Manuscript accepted on :21-11-2022
Published online on: 07-12-2022
Plagiarism Check: Yes
Reviewed by: Dr. Gandham Rajeev
Second Review by: Dr. Pankaj Singh
Final Approval by: Dr. Ian James Martin
Jihan Hussein1* , Hanan Farouk2
, Hanan Farouk2 and Zakaria El-khayat1
and Zakaria El-khayat1
1Medical Biochemistry Department, National Research Centre, 33 El Behouth St., 12622 Dokki, Giza, Egypt
2Therapeutic Chemistry Department, National Research Centre (NRC), 33 El Bohouth St. (Former El- Tahrir St.), Dokki, Cairo, 12622, Egypt
Corresponding Author E-mail: jihan_husein@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2529
Abstract
Nephrotoxicity is a prominent cause of global of injury and mortality. The aim here is to investigate the therapeutic role of selenium in treatment of cisplatin-induced experimental nephropathy. Animals were classified into four groups including cisplatin group in which animals were injected (intraperitoneal) with a single dose of cisplatin, while treated group in which rats injected with cisplatin and then received selenium (0.5 mg /k.g.b.w. / day) orally for ten days , control group , and selenium group in which healthy rats received selenium in a dose of 0.5 mg /k.g.b.w. / day , orally for ten days .After the experimental period, samples (blood and kidney tissues) were collected from each rat to estimate different biochemical and histological parameters using different techniques. Cisplatin significantly increased serum creatinine and urea comparing to control. However, reduction in catalase antioxidant enzyme was recorded in nephrotoxic rats, while marked increase in lipid peroxide (MDA), Advanced Oxidant Protein Product (AOPP), interleukin-1β(IL-1β), ceramide, tumor necrosis factor –α (TNF–α), metalloproteinase -9 (MMP-9)and homocysteine (Hcy) levels was detected as compared to control. Histopathological investigation revealed necrobiotic changes and deterioration in the lining tubular epithelium and tubular cystic dilatation at the cortex and inflammatory cells between the degenerated tubules. Treatment with selenium showed improvement in histopathological picture and corrective effects in all biomarkers under investigation. Nephrotoxicity induced by csplatin in rats is associated with remarkable elevation of oxidative stress, inflammatory markers, and renal histopathological lesions. While, the therapeutic effect of selenium (Se) may be attributed to` the alleviation of ROS-mediated apoptosis. These current results indicated that Se may be offer a promising dietary supplement against nephrotoxicity.
Keywords
Antioxidant; Cisplatin; Inflammatory Markers; Kidney Function; Oxidative Stress; Selenium
Download this article as:| Copy the following to cite this article: Hussein J, Farouk H, El-khayat Z. Therapeutic Efficacy of Selenium in Management of Hyperhomocytenemia in Cisplatin-Induced Nephrotoxicity. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Hussein J, Farouk H, El-khayat Z. Therapeutic Efficacy of Selenium in Management of Hyperhomocytenemia in Cisplatin-Induced Nephrotoxicity. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3HhJc3R |
Introduction
Chemotherapy drug including cisplatin has a huge number of side effects such as nephrotoxicity; which is considered an important disease in the course of chemotherapy. Up to 60% of all cancer cases in the hospitals also have acute kidney injury (AKI) that is linked with a significant rate of mortality. Several mechanisms were involved for the development of nephrotoxicity such as DNA damage, mitochondrial dysfunction, inflammation, and oxidative stress; in addition to tubular epithelial cells cytotoxicity1.Cis-diamminedichloroplatinum II (Cisplatin) is a chemotherapeutic drug which is used for the treatment of many solid tumors, such as neck, head, lung, breast, ovary, and testis. Whereas cisplatin induces numerous toxicities including myelosuppression, ototoxicity, gastrotoxicity, and allergic reactions, the main side effect is nephrotoxicity1. Nephrotoxicity of cisplatin has been documented since its consent for clinical use from more than 34 years ago. Cisplatinis associated with various types of diseases such as hypomagnesemia, hypocalcemia, fanconi-like syndrome, acute kidney injury (AKI), renal tubular acidosis, and hyperuricemia2. However, the most severe and the more common side effects is AKI. The pathophysiological occurrences of cisplatin-induced KI include successive induction of renal vasoconstriction, reduction of the renal plasma flow, decline in the glomerular filtration rate, and elevation of kidney function in addition to a reduction in serum potassium and magnesium. The basis of cisplatin-induced nephrotoxicity has been studied. A dynamic inflammatory response and stimulation of inflammasomes are also observed. 1
Selenium (Se) which is an essential trace element, considered as an efficient constituent of numerous enzymes such as glutathione peroxidases 3 , can effectively eliminate free radicals, protect organs and tissues from oxidative stress damage and apoptosis 4.Se can play an important role in protecting the renal cells from various poisons-induced damage by elevating selenase expression in the organism, scavenging ROS, and obstructing cell apoptosis 3,5,6 .To our knowledge , it is the first study investigates the relation between cisplatin induced renal toxicity and homocysteine level .
So, this study was designed to assess the mechanism /s underlined the role of cisplatin to induce nephrotoxicity in rats and the ameliorative effect of selenium as supplement essential trace element in nephrotoxic rats, through determination of kidney functions, some pro-inflammatory markers, AOPP (advanced oxidative protein product) and oxidative stress markers, metalloproteinase -9(MMP-9), ceramide as well as homocysteine.
Materials and Methods
Materials
Chemicals
Cisplatin (1mg/mL) was purchased from a local pharmacyandSodium selenite (Na2SeO3) was purchased from Merck Chemical Inc. (Darmstadt, Germany). All other using chemicals were HPLC grade and purchased from Sigma-Aldrich, Germany.
Animals
Forty male albino rats (140±10 g) were purchased and housed in 22 ± 2 °C, under a 12-h light/12-h dark; animals were housed in stainless steel cages, and the they allowed for acclimatization for a period of 7-10 days before the experiment; rats were freely allowed to access water and food. Animal procedures were followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985) and the experiment followed the recommendations and guidelines of the ethical commette of the National Research Centre ( NRC).
Induction of nephrotoxicity
Animals were injected with cisplatin (intraperitoneal injection with10 mg/kg body weight once) as modified from the previous method 7.
Experimental design
Forty rats were distributed randomly into four groups ( 10 rats each ) as follows:
Group I (control group): normal rats received water.
Group II (selenium group): normal rats received selenium (0.5 mg/kg b.w. as Na2SeO3 dissolved in water) orally for ten days
Group III (cisplatin group): normal rats received cisplatin then received water daily for ten days.
Group IV (treated group): normal rats received cisplatin and then received selenium (0.5 mg/kg b.w. as Na2SeO3 dissolved in water) orally for ten days 8.
After the experimental period (10 days); animals were fasted overnight and blood was collected from the orbital vein, centrifuged at 4000 rpm for 10 min. Serum samples were stored in -80 oC for subsequent analysis. Kidney was removed quickly from all animals and washed with ice-cold saline; and then divided into two parts: The first one was for determination of biochemical parameters and the other part was for histopathological examination.
Preparation of kidney homogenate
Kidney tissues were homogenized using phosphate buffer at ( pH 7.4) and centrifuged for 10 min at 4000 rpm using cooling centrifuge that was adjusted at 4 ºC. Supernatant was then separated and used for estimation of catalase and lipidperoxide (MDA) 9, 10. However, other biomarkers under investigation are carried out in serum.
Biochemical analysis
Blood urea and serum creatinine were estimated as described previously11.
Oxidant / antioxidant parameters
Catalase (CAT) activity was evaluated by colorimetric method as described by 12.
Malondialdehyde (MDA) were estimated by spectrophotometer colorimetrically according to 13 .In addition , AOPP , the advanced oxidation protein product was assessed by ELISA (NOVA, Bioneovan Co., Ltd) according to the given instructions 14.
Inflammatory markers
IL-1β (interleukin 1β),TNF-α (tumor necrosis factor α),MMP-9 (metalloproteinase -9), and ceramide were evaluated using rats enzyme-linked immunosorbent assay (ELISA) (Bioneovan Co., Ltd., Beijing, China).
Homocysteine (Hcy) estimation
Serum Hcy was estimated according to previous study 15 by high performance liquid chromatography (HPLC) system“Agilent technologies 1100 series, equipped with a quaternary pump “G131A model”. Briefly, serum (400 μ L) were mixed with 40 μL of trichloroacetic acid (TCA) and incubated for 30 min in refrigerator for protein precipitation; this mixture was then centrifuged at 4000 rpm for 10 min at 4 ºC; after the filtration of the supernatant through PVDF syringe filter (pore size 0.45 mm , diameter 25 mm ),twenty μlfrom this supernatant was injected onto HPLC.
HPLC condition
Separation was achieved on the reversed phase (RP) column (C18 X 25, 0.46 cm); thermostat was adjusted at 40º C. The mobile phase consists of 40 mmol/L monobasic sodium phosphate, 8 mmol/L heptane sulfonic acid, and 18%methanol (v/v) (pH3.1). The mobile phase was filtered twice with membrane filter (0.45 mm) and delivered at a flow rate 1 mL/min. UV was set at 260 nm. Serial dilutions of Hcy standard were injected to plot the standard curve and calculate the concentration of each sample using software of Agilent technology.
Histopathological examination
Samples were taken from the kidney of all animals and fixed in formal saline solution (10%) for 24 hours, then washed with a tap water followed by serial dilutions of alcohol (methyl, ethyl and absolute ethyl alcohol) for dehydration before cleaning in xylene and implanted in paraffin at 56˚C in anoven for 24 hours. Paraffin blocks were prepared for sectioning at 4 microns thickness by Leitz rotary microscope. The tissue sections were collected on glass slides, deparaffinized, and stained by hematoxylin & eosin stain for investigation through the light electric microscope 16.
Statistical analysis
Statistical analysis was done for the collected data using SPSS software (version 22)using one way ANOVA to evaluate significant differences between groups; then results were presented as mean ± standard error (SE) and P was considered significant when it ≤ 0.05.
Results and discussion
A significant elevation in the levels of creatinine and urea was detected in nephrotoxic rats reached to 208.57 and 62.0% respectively compared to the normal control levels. While, treatment of nephrotoxic rats induced with cisplatin and treated with selenium recorded amelioration percentages 104.29 % and 34.75%, for creatinine and urea respectively as represented in Table(1). However, treatment with selenium showed insignificant change in healthy treated group compared to untreated control rats.
Serum levels of creatinine and urea are usually used as indicators of kidney functions. Serum creatinine is used as a mirror to reflect the ability of the kidney in cleaning the blood from creatinine and excrete it in the urine. Renal dysfunction also causes an elevation of blood urea nitrogen (BUN) levels since the kidneys in this situation haven’t the ability to clear the blood stream from urea 17 .
The high levels of urea and creatinine in the cisplatin –induced nephrotoxicity was described previously through different mechanisms; tubular injuriousness (cell death by apoptosis or necrosis), renal vasoconstriction, and damages on glomerular parts including basement membrane, capillaries, mesangial cell, podocytes, and parietal cells), and interstitial injury (injuries by inflammatory responses). The stepwise and the complex progressions that lead to renal damage are occured by the elevation of the possibly toxic compounds in the tubular fluid that diffuse into the extremely permeable tubular cells. Cisplatin, the uncharged molecule which has a small molecular weight, is filtered through the glomeruli, taken up by the renal tubular cells and eventually reaches its main gradient in the proximal tubular inner medullae and outer cortices 1. Consequently, these zones are the main situates for cisplatin-induced renal toxicity, which sequentially, causes injury in other tubular parts including the collecting and distal tubules 1.
Table (2), declared significant reduction in the catalase level in nephrotoxic rats reached to 66.44% compared to control level . While noticeable significant increase in MDA and AOPP with percentages 203.96 and 281.32 %, respectively compared to control values. Selenium –treated nephrotoxic rats showed marked improvement in the Catalase, MDA and AOPP levels with percentages of 34.93, 193.65 and 173 .58% respectively. Contrarily, selenium supplementation to the healthy rats showed insignificant change in all studied parameters.
The low level of antioxidant enzyme catalase and the high level of MDA may be due to reactive oxygen species (ROS) directly target the lipid components of the cell membrane causing peroxidation and denaturation of proteins, which finally leads to enzymatic inactivation. The free radicals (FRs) are generated by mitochondria, xanthine-xanthine oxidase, and NADPH oxidase in the cells. After the treatment with cisplatin, ROS are formed through these systems and are associated with the pathogenesis of acute renal injury (ARI) 1. Cisplatin motivates enzymatic activation of glucose-6-phosphate dehydrogenase (G6PD) and hexokinase, that advance the FR production and diminish the production of antioxidant 1. Moreover, cisplatin elevates the intracellular calcium concentration that in turn stimulates NADPH oxidase and ROS creation leading to mitochondrial dysfunction. In addition, it has an inhibitory effect on antioxidant enzymes including SOD, glutathione peroxidase, and catalase 18,19 .
On the other hand, protein content is considered the most leading cause of oxidative stress and, consequently, AOPP are an important factor in renal dysfunction and a significant marker of oxidative stress, where AOPP act as a moderator of oxidative stress. Witko et al.,[1]indicated that AOPP could contribute with the monocyte-mediated inflammatory disorders linked with uremia. Besides, they suggested that AOPP level considered as a dependable marker for assessing the amount of oxidant-mediated protein mutilation in patients with renal dysfunction and for expecting the potential efficiency of therapeutic plans aimed at decreasing such an oxidative stress. In this current work we found that the AOPP level was significantly increased (p≤0.001) owing to the oxidation of protein in nephrotoxic rats. The augmentation in AOPP creates by the plasma proteins oxidation and gathers with coronary and renal diseases 20. In agreement, a concomitant elevation was found between AOPP and the creatinine level demonstrating that AOPP is a good marker for the advancement of chronic renal failure 19.
In nephrotoxic group, a significant increase in inflammatory markers with 209.84, 185.99, 175.40 and 213.12%, respectively for IL-Iβ, TNF-α, ceramide and MMP-9 was observed. Selenium –treated rats showed down regulation in inflammatory markers to nearly the half fold of nephrotoxic levels with ameliorations percentage 177.05, 140.10, 137.70 and 158.82%, respectively. No significant difference was detected in the health rats administered with selenium compared to control rats (Table 3). In agreement with our results, Oh etal.1 demonstrated that, the elevation of renal expression of TNF-α was proved in mouse models of cisplatin nephrotoxicity. The same authors added that, TNF-α inhibitors decreased cisplatin-induced renal injury and histological indication of toxicity and the production of TNF-α by cisplatin is highly dependent upon ROS, NF-κB stimulation and initiation of p38 MAPK47. The expression of inflammatory chemokines and cytokines is augmented in the kidney following the injection of cisplatin21, 22; thus, the expression of IL-1β, IL-18, CX3CL1, and IL-6 was increased in kidney tissue. Additionally, deletion of caspase-1, which is responsible for the creation of IL-1β and IL-18 through stimulation of inflammasomes, reduced cisplatin induced renal injury and neutrophil infiltration in the kidney in vivo 3,23.
It was found that, the renal damage through tubular cell death is a common histopathological feature of cisplatin nephrotoxicity (as showed in Fig.1). Cisplatin-induced nephrotoxicity mechanisms are complicated and cover numerous cellular processes including inflammation oxidative stress, and apoptosis). For example, cell death in the form of both necrosis and apoptosis has been recognized (Photomicrographs 2-4, Fig.1). Numerous apoptotic pathways have been involved in cisplatin-induced renal epithelial cell death including the extrinsic pathway that activated through the tumor necrosis factor alpha (TNF-α), interlukin 1 beta (IL-1β ) and, cell death receptors, in addition to the intrinsic mitochondrial and the endoplasmic reticulum (ER) stress pathways. In the extrinsic pathway, ligands bind to the death receptors on the cell membrane, with enrollment and stimulation of caspase-8, which promotes downstream caspases leading to apoptosis 23.
The present results indicated also significant increase in the level of ceramide in nephrotoxic rats indicated apototosis. Ceramide, the breakdown product of sphingolipid, is associated with a main participant not only in apoptosis but also in cell growth and proliferation 24,25. Elevation of ceramide levels is responsible to numerous procedures of cell stress and it has been revealed to adjust the apoptotic reaction in numerous cell organizations and to apply effective development to oppressive effects in different cell types24 .The high concentrations of ceramide induce mitochondria-dependent apoptosis, exacerbate the synthesis of reactive oxygen species, reduction in ATP level, inhibition of electron transport and releasing of cytochrome c, and activation of caspase-3. Enhancement of ceramide levels in this pathology is the effect of their synthesis de novo or augmentation of sphingomyelin metabolism. The ceramide pathway can directly motivate biochemical alterations in the tissue celebrated at the beginning of disease. In addition, the elevated concentration of ceramide in blood in the pre-clinical period of the illness may spot initial brain alterations25.
Extracellular matrix (ECM) proteins play important roles in tubular injury; MMPs drive proteolytic processes critical to renal basement membrane remodeling. MMP-2 andMMP-9 were detected in glomerulus and proximal tubules of rats26.The current results , declared the high level of MMP-9 in cisplatin –induced nephrotoxicity in rats. It was documented that, proximal tubule cells necessitate adhesion to the basement membrane for regular function and also to evade apoptosis. Preserving collagen IV-integrin interfaces is necessary to regenerate the physiological purposesnext to cellular injury 27 .Closely associatedwith the instruction of collagen-integrin interactions are the MMP-9, which has the capability to destroy basement membrane types IV and V collagens, elastin, aggrecan, and gelatins 26 . In a parallel with the present results, a previous study 28 supported the idea that MMPs mediate acute kidney injury and are involved in changes in the glomeruli and tubular epithelial cells. MMPs are also implicated in ischaemia-reperfusion injury and linked to the elevation of MMP-2 and -9 expression in glomeruli. In an acute kidney allograft rejection, MMP-9 levels augmented during rejection, while MMP-2 levels were reduced 28. Significant high level of Hcy was recorded in Cisplatin induced nephrotoxicity in rats with remarkable percentage reached to 272.74%, compared to control. Selenium –treated nephrotoxic rats showed significant reduction in Hcv level to the half value demonstrated in nephrotoxic rats with improvement percentage 189.18%. However, selenium treated healthy rats showed insignificant difference in its level compared to control (Table 4).
Homocysteine (Hcy),thiol group containing amino acid, is naturally occurred in all individuals. It degraded in the body through two documented pathways, while a slight part is excreted through kidneys. Folic acid , vitamins B6 , and B12 are important factors in these reactions that are necessary for degradation of Hcy. Therefore, Hcy level is influenced by the presence or absence or the reduction of these vitamins. Hyperhomocysteinemia (HHcy) in the present results is associated with chronic renal failure. HHcy also may be attributable to genetic mutations and enzyme shortages in 5, 10-methylenetetrahydrofolate reductase (MTHFR), methionine synthase (MS), and cystathionine β-synthase (CβS). Additionally, HHcy may be due to the deficiency of B6 vitamin that influences methionine metabolism. Furthermore, HHcy can be produced by renal injury 29 .
The overproduction of reactive species in nephrotoxicity induced by cisplatin leads to the oxidative damage 30. ROS plays important roles in regulating the signaling pathways 31. Overproduction of ROS will harm proteins, lipids, and also DNA, and to end withactivation of apoptotic signaling. DNA damage as an important oxidative stress marker will activate downstream apoptotic signaling pathways, such as the DNA damaging signal pathway. P53 , Bcl-2 family/p21,ATR/ATM, all can be stimulated in response to oxidative motivations30 . It was indicated that high level of Hcy could encourage accumulation of ROS leading to the endothelial dysfunction, and damage the vessel wall 32. However, Hcy-induced oxidative damage and underlying mechanism remain subtle.
Treatment of nephrotoxic rats with selenium showed downregulation of inflammatory markers, reduced oxidative damage, corrected the levels of urea and creatinine, normalized level of Hcy. Treatment of antioxidant is considered an important way to restrict cisplatin induced nephrotoxicity . Se, the well-known essential trace element, was reported to provide protection from ROS-induced cell damage 33, 34. Pretreatment with Se can reduce ROS and cell apoptosis that induced by various toxic agents 35. In this study, we aimed to explain whether the beneficial effect of Se on cisplatin -induced nephrotoxicity is related to the reduction of ROS-mediated apoptosis.
Renal injury is mainly attributed to renal dysfunction and increased possibility of death. Se applied the positive influence on the reduction of body weight (BW) induced by streptozotocin in Wistar rats ; thus, injurious effects of STZ caused alkylation of DNA and produced hyperglycaemia and necrotic lesions 36 suggesting that selenium may reduce gluconeogenesis, glycogenolysis, , and also proteolysis to upgrade the level of insulin37.
It was observed that Se enhanced T-2 toxin induced reduction in the BW and kidney coefficient 3. Sealso affects feed utilization through contribution in the carbohydrates, lipids, and proteins metabolism 3 ,that play a significant role in refining the BW and organ coefficient of mice. Histopathological examination in this current results appeared the role of Se in amelioration of tissue construction and showed few focal hemorrhages between the tubules and glomeruli at the cortex as well as some deterioration in the tubular lining epithelium at the cortex compared to control(Photomicrographs 5,6 and 1,Fig.1).In a good agreement with the present results Zhang et al 3 indicated that Se pretreatment reduced T-2- induced tubular lesions. Additionally, they found that Se ameliorated T-2-induced structure lesion of kidney, the ultrastructure of kidney was observed by TEM, and appeared the pretreatment with Se markedly antagonized T-2-induced swelling of mitochondria, loss of mitochondrial cristae, and vacuolation of mitochondria. The thinkable reason is that Se is a constituent of many selenoproteins with redox function 4 that plays a main role in redox regulation. The redox regulation protects the integrity of cell membrane 38, participating in the repair of damaged kidney tissue and protecting it 34.These results showed that Se can improve the damage of renal structure and the kidney development inhibition caused by cisplatin. Pretreatment with Se effectively diminished the renal dysfunction of streptozotocin-exposed rats and cadmium-exposed mice and reduce the T-2-induced increase of renal function biomarkers, suggesting that Se can antagonize the renal dysfunction induced by T-2 toxin 3,4 . At the cellular level 3, illustrated that, pretreatment of nephrotic rats with Se attenuated the apoptosis. ROS is mainly created in mitochondria, and raised ROS levels will abolish the normal construction of cells, resulting in dysfunction. Moreover, ROS is closely linked to mitochondrial membrane damage, which can lead to cell apoptosis 39. Se is involved in the construction of GSH-Px enzyme, which plays a vital role in the elimination of ROS 3 .Se diminished the formation of ROS and the reduced activity of GSH-Px in ochratoxin A-treated porcine kidney epithelial cells and fluorine-treated broiler kidney 6 .Se plays a significant role in human and animal, which neutralizes, eliminates, and blocks the production of ROS, and Se could eliminate ROS through antioxidant GSH-Px 40. Therefore, we speculate that ROS production may be involved in renal dysfunction and excessive apoptosis produced by cisplatin poisoninig, while Se advances normal renal structure and function and avoids apoptosis by eliminating excessive ROS. Mitochondria are the major source of ROS production in cells, in turn, the most adversely affected organelles; excessive ROS can result in mitochondrial dysfunction 30 .
Contrarily, MMP plays a main role in mitochondrial homeostasis by selectively abolishing dysfunctional mitochondria. It is also the motivating power for the passage of ions and proteins, which are necessary for the mitochondria function. The continuous increase or decrease of MMP may be lead to needless loss of cell viability and various pathological changes 41. Apoptosis is closely related to the stability of MMP, when MMP collapses, apoptosis is irreversible 42 . The collapse of MMP can lead to apoptosis of normal cells through mitochondrial pathway 43. We found pretreatment with Se ameliorated cisplatin -induced MMP collapse. A similar antagonistic effect of Se has been found in the NaF treated rat kidney cells (NRK-52E) and cadmium-treated ICR male mice kidney 44. Therefore, it is suggested that Se may protect the kidney by restoring the normal level of MMP and then reducing abnormal apoptosis.
Table 1: Blood urea and serum creatinine levels in different studied groups.
| Parameters
Groups |
Creatinine
(mg/dl) |
BUN
(mmol/L) |
| Group I (control group) | 0.70 ± 0.09 | 4.72 ± 0.32 |
| Group II (selenium group)
%change |
0.6± 0.10
-14.29% |
4.03 ± 0.24
-14.62 |
| Group III (cisplatin group)
%change |
2.16a ± 0.19
208.57% |
7.65a ± 0.34
62.08% |
| Group IV(treated group) % change | 1.43a,b ± 0.04
104.29% |
6.01a,b ± 0.38
34.75% |
P: a significant difference compared to Pa) the control group, Pb) CisPt group.
Table 2: Antioxidant and oxidant parameters in different studied groups.
| Parameters
Groups |
CAT
(U/g tissue) |
MDA
(nmol/g tissue) |
AOPP
(ng/mL)
|
| Group I (control group) | 1.46 ± 0.10 | 12.60 ± 0.80 | 5.30± 0.70 |
| Group II (selenium group)
%change |
1.52± 0.10
+4.11 |
11.20 ± 0.70
-11.11 |
5.10± 0.60
-3.77 |
| Group III (cisplatin group)
%change |
0.49a ± 0.04
-66.44 |
38.30a ± 1.1
+203.96 |
20.21a±1.20
+281.32 |
| Group IV(treated group)
% change |
1.00a,b ± 0.06
34.93 |
13.90a,b ± 0.90
193.65 |
11.01a,b±0.90
173.58 |
P: a significant difference compared to Pa) the control group, Pb) CisPt group.
Table 3: Inflammatory biomarkers in different studied groups.
| Parameters
Groups |
IL-Iβ
(ng/mL) |
TNF-α
(ng/mL) |
Ceramide
(pg/ml) |
MMP-9
(pg/ml) |
| Group I (control group) | 12.20± 0.73 | 15.49 ± 0.90 | 31.30± 1.60 | 22.10 ± 1.70 |
| Group II (selenium group)
%change |
12.40± 0.64
+1.64 |
13.49 ± 0.80
12.91 |
29.80 ± 1.40
-4.79 |
20.5 ± 1.40
-7.24 |
| Group III (cisplatin group)
%change |
37.80a± 1.10
+209.84 |
44.30a ± 2.01
185.99 |
86.20a±2.10
175.40 |
69.20a±2.20
213.122 |
| Group IV(treated group)
% change |
16.20ab± 0.87
177.05 |
22.60ab ± 1.40
140.10
|
43.10ab±1.80
137.70 |
34.10ab ±1.50
158.82 |
P: a significant difference compared to Pa) the control group, Pb) CisPt group.
Table 4: Homocysteine levels in different studied groups.
| Parameters
Groups |
Hcy
ng/ml |
| Group I (control group) | 7.30 ± 0.30 |
| Group II (selenium group)
%change |
6.32 ± 0.20
-13.42 |
| Group III (cisplatin group)
%change |
27.21a± 1.10
+272.74 |
| Group IV(treated group)
% change |
13.40a,b± 0.92
189.18 |
P: a significant difference compared to Pa) the control group, Pb) CisPt group.
Histopathological Results
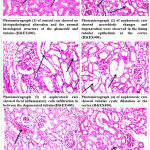 |
Figure 1: Histopathological examination of kidney in different studied groups |
Conclusion
We can concluded that cisplatin induced nephrotoxicity in rats as indicated by the noticeable elevation in creatinine and urea levels , oxidative stress , inflammatory markers, MMP-9 , ceramide, homcystein and renal histopathological lesions. While the alleviating effect of Se on cisplatin -induced nephrotoxicity may be related to the ROS-mediated apoptosis. These results sugges that Se can be used as a dietary additive against the toxicity of cisplatin toxin.
Acknowledgement
Authors are thankful for National Research Centre, Cairo, Egypt for carrying out this work
Authors’ contribution
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by dr.Jihan Hussein, the manuscript was written by Dr. Hanan Farouk , and reviewed by Dr. Zakaria El-Khayat and Dr. Jihan Hussein . All authors read and approved the final manuscript.”
Ethics approval
Animal procedures were followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
Conflict of interest
“The authors declare that they have no competing interests”
Funding Sources
Authors did not receive any fund.
References
- Oh G-S, Kim H-J, Shen A, Lee SB, Khadka D, Pandit A, So H-S: Cisplatin-induced kidney dysfunction and perspectives on improving treatment strategies. Electrolytes & Blood Pressure: E & BP 2014, 12(2):55.
- Miller R, Tadagavadi R: Ramesh g and Reeves WB: Mechanisms of cisplatin nephrotoxicity. Toxins 2010, 2:2490-2518.
- Zhang X, Wang Q, Zhang J, Song M, Shao B, Han Y, Yang X, Li Y: The protective effect of selenium on T-2-induced nephrotoxicity is related to the inhibition of ROS-mediated apoptosis in mice kidney. Biological Trace Element Research 2022, 200(1):206-216.
- Wang Y, Wu Y, Luo K, Liu Y, Zhou M, Yan S, Shi H, Cai Y: The protective effects of selenium on cadmium-induced oxidative stress and apoptosis via mitochondria pathway in mice kidney. Food and Chemical Toxicology 2013, 58:61-67.
- Liu L, Yang B, Cheng Y, Lin H: Ameliorative effects of selenium on cadmium-induced oxidative stress and endoplasmic reticulum stress in the chicken kidney. Biological trace element research 2015, 167(2):308-319.
- Long J, Liu Y, Zhou X, He L: Dietary serine supplementation regulates selenoprotein transcription and selenoenzyme activity in pigs. Biological Trace Element Research 2021, 199(1):148-153.
- Hussein J, El-Naggar ME, Fouda MM, Morsy OM, Ajarem JS, Almalki AM, Allam AA, Mekawi EM: The efficiency of blackberry loaded AgNPs, AuNPs and Ag@ AuNPs mediated pectin in the treatment of cisplatin-induced cardiotoxicity in experimental rats. International Journal of Biological Macromolecules 2020, 159:1084-1093.
- Milošević MD, Paunović MG, Matić MM, Ognjanović BI, Saičić ZS: The ameliorating effects of selenium and vitamin C against fenitrothion-induced blood toxicity in Wistar rats. Environmental toxicology and pharmacology 2017, 56:204-209.
- Hussein J, El-matty DA, El-Khayat Z, Abdel-Latif Y: Therapeutic role of coenzyme Q10 in brain injury during experimental diabetes. Journal of Applied Pharmaceutical Science 2013, 3(6):213-217.
- Medhat D, El-Mezayen HA, El-Naggar ME, Farrag AR, Abdelgawad ME, Hussein J, Kamal MH: Evaluation of urinary 8-hydroxy-2-deoxyguanosine level in experimental Alzheimer’s disease: Impact of carvacrol nanoparticles. Molecular biology reports 2019, 46(4):4517-4527.
- Marsh WH, Fingerhut B, Miller H: Automated and manual direct methods for the determination of blood urea. Clinical chemistry 1965, 11(6):624-627.
- Johansson LH, Borg LH: A spectrophotometric method for determination of catalase activity in small tissue samples. Analytical biochemistry 1988, 174(1):331-336.
- Ohkawa H ON, Yagi K.: Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979, 95:351-358.
- Badawy E, Rasheed W, Elias T, Hussein J, Harvi M, Morsy S, Mahmoud YE-L: Flaxseed oil reduces oxidative stress and enhances brain monoamines release in streptozotocin-induced diabetic rats. Human & Experimental Toxicology 2015, 34(11):1133-1138.
- El-Naggar ME, Hussein J, El-sayed SM, Youssef AM, El Bana M, Latif YA, Medhat D: Protective effect of the functional yogurt based on Malva parviflora leaves extract nanoemulsion on acetic acid-induced ulcerative colitis in rats. Journal of Materials Research and Technology 2020, 9(6):14500-14508.
- Banchroft J, Stevens A, Turner D: Theory and practice of histological techniques Fourth Ed Churchil Livingstone. New York, London, San Francisco, Tokyo:[Google Scholar] 1996.
- Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, Barrios CH, Bergh J, Bhattacharyya GS, Biganzoli L et al: 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Annals of Oncology 2020, 31(12):1623-1649.
- Badary OA, Abdel-Maksoud S, Ahmed WA, Owieda GH: Naringenin attenuates cisplatin nephrotoxicity in rats. Life sciences 2005, 76(18):2125-2135.
- Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B: Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney international 1996, 49(5):1304-1313.
- Marsche G, Frank S, Hrzenjak A, Holzer M, Dirnberger S, Wadsack C, Scharnagl H, Stojakovic T, Heinemann A, Oettl K: Plasma-advanced oxidation protein products are potent high-density lipoprotein receptor antagonists in vivo. Circulation research 2009, 104(6):750-757.
- Faubel S, Lewis EC, Reznikov L, Ljubanovic D, Hoke TS, Somerset H, Oh D-J, Lu L, Klein CL, Dinarello CA: Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1β, IL-18, IL-6, and neutrophil infiltration in the kidney. Journal of Pharmacology and Experimental Therapeutics 2007, 322(1):8-15.
- Lu LH, Oh D-J, Dursun B, He Z, Hoke TS, Faubel S, Edelstein CL: Increased macrophage infiltration and fractalkine expression in cisplatin-induced acute renal failure in mice. Journal of Pharmacology and Experimental Therapeutics 2008, 324(1):111-117.
- Strasser A, O’Connor L, Dixit VM: Apoptosis signaling. Annual review of biochemistry 2000, 69(1):217-245.
- Bitar FF, Mroueh S, El Khatib M, Bitar H, Tarrabain M, El Sabban M, Obeid M, Nasser M, Dbaibo GS: Tissue-specific ceramide response in the chronically hypoxic rat model mimicking cyanotic heart disease. Prostaglandins & other lipid mediators 2003, 72(3-4):155-163.
- Car H, Zendzian-Piotrowska M, Fiedorowicz A, Prokopiuk S, Sadowska A, Kurek K: The role of ceramides in selected brain pathologies: ischemia/hypoxia, Alzheimer disease. Postepy Higieny i Medycyny Doswiadczalnej (Online) 2012, 66:295-303.
- Romero F, Pérez M, Chávez M, Parra G, Durante P: Effect of uric acid on gentamicin‐induced nephrotoxicity in rats–role of matrix metalloproteinases 2 and 9. Basic & clinical pharmacology & toxicology 2009, 105(6):416-424.
- Nony PA, Schnellmann RG: Mechanisms of renal cell repair and regeneration after acute renal failure. Journal of Pharmacology and Experimental Therapeutics 2003, 304(3):905-912.
- Catania JM CG, Parrish AR.:. : Role of matrix metalloproteinases in renal pathophysiologies. . Am J Physiol Renal Physiol 2007, Mar;292( (3)):F905-911.
- Zaric BL, Obradovic M, Bajic V, Haidara MA, Jovanovic M, Isenovic ER: Homocysteine and hyperhomocysteinaemia. Current medicinal chemistry 2019, 26(16):2948-2961.
- Li X, Fang F, Gao Y, Tang G, Xu W, Wang Y, Kong R, Tuyihong A, Wang Z: ROS induced by KillerRed targeting mitochondria (mtKR) enhances apoptosis caused by radiation via Cyt c/caspase-3 pathway. Oxidative Medicine and Cellular Longevity 2019, 2019.
- Idelchik MdPS, Begley U, Begley TJ, Melendez JA: Mitochondrial ROS control of cancer. In: Seminars in cancer biology: 2017: Elsevier; 2017: 57-66.
- Cloonan L, Fitzpatrick KM, Kanakis AS, Furie KL, Rosand J, Rost NS: Metabolic determinants of white matter hyperintensity burden in patients with ischemic stroke. Atherosclerosis 2015, 240(1):149-153.
- Wang Y, Xiao X, Zhan X: Antagonistic effects of different selenium sources on growth inhibition, oxidative damage, and apoptosis induced by fluorine in broilers. Poultry science 2018, 97(9):3207-3217.
- Bas E, Naziroglu M: Selenium attenuates docetaxel-induced apoptosis and mitochondrial oxidative stress in kidney cells. Anti-Cancer Drugs 2019, 30(4):339-346.
- Jin X, Xu Z, Zhao X, Chen M, Xu S: The antagonistic effect of selenium on lead-induced apoptosis via mitochondrial dynamics pathway in the chicken kidney. Chemosphere 2017, 180:259-266.
- Roy S, Dontamalla SK, Mondru AK, Sannigrahi S, Veerareddy PR: Downregulation of apoptosis and modulation of TGF-β1 by sodium selenate prevents streptozotocin-induced diabetic rat renal impairment. Biological trace element research 2011, 139(1):55-71.
- Muecke R, Schomburg L, Buentzel J, Kisters K, Micke O, Elements GWGT, Oncology-AKTE Ei: Selenium or no selenium-that is the question in tumor patients: a new controversy. Integrative Cancer Therapies 2010, 9(2):136-141.
- Keswani T, Chowdhury S, Mukherjee S, Bhattacharyya A: Palladium (II) complex induces apoptosis through ROS-mediated mitochondrial pathway in human lung adenocarcinoma cell line (A549). Current Science 2014:1711-1719.
- Wang X-j, Chen W, Fu X-t, Ma J-k, Wang M-h, Hou Y-j, Tian D-c, Fu X-y, Fan C-d: Reversal of homocysteine-induced neurotoxicity in rat hippocampal neurons by astaxanthin: Evidences for mitochondrial dysfunction and signaling crosstalk. Cell death discovery 2018, 4(1):1-11.
- L. D. Zorova VAP, E. Y. Plotnikov, D. N. Silachev, I. B. Pevzner, S. S: Anal Biochem 2018, 552:50-59.
- Park C, Cha H-J, Hong SH, Kim G-Y, Kim S, Kim H-S, Kim BW, Jeon Y-J, Choi YH: Protective effect of phloroglucinol on oxidative stress-induced DNA damage and apoptosis through activation of the Nrf2/HO-1 signaling pathway in HaCaT human keratinocytes. Marine Drugs 2019, 17(4):225.
- Yang Y, Wang G, Wu W, Yao S, Han X, He D, He J, Zheng G, Zhao Y, Cai Z: Camalexin induces apoptosis via the ROS-ER stress-mitochondrial apoptosis pathway in AML cells. Oxidative medicine and cellular longevity 2018, 2018.
- Gao J, Tian X, Yan X, Wang Y, Wei J, Wang X, Yan X, Song G: Selenium exerts protective effects against fluoride-induced apoptosis and oxidative stress and altered the expression of Bcl-2/caspase family. Biological Trace Element Research 2021, 199(2):682-692.







