Manuscript accepted on :23-01-2023
Published online on: 20-02-2023
Plagiarism Check: Yes
Reviewed by: Dr. Fatma Çavuş Yonar
Second Review by: Dr. Niharika Kondepudi
Final Approval by: Dr. Ian James Martin
Anand Ramasamy*  and K. Kathiresan
and K. Kathiresan
Department of Pharmacy, Annamalai University, Annamali Nagar, Tamil Nadu, India.
Corresponding Author E-mail: pharmacyanand@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2586
Abstract
The Ficus benghalensis of the family Moraceae, often known as the banyan or "Bargad" in Hindi. In traditional medicine, banyan tree pieces are used to cure several conditions, including ulcers, diabetes, bleeding, diarrhea, dysentery, and burning. The aerial root latex is useful for treating rheumatism, haemorrhoids, wounds, boils, inflammation, and skin disorders. The objective of the current investigation was to determine its potential toxicity. Ethyl acetate extracts of Ficus benghalensis aerial roots were tested for acute oral toxicity in female Wistar rats at a level of 5000 mg/Kg body wt. The rats were evaluated for death, behavioural abnormalities, neuro-motor abnormalities, body weight, and water-feed intake patterns throughout the 14-day trial. The kidney and liver functions were evaluated using blood biochemical indicators, and these organs' histological compositions were also examined. It was discovered that Ficus benghalensis aerial root ethyl acetate extract did not induce any harmful effects or mortality at the provided dose. In comparison to the corresponding control group, there were no abnormalities seen in any specified parameter in the rats. For Ethyl Acetate Extracts of Ficus benghalensis Aerial Roots, the potential oral fatal dose is, therefore, greater than 5000 mg/Kg body wt. Hence, our findings are evidence that the aerial root extract was safe for oral consumption and also suitable for in-vivo biological screening.
Keywords
Acute Oral Toxicity; Aerial Roots; Ethyl Acetate Extracts; Ficus benghalensis
Download this article as:| Copy the following to cite this article: Ramasamy A, Kathiresan K. Acute Oral Toxicity Study of Ethyl Acetate Extracts of Ficus benghalensis Aerial Roots. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Ramasamy A, Kathiresan K. Acute Oral Toxicity Study of Ethyl Acetate Extracts of Ficus benghalensis Aerial Roots. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3xCIPL4 |
Introduction
Natural goods, originating from “plant, animal, or marine sources”, are increasingly used as health additions, supplements, and disease prevention agents. The idea of using “food as medicine” has developed and has been used for centuries. Ancient literature makes it clear that natural organic cures were the customary approach among the “not modern” people for “disease prevention and treatment”. It is genuinely universal, as seen by the use of natural product therapies in various parts of the world, mostly under the designations of Traditional Chinese Traditional and Ayurveda medicine. Titles vary, but they all rest on the same fundamental tenet of employing substances from nature to treat illnesses and ward against them.
For thousands of years, therapeutic substances have been found in nature. Even today, many impoverished nations still heavily rely on plant-based materials for therapeutic treatments in primary healthcare. The World Health Organization (WHO) states that plant extracts or their active components are extensively used in traditional medicine. According to estimates from the WHO, around 80% of the population in underdeveloped nations rely on traditional medicine for their essential medical requirements 1.
As a continuation, more scientific investigations have focused on locating and isolating bioactive elements from their natural origins. Despite the effective development of pharmaceuticals from these sources, most synthetic medicines may be accompanied by discomfort brought on by the adverse effects, which has sparked interest in and use of natural resources with potential medical applications. The primary class of natural sources that contribute is medicinal plants.
The “development and introduction of multiple forms of a single product” derived from a given plant material and added claims such as the “use of advanced processing technologies” and “products enriched with specific bioactive components” have been fueled by the large and growing consumer block for “natural medicinal plants in the forms of food or health supplements, health drinks or tonic, and many other forms”. The various process factors used to create these products cause either qualitative or quantitative alterations in the biological and chemical properties of the plant mass 2,3. Most research focuses on developing and improving extraction techniques to extract the most biologically active material possible. However, it is crucial to evaluate the toxicity profile of such products 4. It becomes even more crucial given that most people believe that all natural goods are risk-free, readily available, and consumable 5 and that most of these items are labelled as supplements. Therefore, it is crucial to have “accurate chemical, toxicological, and safety data” when using plants that have long been used to support health claims.
The Ficus benghalensis of the family Moraceae, 6 often known as the banyan or “Bargad” in Hindi, is a large deciduous tree and grows in the forests of the Sub-Himalayan area, the Deccan, and South India and it is found all throughout India. It grows in evergreen environments and can be found all year long 7,8. Banyan tree pieces are utilized in traditional medicine to treat various illnesses. Burning, haemorrhages, diarrhoea, dysentery, diabetes, ulcers, and skin problems can all be treated with the bark. The leaves can treat skin allergies, leprosy, ulcers, and other conditions. For diarrhea and dysentery, aerial root buds and decoction are used. Rheumatism, haemorrhoids, wounds, boils, inflammation, and skin conditions can all benefit from the aerial root latex 9. It is administered externally for body and body discomfort, toothache, and diabetes, in addition to being used to stop menstruation. In Europe and South East Asian nations, pharmacies and supermarkets effectively sell Ficus benghalensis goods.
However, the toxicity data and chemical ingredient profile of such a product are not yet accessible. An early yet crucial study that may provide insight into a substance’s overall safety is one on acute toxicity. Animals are typically given a single, high dose of the substance, and their behaviour, motor-neuronal alterations, and mortality are monitored 10. The safety window for use can be determined by calculating the median lethal dosage (LD50).This study’s objective is to assess the acute oral toxicity of ethyl acetate extracts of Ficus benghalensis aerial roots.
Materials and Methods
Plant Collection
Ficus benghalensis fresh aerial roots were obtained from the outskirts of Namakkal city (Tamil Nadu, India). Authentication and taxonomical identification of plant material was done and a voucher for specimen was deposited at the herbarium in the Department of Pharmacy, Annamalai University.
Preparation of extracts
The aerial root extract was made using a series of sequential extractions with several organic solvents, including Pet ether, Chloroform, Ethyl acetate, Acetone, Methanol, and Water (1:10 w/v), which had increasing degrees of polarity. Aerial root powder (200 g) was packed in the Soxhlet apparatus, and petroleum ether (2 litres) was added and refluxed for 7 h in the round-bottomed flask. After that, a vacuum rotary evaporator was used to dry the aerial root extract. The dried consecutive petroleum ether extract (yield 2.0153%) was then kept in a freezer at 4 degrees Celsius for later research. Plant material that had been extracted using petroleum ether was thoroughly dried before being subjected to additional extraction steps using a Soxhlet device and various increasing polarity solvents. The extracts were dried and stored. The ethyl acetate extract was used for further acute toxicity.
Experimental Animals
8 weeks old weighting 250–300 g, 6 female wistar rats were acquired from the Department Animal House. Before dosing, all rats had a one-week acclimatization period to the laboratory environment. The rats were kept in a climate-controlled environment with a “12/12 h light-dark cycle” at 24+/− 2 °C. Each polycarbonate cage held 6 rats, who had unlimited access to rodent food and water. The rats underwent as little anguish as possible.
Reagents and Chemicals
All the chemicals were procured from Sigma-Aldrich and Fischer Scientific, India.
Acute Toxicity Study (OECD 423)
According to steps indicated by the “Organization for Economic Co-operation and Development (OECD) 43” [11,12], the acute oral toxicity test was carried out on 6 female wistar rats. The animals were divided equally (3 each) into control (getting only distilled water) and treatment (Ethyl Acetate Extracts of Ficus benghalensis Aerial Roots) groups after being randomly chosen. The animals were weighed after an overnight fast, and then separate oral doses of 5000 mg/kg of ethyl Ficus extract and a vehicle regulation were administered. The animals were then observed for 2 weeks/14 days.
2 Groups were there
Control group: Distilled water (untreated)
Treatment group: 5000mg/Kg of Ficus extract PO
Body Weight and Cage-side observations
All animals received food and drink after 60 min. The treated rats were then observed after 30 min following treatment, hourly for 8 h, and daily for the following 14 days. All results were noted for each animal that received treatment. All the animals were weighed regularly throughout the experiment, and visual observations were made for signs of morbidity and mortality, such as fur and skin changes, mucous membrane and eye changes, salivation, respiration, urination (colour), faeces consistency, somatomotor activity, behavioural patterns (such as moving around, being drowsy, having seizures), sleep and itching, physical changes (such as immobility, tremors), and morbidity. Daily assessments of the amount of food and drink consumed were noted during the 2 week trial study. The remaining feeds where Weight of the remaining feeds was deducted from the baseline wt. to determine the food intake. Each treatment group’s water consumption was also recorded.
Biochemical Analysis
Before drawing blood, each rat received one drop of tetracaine, an eye anaesthetic. Using heparinised micro-haematocrit tubes, “250 μL bloods were drawn from the retro-orbital sinus” on Day 1 and Day 14 two minutes apart. The levels of urea, creatinine, ALT, AST, total protein, and bilirubin in blood serum were determined using an automated blood chemistry analyser.
Histopathology
At the conclusion of the experiment, under Thiopental sodium anaesthesia, the rats were euthanized, and the liver, kidney, and heart were removed for histological analysis. Organs were cleaned with regular saline, dried with blotting paper and their weight was measured. The formula “(weight of organ/bodyweight of rats on the day of sacrifice)*100%” [13] was used to compute the “relative weight index of each organ to its body weight.”
Then 10% formalin was used to fix the organs. Sections with a thickness of 5 μm were cut from the embedding-fixed tissues. Under a light microscope, the slices were stained with haematoxylin and eosin was examined.
Statistics
Analysis was done using SPSS and the one-way ANOVA and Dunnett tests.
Results
Study of Acute Toxicity
Cage Side Observation
No mortality was noted in any rat in the treatment group, and all of the provided Ficus extract samples resulted in normal behavioural, motor, and neural functions. When compared to the control group, the monitoring of the treatment rats’ skin and fur, eyes, behavioural patterns such locomotion and posture, and ANS and CNS activity were unaltered. (Table 1) This demonstrated that the oral LD50 i.e., median lethal dose of Ficus benghalensis aerial roots ethyl acetate extracts was larger than 5000 mg/Kg body wt.
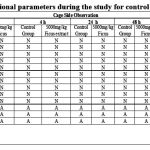 |
Table 1: Observational parameters during the study for control and treatment group. |
Measurement of Body Wt.
Figure 1 shows the composite mean body weight of the rats fed distilled water and 5000 mg/kg of Ficus extracts (EFB). There were no significant differences in the mean relative organ weight gain in Table 2 or the individual rat body weights after EFB administration in Figure 1.
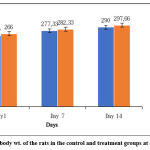 |
Figure 1: Mean body wt. of the rats in the control and treatment groups at days 1, 7 and 14. |
Table 2: Mean body wt. of the rats in the control and treatment groups at days 1, 7 and 14.
| Control Group | 5000mg/kg Ficus extract | |
| Kidney | 1.18 ±0.05 | 1.24±0.09 |
| Liver | 5.74±0.09 | 6.13±0.12 |
| Heart | 0.59±0.17 | 0.65±0.21 |
Throughout the course of the trial, no statistically significant differences were seen in the body wt. of the rats in the treatment group compared to the control. (p value greater than 0.05) The rats’ habit of feeding and drinking was regular and steady for the course of the experiment.
Biochemical analysis
Table 3: Mean of biochemical parameters of rats in both groups at the end of the study.
| Parameters | Units | Control Group | 5000mg/kg Ficus extract |
| Urea | mg/dl | 40 ±1.15 | 42 ± 1.12 |
| Creatinine | mg/dl | 0.67 ± 0.01 | 0.72 ±0.02 |
| ALT | U/L | 52 ± 1.76 | 59 ± 1.54 |
| AST | U/L | 110 ± 1.15 | 121 ± 1.25 |
| Total Protein | G/dl | 5.14 ± 0.02 | 5.62 ± 0.04 |
| Bilirubin | mg/dl | 1.2 ± 1.17 | 1.6 ± 1.15 |
Table 3 displays the mean of biochemical parameters of rats in both groups at the end of the study after the single oral treatment with 5000 mg/Kg body weight of Ficus extract in the rats. It displays bilirubin, urea, creatinine, ALT, and AST. Serum levels between the treatment and control groups were identical. Statistically insignificant (p> 0.05) differences between the measured parameters and the corresponding control groups were found. According to our research, the liver and kidneys were not harmed by the Ficus extracts.
Histopathology and Relative Organ Weight
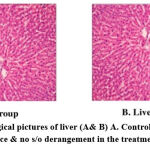 |
Figure 2: Histopathological pictures of liver (A& B) A. Control; B. Test; Normal gross appearance & no s/o derangement in the treatment group. |
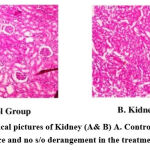 |
Figure 3: Histopathological pictures of Kidney (A& B) A. Control; B. Test; Normal gross appearance and no s/o derangement in the treatment group. |
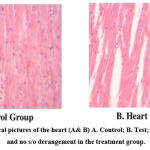 |
Figure 4: Histopathological pictures of the heart (A& B) A. Control; B. Test; Normal gross appearance and no s/o derangement in the treatment group. |
“Stained micro section organs” were observed in a blinded fashion. The assessors were not informed of any treatment prior before examination and stained mass analysis. The primary metabolic targets of any harmful medicine are the liver, heart &kidneys, which are vital.
When compared with the corresponding control, microscopic examination of the sections from the rats’ liver, kidneys, and hearts didn’t reveal any disorder lies or aberrant changes in histopathology.(Figures 2– 4) On a physical examination, the organs appeared and felt normal.
Table 4: Mean of relative organ weight of rats in both groups at the end of the study.
| Control Group | 5000mg/kgFicus | |
| Kindney | 1.18±0.05 | 1.24±0.09 |
| Liver | 5.74±0.09 | 6.13±0.12 |
| Heart | 0.59±0.17 | 0.59±0.21 |
Rats given Ficus extracts did not significantly differ in their relative organ weight indices for their hearts, kidneys, or livers from the corresponding control groups (Table 4).
Discussion
Since ancient times, medicinal plants have been used to treat various ailments [14]. As the WHO advocates appropriate ethno medicinal usage and indicates the safety evaluation of herbal medications [15-18], phytotherapy is becoming more and more popular. FDA and WHO place a strong emphasis on conducting research with a scientific foundation to verify the effectiveness and safety of using herbal medicines [19]. Validating the safety of herbal medicines requires a preliminary toxicological study. Although Ficus benghalensis aerial roots offer beneficial pharmacological effects, there hasn’t been a thorough understanding of their toxicity potential. Following OECD guidelines 423, this study was therefore conducted to evaluate the acute toxicity of ethyl acetate extracts of Ficus benghalensis aerial roots in animal models. This must ascertain “the safer dose range to manage the clinical signs and symptoms of the drugs” [20]. Wistar female rats were used in this investigation.
Clinical signs and symptoms are the main findings among many other indicators of toxicity and reveal the hazardous effects of medications on important bodily organs [21]. Food and water intake were normal over the 14-day acute toxicity study period, and no appreciable changes were seen in body weight. This implies that lipid, carbohydrate, and protein metabolism inside an animal’s body is normal given the significant roles that these nutrients play in various physiological processes [22-24]. The important organs of our body- the liver, heart, kidney, spleen, & lungs, are the main metabolic targets of any hazardous chemical [25]. In comparison to the control group, no lesions were discovered in the heart, kidney, or liver when the animals were euthanized at the conclusion of the study. The organs did not exhibit any necropsy or aberrant morphological alterations upon gross inspection. When compared to the tissues of the control rats, the analysis (microscopic) of the H&E stained sections revealed no alterations.
In comparison to the rats in the vehicle control group, the “organ to body weight index “of the Ficus extract group showed no statistically significant differences. “Chemicals are categorised into five classes based on their LD50 values, according to the worldwide agreed classification system [26]. The Ficus benghalensis ethyl acetate extract can be put into group 5 (LD50 > 5000 mg/kg), which is the least harmful category.
In this toxicity investigation, several significant biochemical data were also included. The kidney, liver, and heart are the main organs vulnerable to drug toxicity. Serum creatinine, total protein levels and urea levels were measured to assess kidney function, and the levels of AST and ALT were measured to evaluate liver integrity. The experiment’s findings revealed that both the Ficus extract-treated rats’ kidney and liver functions were unaffected, just like the control. Biochemical levels did not change statistically significantly between untreated and treated rats. These results imply that Ficus extracts did not have any negative impact on the rats’ kidney, heart, and liver.
All the rats in this study displayed comparable growth in weight that followed a typical pattern. Treatment groups didn’t see any weight loss or gain, indicating that the Ficus extracts did not significantly alter the rats’ appetites or have a negative impact on their overall health or metabolic development. It was also observed that the rats’ feed and water consumption from the start of the experiment to its conclusion followed a typical and consistent pattern. Administration of Ficus extract did not significantly (p> 0.05) change the pattern of body weight or feed consumption, indicating that the plant’s aerial roots did not negatively affect the rats’ growth and development. These results imply that Ficus extracts did not have any negative impact on the rats’ kidney, heart and liver.
The ethyl acetate extract of Ficus benghalensis aerial roots was found to had a “no observed adverse effect limit (NOAEL)” of 5000 mg/Kg body wteight/day according to the results of this acute oral toxicity investigation. To prove its safety when used for an extended period, additional toxicity assessments such as “sub-acute, chronic, or genotoxic studies” employing repeated doses of Ficus aerial root extract should be carried out.
Conclusion
The Ficus benghalensis aerial roots’ ethyl acetate extracts comprise pharmacologically active substances with proven positive pharmacologic effects.
No mortality was noted in any rat in the treatment group, and all of the provided Ficus extract samples resulted in normal behavioural, motor, and neural functions. All the parameters checked remained either unchanged or statistically insignificant compared to the control group. Even at 5000 mg/kg body weight (a high dose), the Ethyl Acetate Extracts of Ficus benghalensis Aerial Roots did not show any overt harmful effects. This demonstrated that the oral LD50 i.e., median lethal dose of Ficus benghalensis aerial roots ethyl acetate extracts was larger than 5000 mg/Kg body weight.
The Ficus benghalensis aerial root ethyl acetate extracts are therefore believed to be safe for usage as an oral health supplement and our findings are evidence that the aerial root extract was also suitable for in-vivo biological screening. To increase understanding, sub-acute and chronic toxicity studies should examine the safety of administering doses repeatedly.
Conflict of Interests
In relation to the publishing of this paper, the author hereby certifies that there are no competing interests.
Funding Sources
The authors didn’t receive any financial support for this study
References
- Ekor M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol 2014; 4:177.
CrossRef - H. Sellami, W. A. Wannes, I. Bettaieb et al., “Qualitative and quantitative changes in the essential oil of Laurusnobilis L. leaves as affected by different drying methods,” Food Chemistry, vol. 126, no. 2, pp. 691–697, 2011.
CrossRef - A. Wojdyło, A. Figiel, K. Lech, P. Nowicka, and J. Oszmiański, “Effect of convective andvacuum-microwave drying on the bioactive compounds, color, and antioxidant capacity of sour cherries,” Food and Bioprocess Technology, vol. 7, no. 3, pp. 829–841, 2014.
CrossRef - D. J. Newman and G. M. Cragg, “Natural products as sources of new drugs over the 30 years from 1981 to 2010,” Journal of Natural Products, vol. 75, no. 3, pp. 311–335, 2012.
CrossRef - P. Gregory, D. J. Hein, M. A. Malesker, and L. E. Morrow, “Over the counter nutritional supplements: implications for critically ill patients,” in Diet and Nutrition in Critical Care, pp. 1005–1016, Springer, 2015.
CrossRef - Govindan V, Francis GS. Qualitative and quantitative determination of secondary metabolites and antioxidant potential of Ficus benghalensis Linn seed. Int J Pharm PharmSci 2015;7:118-24.
- Gayathri M, Kannabiran K. Antidiabetic and ameliorative potential of Ficus benghalensis bark extract in streptozotocin induced diabetic rats. Indian J ClinBiochem 2008;23:394-400.
CrossRef - Patil VV, Patil VR. Ficus benghalensis Linn.-an overview. Int J Pharma Bio Sci 2010;1:1-11.
- Patel MA, Patel PK, Patel MB. aqueous extract of Ficus benghalensis Linn. Bark for inflammatory bowel disease. J.young pharm 2010;2:130-6.
CrossRef - G. L. Kennedy Jr., R. L. Ferenz, and B. A. Burgess, “Estimation of acute oral toxicity in rates by determination of the approximate lethal dose rather than the LD50,” Journal of Applied Toxicology, vol. 6, no. 3, pp. 145–148, 1986.
CrossRef - W. Diener, U. Mischke, E. Schlede, D. Kayser. The biometric evaluation of the OECD modified version of the acute toxic category method (oral)Arch. Toxicol., 69 (1995), pp. 729-734
CrossRef - U. Saleem, Sunita Mary. Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharummunja Roxb .roots in albino mice as per OECD 425 TGToxicol. Rep., 4 (2017), pp. 580-585
CrossRef - R. S. Sellers, D. Morton, B. Michael et al., “Society of Toxicologic Pathology position paper: organ weight recommendations for toxicology studies,” Toxicologic pathology, vol. 35, no. 5, pp. 751–755, 2007.
CrossRef - W. Ridtitid, C. Sae-Wong, W. Reanmongkol, M. Wongnawa. Antinociceptive activity of the methanolic extract of Kaemp feriagalangalinn.In experimental animals.J Ethnopharmacol., 118 (2) (2008), pp. 225-230
CrossRef - WHO. Research Guidelines for Evaluating the Safety and Efficacy of Herbal Medicines. World Health Organization (1993), p. 94
- G.P. Daswani, S. Brijesh, J.T. Birdi. reclinical testing of medicinal plants: advantages and approaches. Workshop Proceedings on Approaches towards Evaluation of Medicinal Plants Prior to Clinical Trial Citeseer (2006)
- S.O. Ogbonnia, G.O. Mbaka, E.N. Anyika, O.M. Osegbo, N.H. Igbokwe. Evaluation of acute toxicity in mice and subchronic toxicity of hydroethanolic extract of chromolaenaodorata (l.) king and robinson (fam. Asteraceae) in rats. ABJNA, 1 (5) (2010), pp. 859-865
CrossRef - Y.K. Vaghasiya, V.J. Shukla, S.V. Chanda. Acute oral toxicity study of Pluchea argutaboiss extract in mice. J. Pharmacol. Toxicol., 6 (2) (2011), pp. 113-123
CrossRef - R.W. Setzer, C.A. Kimmel. Use of noael, benchmark dose, and other models for human risk assessment of hormonally active substances.Pure Appl. Chem., 75 (11-12) (2003), pp. 2151-2158
CrossRef - U. Saleem, B. Ahmad, M. Ahmad, A. Erum, K. Hussain, N.I. Bukhari. Is folklore use of euphorbia helioscopia devoid of toxic effects?.Drug Chem. Toxicol., 39 (2) (2016), pp. 233-237
CrossRef - L.J. Subramanion, Z. Zakaria, Y. Chen, Y.L. Lau, L.Y. Latha, S. Sasidharan. Acute oral toxicity of methanolic seed extract of cassia fistula in mice. Molecules, 16 (6) (2011), pp. 5268-5282
CrossRef - P.O. Iversen, G. Nicolaysen. Water–for life.Tidsskrift for Den Norske Laegeforening: tidsskrift for praktiskmedicin, nyraekke, 123 (23) (2003), pp. 3402-3405
- K.R. Stevens, L. Mylecraine. Issues in chronic toxicology.Principles Methods Toxicol., 3 (1994), p. 673
- Z. Gregus, C.D. Klaassen. Mechanisms of toxicity.Casarett and Doull’s toxicology: The Basic Science of Poisons, 6 (2001), pp. 35-82
- C.S. Auletta. Acute, Subchronic and Chronic Toxicology.CRC Press, London (1995)
- Secretariat United Nations. Economic Commission for Europe. Globally Harmonized System of Classification and Labelling of Chemicals (ghs).United Nations Publications, 2009) (2017).







