnaba L, Nitiema M, Ouédraogo S, Auger C, Schini-Kerth VB, Bernard B. Endothelium-independent vasorelaxation by dichloromethanolic fraction from Anogeissus leiocarpa (DC) Guill. Et Perr. (Combretaceae) bark of trunk on porcine coronary artery rings: Involvement of [Ca2+]i decreased and phosphodiesterases inhibition. African J Pharm Pharmacol 2019; 13: 25–35.
CrossRef - Yan H, Zhang MZ, Wong G, Liu L, Kwok YS (Shelia), Kuang SJ et al. Mechanisms of U46619-induced contraction in mouse intrarenal artery. Clin Exp Pharmacol Physiol 2019; 46: 643–651.
CrossRef - Wenceslau CF, McCarthy CG, Earley S, England SK, Filosa JA, Goulopoulou S et al. Guidelines for the measurement of vascular function and structure in isolated arteries and veins. https://doi.org/101152/ajpheart010212020 2021; 321: H77–H111.
CrossRef - Konja D, Luo C, Sun WY, Yang K, Man AWC, Xu A et al. Assessment of Vascular Tone Responsiveness using Isolated Mesenteric Arteries with a Focus on Modulation by Perivascular Adipose Tissues. JoVE (Journal Vis Exp 2019; 2019: e59688.
CrossRef - Marques AAM, da Silva CHF, de Souza P, de Almeida CLB, Cechinel-Filho V, Lourenço ELB et al. Nitric oxide and Ca2+-activated high-conductance K+ channels mediate nothofagin-induced endothelium-dependent vasodilation in the perfused rat kidney. Chem Biol Interact 2020; 327. doi:10.1016/J.CBI.2020.109182.
CrossRef - Garland CJ, Dora KA. EDH: endothelium-dependent hyperpolarization and microvascular signalling. Acta Physiol 2017; 219: 152–161.
CrossRef - Hort MA, Brighente IMC, Pizzolatti MG, Ribeiro-do-Valle RM. Mechanisms involved in the endothelium-dependent vasodilatory effect of an ethyl acetate fraction of Cyathea phalerata Mart. in isolated rats’ aorta rings. J Tradit Complement Med 2020; 10: 360–365.
CrossRef - Belemnaba L, Ouédraogo S, Auger C, Chataigneau T, Traore A, Guissou IP et al. Endothelium-Independent and Endothelium-Dependent Vasorelaxation by a Dichloromethane Fraction from Anogeissus Leiocarpus (DC) Guill. Et Perr. (Combretaceae): Possible Involvement of Cyclic Nucleotide Phosphodiesterase Inhibition. African J Tradit Complement Altern Med 2013; 10: 173.
CrossRef - Knox M, Vinet R, Fuentes L, Morales B, Martínez JL. A Review of Endothelium-Dependent and -Independent Vasodilation Induced by Phytochemicals in Isolated Rat Aorta. Anim 2019, Vol 9, Page 623 2019; 9: 623.
CrossRef - Soto-Blanco B. Herbal glycosides in healthcare. Herb Biomol Healthc Appl 2022; : 239–282.
CrossRef - Moreno-García KL, Antunes-Ricardo M, Martínez-Ávila M, Milán-Carrillo J, Guajardo-Flores D. Evaluation of the antioxidant, anti-inflammatory and antihyperglycemic activities of black bean (Phaseolus vulgaris L.) by-product extracts obtained by supercritical CO2. J Supercrit Fluids 2022; 183: 105560.
CrossRef








