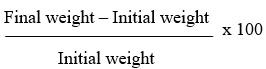Manuscript accepted on :23-11-2022
Published online on: 30-11-2022
Plagiarism Check: Yes
Reviewed by: Dr. Amit Gupta
Second Review by: Dr. Nicolas Padilla
Final Approval by: Dr. Anton R Kiselev
Enye Linus Anderson1* , Saka Olusola Stephen1
, Saka Olusola Stephen1 , Fakunle Bankole Peter1
, Fakunle Bankole Peter1 , Fafure Adedamola Adediran1
, Fafure Adedamola Adediran1  , Abijo Ayodeji Zabdiel3 and Arayombo Babatunde. E2
, Abijo Ayodeji Zabdiel3 and Arayombo Babatunde. E2
1Department of Anatomy, College of Medicine and Health Sciences, Afe Babalola University Ado Ekiti, Nigeria
2Department of Anatomy and Cell Biology, College of Health Sciences, Obafemi Awolowo University Ile Ife, Nigeria
3Department of Anatomy, Faculty of Basic Medical Sciences, Babcock University, Illehan-Remo, Nigeria.
Corresponding Author E-mail: enyelinus@abuad.edu.ng
DOI : https://dx.doi.org/10.13005/bpj/2579
Abstract
Cyclophosphamide is a synthetized drug and alkylating agent used for treating cancer. In this research, cyclophosphamide's effect on the testicles of adult male Wistar rats will be assessed, as well as the impact of alkaloid extract on the induced damage. Twenty-four healthy male wistar rats weighing between 132g to 168g of the same species of rattus Norvegicus were used. Rats were randomly assigned to 4 groups, A through D (n=7 in each). Group A was given distilled water. Group B were administered with Cyclophosphamide 150mg/ kg intra-peritoneal route for seven days. Group C was administered with Cyclophosphamide 150 mg/kg and 50 mg / kg of Alkaloid concomitantly for a week and Group D was administered Alkaloid for 7 days and then Cyclophosphamide of 150 mg /kg for 7 days. There was a significant difference in weight change, according to one way ANOVA result (F = 175.9; p < 0.001), testosterone activity (F = 7.019; p = 0.0125), follicle stimulating hormone activity (F = 13.27; p = 0.0018), sperm motility (F = 11.95; p = 0.0025) in group B (cyclophosphamide only) as compared to control and across all experimental groups. Cyclophosphamide administration was observed to have a negative effect on the testicular histology and immunohistochemical results. The administration of alkaloids both concomitantly and as a pre-treatment helped to counteract the effect of the drug. In conclusion, the administration of cyclophosphamide should be strictly monitored and given in low doses alongside alkaloid to prevent toxic effect.
Keywords
Ameliorative; Alkaloid; Cyclophosphamide; Dioscorcia bulbifera
Download this article as:| Copy the following to cite this article: Anderson E. L, Stephen S. O, Peter F. B, Adediran F. A, Zabdiel A. A, Babatunde E. A. The Microanatomical Study of Effect of Alkaloid Isolate of Dioscorea Bulbifera Bulbis on Cyclophosphamide Induced Testicular Damage in Adult Male Wistar Rats. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Anderson E. L, Stephen S. O, Peter F. B, Adediran F. A, Zabdiel A. A, Babatunde E. A. The Microanatomical Study of Effect of Alkaloid Isolate of Dioscorea Bulbifera Bulbis on Cyclophosphamide Induced Testicular Damage in Adult Male Wistar Rats. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3OLNQJ4 |
Introduction
Cyclophosphamide, a commonly used synthetic anticancer medication, is an alkylating compound that adds an alkyl (CnH2n+1) group to DNA1. During the phases (G2 and S) of the cell cycle, it causes damage by permanently crosslinking DNA strands1. Cyclophosphamide is used to treat a variety of different types of cancer, including breast cancer, multiple myeloma, and neuromyelitis optica, amongst others2,3,4. Despite its many positive effects, research has shown that it can also cause nausea, vomiting, thrombocytopenia, mucosal ulcerations, baldness, ovarian failure, growth retarded infants, and aberrant sperm production in Sprague Dawley rats1. Studies were done to find ways to lessen the toxicity of cyclophosphamide without compromising its efficacy. In one study, researchers found that giving cyclophosphamide in very small doses through drinking water was both safe and effective.1.
Cyclophosphamide is also known to inhibit oogenesis in females and spermatogenesis in men, causing infertility in both sexes5.
It has been demonstrated that cyclophosphamide impairs fertility and reproduction by causing oxidative stress in the reproductive organs. In addition, it causes decreased sperm counts in men6 and ovarian failure in women, as well as damaging morphological alterations in the uterus and ovaries7. The hepatic cytochrome-P450 enzymes convert cyclophosphamide into acrolein and phosphoramide very efficiently8,9,10. Phosphoramide is responsible for the anticancer and immunosuppressive effects of cyclophosphamide, while acrolein is responsible for the toxicity to healthy cells. Additional studies have indicated that acrolein in cyclophosphamide promotes oxidative damage. It causes toxicity by generating an excessive amount of reactive oxygen species (ROS), which can result in protein adducts and DNA damage11. Additionally, ROS generates some membrane damage and mitochondrial dysfunction11. Therefore, cyclophosphamide acts on ROS and disrupts redox equilibrium8 to induce oxidative stress.
Alkaloid isolate from Dioscorea bulbifera has been used in both traditional and modern medicine for its anti-mutagenic or anti-carcinogenic activity, as well as its antibacterial, antifungal, and antiviral properties12. Alkaloids have numerous pharmacological effects and are used as stimulants of the central nervous system (brucine), anticholinergic drugs (tropane)13, muscle relaxants, and antimalarial medications (Morphine)14. This study examines the effect of cyclophosphamide on the testis of adult male Wistar rats following administration of an alkaloid isolate from Dioscorea bulbifera.
Methodology
Rat Management and Care
Male Wistar rats ranging in weight from 132 to 168 g were procured from the animal holdings unit of the Department of Anatomy, Ekiti State University, Ado-Ekiti, for this study and used for this research. The rats were allowed to drink as much clean water and eat regular laboratory pellets as they desired. After acclimating for a week, the animals were separated into four groups according to their weight. Research, Ethics, and Grant Committees of the Faculty of Basic Medical Sciences at Afe Babalola University, Ado Ekiti (ABUAD) approved all animal procedures for this study with approval number REC/FBMS/ABUAD/21/35 per the guiding principles for the care and use of animals and the principles of animal research.
Experimental Design
The control group (Group A) received 0.14 mL of distilled water daily for 14 days. Cyclophosphamide 150 mg/kg (i.p.) for 7 days was given to Group B. Group C received cyclophosphamide 150 mg/kg (i.p.) and alkaloid isolate 50 mg/kg (p.o.) concurrently for 7 days, while Group D received alkaloid isolate 50 mg/kg (p.o.) for 7 days and cyclophosphamide 150 mg/kg (i.p.) delivered on day 7. The total time of the trial was 14 days.
Dioscorea bulbifera Extraction Procedure
Freshly obtained Dioscorea bulbifera bulbils were acquired at the Ado-Ekiti local market. The leaves and bulbils were submitted to the herbarium at the Department of Botany of Obafemi Awolowo University in Ile-Ife, where they were assigned a voucher specimen number (IFE-18037). The bulbils were cleaned, peeled, sliced thinly, diced, and air-dried. Using a mortar and pestle, the bulbils were ground into a fine powder. In a 2-liter beaker, 500g of the material was infused. After adding 2.5L of 10% acetic acid in ethanol, the mixture was constantly stirred for four hours at 50°C in a water bath. At 50°C, this was subsequently filtered and concentrated to a quarter of its initial volume. The extract was treated with 100mL of concentrated ammonium hydroxide until the precipitation ceased. After allowing the entire solution to settle, the precipitate was collected, filtered, and washed with 2 molar ammonium hydroxide. The extra alkaloid (residue) was gathered after drying15.
Drug (Cyclophosphamide injection)
The cyclophosphamide used in this research was purchased from the Obafemi Awolowo University Teaching Hospital pharmacy in Ile-Ife, Nigeria, and was manufactured by Biochem Pharmaceutical Industries Ltd. at Ackruti Star, Unit No. 103, MIDC, Andheri (E), Mumbai, 400 093.
Animal Sacrifice and Weight Determination
In the beginning, midway through, and conclusion of the experiment, body weights were recorded. Each animal was sacrificed via cervical dislocation on the last day of the trial. Blood samples were taken via intracardiac puncture. Serum was separated from blood samples by centrifugation; the samples were stored in simple sample vials. Researchers performed biochemical tests on the collected serum. Testicles were extracted, measured with a Gallenhamp electronic balance (MP 10001), and then preserved in 10% Bouin’s solution for histological analysis. Percentage weight change (%) was calculated using:
Histological and Histochemical Methods
Hematoxylin and Eosin staining technique was used to analyze the general histoarchitecture of the testicular tissues while the Masson Trichrome (MT) technique was used for the detection of collagen fibers and reticular fibers were demonstrated using Gordon and Sweet’s silver staining.
Hematoxylin and Eosin staining procedure
The tissues were hydrated using descending degrees of xylene after being dewaxed. Harris Haematoxylin was used to stain the sections for five minutes, after which they were rinsed in alcohol.
The sections were blued for 10 minutes in running tap water after being briefly differentiated in 1% alcohol. The sections were counter-stained in 1% aqueous eosin of alcohol and taken into xylene and then mounted using DPX and cover slip16.
Masson’s Trichrome procedure
The section was deparaffinized, then washed in distilled water after being rehydrated with 100%, 95%, and 70% alcohol. Use Weigert’s iron hematoxylin working solution to stain for 10 minutes, then rinse for 10 minutes under warm running water before rinsing with distilled water. Before differentiating the collagen in phosphomolybdic-phosphotungstic acid solution, stain the collagen for 10 to 15 minutes with Biebrich scarlet-acid fuchsin solution. Rinse off the stain with distilled water once it has lost its red color. To stain, add parts immediately to an aniline blue solution and let them stain for 5 to 10 minutes. Shortly rinse with distilled water before differentiating for 2 to 5 minutes in a solution of 1% acetic acid. Use distilled water to clean. Dehydrate fast using 95 percent alcohol, absolute alcohol, and clear in xylene (these steps will remove Biebrich scarlet-acid fuchsin staining). Mount with resinous mounting medium17.
Biochemical Analysis
Biochemical analyses were carried out to assay the activities of Testosterone and Follicle Stimulating Hormone (FSH). The rats’ blood was collected from the heart and allowed to clot. And then centrifuge at 3000r/min for 30 minutes. The collected serum was used to analyze hormone measurement. The testosterone and follicle-stimulating hormone were analyzed by the ELISA method using rat FSH ELISA kits.
Sperm Motility
On the cover glass, there was a drop of diluted semen. To create a hanging drop, the cover glass was set on a cavity slide and turned over. Vaseline was used to seal the coverslip’s edges, and a compound microscope set to a magnification of 450x was used to view the hanging drop. The preparation was assessed at regular intervals to determine how long, in minutes, the final motile sperm had been moving. Two independent observers evaluated the motility of each animal using two different hanging drop setups. The average was calculated using the data for each animal.18.
Sperm Viability
Two drops of heated Eosin/Nigrosin stain were put to the semen on a previously warmed slide, and after letting it dry with air, the slide was examined under a microscope with a magnification setting of x400 to ensure a uniform smear. The active sperm cells were unaffected by the dye, and only the dead ones absorbed it. The Proportion was estimated by counting stained and unstained sperm19.
Photomicrography
A binocular microscope from Olympus was utilized. One of the oculars was equipped with a 5,1-megapixel MV550 microscope research camera. This was connected to a PC running image capture and analyzing software. After being placed over the slide, the immersion oil was modified progressively to correspond with the X100 oil immersion target. The technology was adjusted to achieve clarity and resolution. The images were captured and saved to a computer.
Quantification of staining intensity
Masson trichrome and CD68 staining intensity were analyzed and quantified using Java image processing and analysis (ImageJ). Using ImageJ, grayscale versions of imported RGB images were created. After thresholding of areas with staining, the software was used to calculate the pixel value of each pixel in grayscale images and changes the pixel value to brightness value or gray value. This enables the software to quantify the intensity of stains. The acquired values were then statistically evaluated.20
Data Analysis
After analyzing the data using a one-way ANOVA, we performed the Student Newman-Keuls (SNK) post hoc test to determine significant differences in the means of the different groups. Statistical analysis was performed using Graph Pad Prism 5.(Version 5.03, Graphpad Inc.). The level of significance was set at p< 0.05..
Results
Analysis of Sperm, Biochemical data, and percentage Weight change
Analysis of results revealed that, there were significant reductions in weight change (F = 175.9; p < 0.001), testosterone concentration (F = 7.019; p = 0.0125), follicle-stimulating hormone concentration (F = 13.27; p = 0.0018), sperm motility (F = 11.95; p = 0.0025), life/dead ratio (F = 7.378; p = 0.0108) across the experimental groups. The post hoc analysis showed that data in group B (cyclophosphamide only) were significantly lower than in the positive control (group A). This reduction induced by cyclophosphamide was reversed by pre-treatment of alkaloid administration in group D (p<0.05) (Figures 1, 2, 3, 4, and 5).
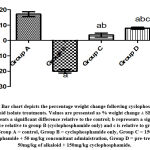 |
Figure 1: Bar chart depicts the percentage weight change following cyclophosphamide and alkaloid isolate treatments. Values are presented as % weight change ± SEM (n=7). |
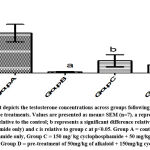 |
Figure 2: Bar chart depicts the testosterone concentrations across groups following cyclophosphamide and alkaloid isolate treatments. Values are presented as mean± SEM (n=7). |
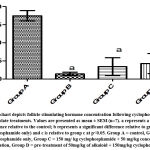 |
Figure 3: Bar chart depicts follicle stimulating hormone concentration following cyclophosphamide and alkaloid isolate treatments. Values are presented as mean ± SEM (n=7). |
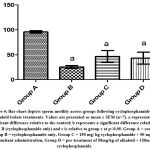 |
Figure 4: Bar chart depicts sperm motility across groups following cyclophosphamide and alkaloid isolate treatments. Values are presented as mean ± SEM (n=7). |
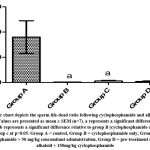 |
Figure 5: Bar chart depicts the sperm life-dead ratio following cyclophosphamide and alkaloid isolate treatments. Values are presented as mean ± SEM (n=7). |
Testis Histopathology
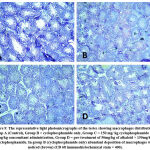
The normal histological features seen in the control group are; basement membrane, seminiferous tubules, Leydig cells, Spermatogonia, and interstitial tissue (figure 6 A). In the cyclophosphamide-only group degenerated cells were seen which indicated atrophy. In the groups treated with the alkaloid isolate, little debris of degenerated cells was seen. The toxic effect of the cyclophosphamide was ameliorated by the alkaloid as they were administered together (figures 6 D and E).
Normal histoarchitecture of testis tissue was evident with no traces of collagen deposition in the control group (figure 7 A). Also, collagen fibers’ distribution was observed around the basement membrane, interstitial tissue, and seminiferous tubule in the cyclophosphamide group (figure 7 B). Sparse distribution of collagen fibers was seen in the seminiferous tubule and basement membrane in group C and group D (figures 7C and 7D) and (figures 7 and 8).
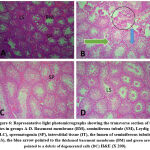 |
Figure 6: Representative light photomicrographs showing the transverse section of the testes in groups A-D. |
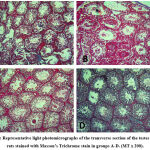 |
Figure 7: Representative light photomicrographs of the transverse section of the testes of Wista rats stained with Masson’s Trichrome stain in groups A-D. (MT x 200). |
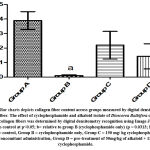 |
Figure 8: Bar charts depicts collagen fiber content across groups measured by digital densitometry of collagen fiber. |
Immunohistochemical findings
CD68 revealed proper macrophage cells’ distributions in the testes (Fig. 9A). Testes from cyclophosphamide-treated rats stained positively with anti-CD68 antibody (Fig. 9 B). The CD68 distribution was nearly normal in rats given 150 mg/kg cyclophosphamide plus 50 mg/kg alkaloid concurrently, but it was significantly reduced in the pre-treatment group given 50mg/kg of alkaloid + 150 mg/kg cyclophosphamide. (Figures 9C, 9D) Macrophage cell distribution in the heart was analyzed using digital densitometry to determine the percentage of CD68 immunopositive areas (Table 1).
Table 1: Effects of alkaloid on cyclophosphamide in testes in changes on macrophage distribution. n = 7, values are expressed as % area of CD 68 ± SEM. a = relative to control at p<0.05; b= relative to group B (cyclophosphamide only) (p < 0.0001; F = 45.92). Group A = control, Group B = cyclophosphamide only, Group C = 150 mg/ kg cyclophosphamide + 50 mg/kg concomitant administration, Group D = pre-treatment of 50mg/kg of alkaloid + 150mg/kg cyclophosphamide.
| Groups | Percentage Area of CD 68 Immunohistochemistry (%) |
| A (Control) | 0.3000 ± 0.241 |
| B (Cyclophosphamide only) | 8.103 ± 0.592a |
| C (Cyclo + 50 mg kg-1 of Alkaloid) | 3.720 ± 0.591ab |
| D (100 mg kg-1 of Alkaloid + cyclo) | 3.153 ± 0.384ab |
Discussion
We investigated the ameliorative effects of Alkaloid isolate of Dioscorea bulbifera on the toxic effect of cyclophosphamide on the testis of adult Wistar rats in this study. Alkaloid has been known to be beneficial21 and this current study was implemented to demonstrate the protective impact of alkaloid following the administration of cyclophosphamide in Wistar rats.
In this study, group B showed a significant reduction in percentage weight change and the percentage weight change was highest in the control group. This could be explained by the dosage of cyclophosphamide administered, and this is supported by the research of Bridges, which reported that the popular anti-cancer drug cyclophosphamide caused considerable weight loss22. The beneficial effect of alkaloids against cyclophosphamide poisoning concerning body weight was confirmed by this study and the best result was seen when administered as a pre-treatment23.
The study showed a drastic reduction in testosterone levels in group B rats while it was at its highest in the control group. This was explained by the dosage of the cyclophosphamide administered to the rats and it is supported by the research of Drobnis and Kaya, which reported the immunosuppressive ability of cyclophosphamide on the male reproductive system including testosterone levels6, 24. A little counter effect was seen by the alkaloids to prevent low testosterone levels and this contradicts previous research where testosterone level was lowered after the administration of alkaloid25. FSH level was also at its lowest in group B rats and highest in the control group. The low FSH levels was supported by a previous research26. The protective effect of Alkaloid was confirmed as it countered the effect of the cyclophosphamide which was in accord with the research of Udoh et al.,27.
Sperm motility was reduced in group B rats and was at its highest in the control group. The low sperm motility is also confirmed by previous research which shows the toxic effect of cyclophosphamide on sperm motility28. The protective ability of Alkaloids was seen as it acts to counter the toxic effect of the cyclophosphamide and supported by the research of Alves et al.,29. The Life/Death ratio was highest in group A and lowest in group B; there was no significant difference between groups B, C, and D, indicating that alkaloids had minimal impact on the toxic action of cyclophosphamide on the Life/Death ratio.
Normal histological features were seen in the control group compared to the toxic group B rats where degenerated cells were noticed as a result of the administration of cyclophosphamide which is supported by the research of Bakhtiary et al.,30 stated that oxidative stress could have caused the decrease in thickness at the testicular capsule which induced inflammatory mechanisms due to the secretion of mast cells. In the groups treated with alkaloid little debris of degenerated cells were seen, showing the ameliorative properties of alkaloids on the toxic effect of cyclophosphamide as they were administered together. Collagen fibers’ distribution was observed around the basement membrane, interstitial tissue and seminiferous tubule in the cyclophosphamide group B showed the damage in group B which was in accord with the research of Almasry et al31.
Early on, it had been documented that macrophages dominated the degenerated area20 of testes. Due to the antioxidant nature of alkaloid32, this study has shown that CD 68 was strongly positive in group B and lowered in groups C and D. However, the distribution of CD68 expression in the testes tissues is resisted by its ability to operate as radical scavengers.
Conclusion
The findings of this study demonstrated that cyclophosphamide negatively affects the testis’ histology, immunohistochemistry, and hormones like testosterone, FSH, Sperm motility, Sperm Life/Death ratio, and body weight. This study also showed the ability of alkaloid to counteract the toxic effect of cyclophosphamide on the testis.
Conflict of interest
The authors have no conflict of interest to declare.
Funding Sources
There is no funding sources.
References
- Gintanjali K, Renu C, Mahindra N. Effect of cyclophosphamide on liver in albino rats: A comparative dose dependent histomorphological study. International Journal of Biomedical and Advance Research 2017; 8: 102-107
- Wang L, Liu K, Tan X, Zhou L, Zhang Y, Liu X, Fu Y, Qiu W, Yang H. Remedial effect of intravenous cyclophosphamide in corticosteroid-refractory patients in the acute phase of neuromyelitis optica spectrum disorder- related optic neuritis. Frontiers in Neurology 2021; 11: 1-11(612097). doi: 10.3389/fneur.2020.612097
CrossRef - Nakatsukasa K, Koyama H, Oouchi Y, Imanishi S, Mizuta N, Sakaguchi K, Fujita Y, Fujiwara I, Kotani T, Matsuda T, Fukuda K, Morita M, Kawakami S, Kadotani Y, Konishi E, Yanagisawa A, Taguchi T. Docetaxel and cyclophosphamide as neoadjuvant chemotherapy in HER2-negative primary breast cancer. Breast cancer (Tokyo, Japan), 2017; 24(1), 63–68. doi: 10.1007/s12282-016-0666-7
CrossRef - Garderet L, Kuhnowski F, Berge B, Roussel M, Escoffre-Barbe M, Lafon I, Facon T, Leleu X, Karlin L, Perrot A, Moreau P. Pomalidomide, cyclophosphamide, and dexamethasone for relapsed multiple myeloma. Blood. The Journal of the American Society of Hematology 2018;132(24):2555-63. doi: 10.1182/blood-2018-07-863829
CrossRef - Abarikwu S. O, Otuechere C. A, Ekor M, Monwuba K, Osobu D. Rutin Ameliorates Cyclophosphamide-induced Reproductive Toxicity in Male Rats. Toxicology international2012; 19(2), 207–214. https://doi.org/10.4103/0971-6580.97224
CrossRef - Drobnis E. Z, Nangia A. K. Immunosuppressants and Male Reproduction. Advances in experimental medicine and biology 2017; 1034, 179–210. doi: 10.1007/978-3-319-69535-8_12
CrossRef - Melekoglu R, Ciftci O, Eraslan S, Cetin A, Basak N. Beneficial effects of curcumin and capsaicin on cyclophosphamide-induced premature ovarian failure in a rat model. Journal of ovarian research 2018; 11(1), 33. doi: 10.1186/s13048-018-0409-9.
CrossRef - Ekeleme-Egedigwe C. A, Famurewa A. C, David E. E, Eleazu C. O, Egedigwe U. O. Antioxidant potential of garlic oil supplementation prevents cyclophosphamide-induced oxidative testicular damage and endocrine depletion in rats. Journal of Nutrition & Intermediary Metabolism 2019;18:100109.
CrossRef - Oyagbemi A. A, Omobowale T. O, Saba B, Adedara I. A, Olowu E. R, Akinrinde A. S, Dada R. O. Gallic acid protects against cyclophosphamide‐induced toxicity in testis and epididymis of rats. Andrologia 2016; 48(4), 393-401. doi: 10.1111/and.12459
CrossRef - Nafees S, Ahmad S. T, Arjumand W, Rashid S, Ali N, Sultana S. Modulatory effects of gentisic acid against genotoxicity and hepatotoxicity induced by cyclophosphamide in Swiss albino mice. Journal of Pharmacy and Pharmacology2012; 64(2), 259-267. doi: 10.1111/j.2042-7158.2011.01393.x
CrossRef - Zirak M. R, Mehri S, Karimani A, Zeinali M, Hayes A. W, Karimi G. Mechanisms behind the atherothrombotic effects of acrolein, a review. Food and Chemical Toxicology 2019; 129, 38-53. doi: 10.1016/j.fct.2019.04.034
CrossRef - Warowicka A, Nawrot R, Goździcka-Józefiak A. Antiviral activity of berberine. Archives of virology 2020; 165(9), 1935–1945. https://doi.org/10.1007/s00705-020-04706-3
CrossRef - Kwakye G. F, Jiménez J, Jiménez J. A, Aschner M. Atropa belladonna neurotoxicity: Implications to neurological disorders. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 2018; 116: 346–353. https://doi.org/10.1016/j.fct.2018.04.022
CrossRef - Badri S, Basu V. R, Khader B, Chandra K, Anasuya D. A Review on Pharmacological Activities of Alkaloids. World Journal of Current Medical and Pharmaceutical Research 2019; 1(6), 230-234. https://doi.org/10.37022/WJCMPR.2019.01068
CrossRef - Obadoni O, Ochuko P. O. Phytochemical Studies and Comparative Efficacy of the Crude Extracts of Some Homeostatic Plants in Edo and Delta States of Nigeria. Global Journal of Pure and Applied Science 2001; 8, 203-208
CrossRef - Drury R. A, Wallington E. A. Carleton’s histological technique 5th ed. New York: Churchill Livingstone
- Garvey W. Modified elastic tissue-Masson trichrome stain. Stain technology 1984; 59(4):213-6. doi: 10.3109/10520298409113858
CrossRef - Seyma O. K, Emre K, Seyfettin G, Seval Y, Ilyas M. C. Effect of Starvation and Refeeding on Spermatological Parameters and Oxidative Stress in Rats. International Journal of Health Sciences 2019; 7 (2): 21-28
- Liang J. A. Fertility and Infertility in Domestic Animas. 3rd Ed, 1979 Baillieere Tindall, London
- Komolafe O. A, Arayombo B. E, Abiodun A. A, Saka O. S, Abijo A. Z, Ojo S. K, Fakunle O. O. Immunohistochemical and histological evaluations of cyclophosphamide-induced acute cardiotoxicity in wistar rats: The role of turmeric extract (curcuma). Morphologie: bulletin de l’Association des anatomistes 2020; 104(345), 133–142. doi: 10.1016/j.morpho.2019.10.047
CrossRef - Suntar I, Khan H, Patel S, Celano R, Rastrelli L. An Overview on Citrus aurantium: Its Functions as Food Ingredient and Therapeutic Agent. Oxidative medicine and cellular longevity, 2018:7864269. doi: 10.1155/2018/7864269.
CrossRef - Bridges D. Weight loss effects of methotrexate and cyclophosphamide. Oncotarget 2017; 8(3), 5640. doi: 10.18632/oncotarget.14569
CrossRef - Mahmoud A. M, Hozayen W. G, Ramadan S. M. Berberine ameliorates methotrexate-induced liver injury by activating Nrf2/HO-1 pathway and PPARγ, and suppressing oxidative stress and apoptosis in rats. Biomedicine & Pharmacotherapy2017; 94, 280-291. doi: 10.1016/j.biopha.2017.07.101
CrossRef - Kaya C, Barbaros Baseskioglu A, Yigitaslan S, Yasemin Ozatik F, Ozatik O, Uslu S. The therapeutic potential of amifostine on cyclophosphamide-induced testicular dysfunction in rats: An experimental study. International journal of reproductive biomedicine 2019; 17(4), 245–252. doi: 10.18502/ijrm.v17i4.4549
CrossRef - Ismalia K. R, Pangkahila W, Sriwidyani N. P. Oral administration of Bali Robusta coffee (Coffea canephora) extract prevented the reduction of Leydig cells and testosterone levels in male Wistar rats (Rattus norvegicus) with excessive physical training. Neurologico Spinale Medico Chirurgico 2021; 4(1), 37-41.
CrossRef - Zhang B. F, Hu Y, Liu X, Cheng Z, Lei Y, Liu Y, Zhao X, Mu M, Yu L, Cheng M. L. The role of AKT and FOXO3 in preventing ovarian toxicity induced by cyclophosphamide. PloS one 2018; 13(8), e0201136. doi: 10.1371/journal.pone.0201136
CrossRef - Udoh P. B, Udoh F. V, Umoren E. B, James U. W, Okeke C. P, Agwu B. Effect of caricapryl-99 seed alkaloid extract on the serum levels of sex hormones and pituitary gonadotrophins in male albino rats. Nigerian journal of physiological sciences : official publication of the Physiological Society of Nigeria 2009; 24(1), 13–15. doi: 10.4314/njps.v24i1.46375
CrossRef - Iqubal A, Syed M. A, Najmi A. K, Ali J, Haque S. E. Ameliorative effect of nerolidol on cyclophosphamide-induced gonadal toxicity in Swiss Albino mice: Biochemical-, histological- and immunohistochemical-based evidences. Andrologia 2020; 52(4), e13535. doi: 10.1111/and.13535
CrossRef - Alves N. C, Diniz S. A, Viegas R. N, Cortes S. F, Costa E. D, Freitas M. M, Martins-Filho O. A, Araújo M, Lana Â, Wenceslau R. R, Lagares M. A. Addition of caffeine to equine thawed sperm increases motility and decreases nitrite concentration. Andrologia 2021; 53(2), e13918. doi: 10.1111/and.13918
CrossRef - Bakhtiary Z, Shahrooz R, Ahmadi A, Soltanalinejad F. Protective effect of ethyl pyruvate on testicular histology and fertilization potential in cyclophosphamide treated mice. Veterinary research forum: an international quarterly journal 2020; 11(1), 7–13. doi: 10.30466/vrf.2018.91253.2047
- Almasry S. M, Hassan Z. A, Elsaed W. M, Elbastawisy Y. M. Structural evaluation of the peritubular sheath of rat’s testes after administration of ribavirin: a possible impact on the testicular function. International journal of immunopathology and pharmacology2017; 30(3), 282-296. doi: 10.1177/0394632017726261
CrossRef - Adedayo B. C, Oyeleye S. I, Okeke B. M, Oboh G. Anti‐cholinesterase and antioxidant properties of alkaloid and phenolic‐rich extracts from pawpaw (Carica papaya) leaf: A comparative study. Flavour and Fragrance Journal2021; 36(1), 47-54