Manuscript accepted on :29-11-2022
Published online on: 16-12-2022
Plagiarism Check: Yes
Reviewed by: Dr. Nishu Raina
Second Review by: Dr. Hany Akeel
Final Approval by: Dr. Anton R Kiselev
S. O. Maslennikov1 , M. L. Golovakha1
, M. L. Golovakha1 , I. F. Belenichev2
, I. F. Belenichev2 and L. V. Makyeyeva3*
and L. V. Makyeyeva3*
1Department of Traumatology and Orthopedics, Zaporizhzhia State Medical University, Zaporizhzhia, Ukraine
2Department of Pharmacology and Medical Recipes with the Course of Normal Physiology, Zaporizhzhia State Medical University, Zaporizhzhia, Ukraine
3Department of Histology, Cytology and Embryology, Zaporizhzhia State Medical University, Zaporizhzhia, Ukraine
Corresponding Author E-mail: lyudmylamakyeyeva@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2537
Abstract
Restoration and strengthening of soft tissue structures of the musculoskeletal system underlie the principles of regenerative medicine to improve treatment results and prevent possible complications. Influencing various parts of the regenerative process, it is possible to correct the recovery time, the morphological properties of the scar, and, as a result, the clinical results of treatment. We have proposed using a polypropylene mesh (PM) impregnated with a metabolitotropic solution to restore damaged and strengthen weakened structures. The work aimed to study the effect of PM with metabolite impregnation on the regeneration process of soft tissues. A series of morphological and biochemical studies were carried out on laboratory animals. The degree of inducible nitric oxide synthase (iNOS) and endothelial nitric oxide synthase (eNOS) mRNA expression was studied as indicators of tissue ischemia and their trophism in correlation with morphological changes in the zone of mesh implantation. Using a PM with a metabolitotrope increases the expression of eNOS mRNA by 10.8 times, and iNOS mRNA - reduces by 3.6 times. Morphologically coated PM causes a pronounced inflammatory effect, followed by a pronounced proliferation of connective tissue fibers around the mesh elements. Particular attention is drawn to the growth of the microvasculature and the increase in the number of anastomoses - 10.3% of the relative area compared to 8.8% in the group using PM without a metabolite. The metabolitotropic impregnation improves oxygenation and trophism of damaged soft tissues, which helps to stimulate regeneration.
Keywords
Nitric Oxide Synthase; Metabolites; Polypropylene mesh; Regeneration; Soft Tissue
Download this article as:| Copy the following to cite this article: Maslennikov S. O, Golovakha M. L, Belenichev I. F, Makyeyeva L. V. Regenerative Properties of Polypropylene Mesh Coated with Thiotriazoline and L-arginine. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Maslennikov S. O, Golovakha M. L, Belenichev I. F, Makyeyeva L. V. Regenerative Properties of Polypropylene Mesh Coated with Thiotriazoline and L-arginine. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3YE37QS |
Introduction
Like other medical fields, modern orthopedics and traumatology is evolving toward the application of regenerative medicine technology. Currently, there are many directions for stimulating cell growth and regeneration. In orthopedics, there are many examples of the necessity to restore the soft tissue components (joint capsule, in case of arthroplasty; tendons in case of ACL reconstruction etc.) in the shortest possible time to obtain high functional results to maintain a high quality of patients’ life. New operational techniques and materials are being developed to achieve these objectives.
We have proposed a method for enhancing tissue regeneration using a polypropylene mesh subjected to preoperative impregnated with a solution containing Thiotriazoline and L-arginine in the original ratio and concentrations 1. The range of polypropylene mesh implants for surgery includes mesh with different coatings and impregnation that accelerate regeneration. Given the possibility of using various biological agents on the mesh implant, its use to stimulate proliferative and regenerative processes in soft tissues may be very promising.
The polypropylene mesh in the implantation zone causes a cascade of histological changes regulated by pathophysiological processes characteristic of any injury or surgical intervention 2. The process of regeneration and integration of the mesh largely depends on the characteristics of the mesh implant, but in general, they go through similar stages 3.
We assumed that if the vascular component in the area of damage is affected, reducing tissue ischemia and improving their trophism and oxygenation, it is possible to increase proliferation due to the influx of anabolites and the excretion of decay products. Thus, it is hypothetically possible to accelerate the processes of tissue integration, increase the strength of scar tissue, and, as a result, gain better clinical results.
The aim of the work was to study the effect of a polypropylene mesh impregnated with a solution of Thiotriazoline and L-Arginine on the regenerative process of soft tissues.
Materials and Methods
In our studies, we used a polypropylene mesh with an average density of 52 g/m2 due to the need to combine the rigidity and elastic properties of the implant. The choice of a mesh with a pore size of 0.75 mm was due to the maximum efficiency of the retention of the solution due to its surface tension. The process of covering and impregnating the mesh takes 48 hours. The sterile mesh is gradually immersed in a prepared sterile metabolite solution at a temperature of 20-22o C and with the help of a reciprocating shaker, it is impregnated during the specified time. The biomechanical characteristics of the mesh are presented in Table 1. It should be noted that the metabolite solution does not change the physical and mechanical properties of the mesh.
Table 1: Biomechanical properties of polypropylene mesh.
| Parameter (unit of measurement) | Indicator |
| Fiber diameter (mm) | 0,15 |
| Density (g / m2) | 52 |
| Elasticity (%) | 4-16 |
| Surface porosity (%) | 50,3±1,6* |
| Volume porosity (%) | 82,9±0,3* |
| Pore size (mm × mm) | 0,75×0,75 |
| Mechanical properties when tested for longitudinal tension | |
| Maximum force (N / cm) | 84,8±15,0* |
| Resistance to rupture(N / mm) | 186±7* |
| Stiffness (N / mm) | 3,6±0.4* |
| Mechanical properties in the transverse tensile test | |
| Maximum force (N / cm) | 41,6±5,4* |
| Resistance to rupture (%) | 274±6* |
| Stiffness (N / mm) | 1,1±0,1* |
* Mean±m
The mesh can be stored and transported in a sterile sealed package pre-filled with a metabolite based on Thiotriazoline and L-arginine to avoid solidification in the pores and on the surface of the mesh (Fig.1).
A comparative experimental study was conducted on laboratory animals. The Local committee approved experimental studies on bioethics; the study was carried out following the Law of Ukraine “On Scientific and Technical Activities” and following the Council of Europe Convention “On the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes” Strasbourg 1985 and EU Directive 2010/63/EU for animal experiments and ‘Principles of Laboratory Animal Care (NIH Publication Vol 25, No. 28 revised 1996). An experimental study was carried out on 25 rabbits of both sexes, weighing 694±27g, which were kept under the same conditions on a standard diet with free access to water with a natural change of day and night. Under sterile conditions, in the presence of a veterinary doctor under intramuscular anesthesia, surgical intervention was performed on the knee joint in the amount of arthrotomy and the creation of a capsule defect of 4-5mm in size (Fig. 2).
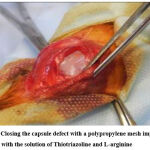 |
Figure 2: Сlosing the capsule defect with a polypropylene mesh impregnated with the solution of Thiotriazoline and L-arginine |
The animals were divided into three groups. In the study group (Group A, n=10), a polypropylene mesh was implanted with an adhered solution of Thiotriazoline and L-arginine; in the comparison group (Group B, n=10), the capsule defect was closed with the implantation of an uncoated polypropylene mesh, reference group (Group C, n=5, only for qRT-PCR study) in which the capsule defect was closed without the use of implants. In this case, a standard regenerative process proceeded, and the results of enzyme expression were regarded as a baseline about which the expression changes were evaluated. Since the enzymatic activity could either increase or decrease from the baseline, its value was 1.0.
Morphological examination of the knee joint tissues was performed on the material obtained from groups A and B in experiments 7 and 14 days after plastic surgery of the joint capsule. The material was fixed in a 10% neutral formalin solution; dehydration was performed in an ascending battery of alcohols. The pieces were filled with a mixture of paraffin-rubber-wax in a ratio of 20: 1: 1. The obtained paraffin blocks were used for sectioning – thickness 4 μm, which were stained with hematoxylin and eosin according to Mason. High-power microscopic view analysis of histological specimens was performed by light microscopy on a microscope “Biolam” at different magnifications. Relative cell area was measured using ImageJ software.
To determine the reparative characteristics of the process, the activity of the inducible nitric oxide synthase(iNOS) and endothelial nitric oxide synthase (eNOS) enzymes was assessed by determining the nature of their mRNA expression.
The method of Polymerase Chain Reaction with reverse transcription in Real Time (qRT-PCR) was used to assess the state of expression of iNOS mRNA and eNOS mRNA; The Molecular genetic research included several stages. Isolation of total RNA from rabbit blood was carried out using the TrizolRNAPrep100 kit, which contains the following reagents: Trizol reagent and Extra Gene E. RNA is isolated according to the recruitment protocol. For reverse transcription(DNA synthesis), the “Reagent kit for reverse transcription (RT-1)” was used. The preparation and performance of the reaction were carried out following the kit protocol. Amplification CFX96 TM Real-TimePCR Detection Systems and a set of reagents for performing qRT-PCR in the presence of SYBRGreenR-402 were used to ascertain the level of expression of the under investigation genes. The final reaction of the mixture for amplification included SYBRGreen dye, SynTaq DNA polymerase with antibodies inhibiting the enzyme activity, 0.2 µl of forward and reverse specific primers, dNTP-deoxynucleoside triphosphates, 1 µl of a template (cDNA). The reaction mixture was brought to a total volume of 25 μl by adding deionized H2O. Specific primer pairs (5′-3′) for the analysis of the studied and reference genes were selected using the PrimerBlast software (www.ncbi.nlm.nih.gov/tools/primer-blast). Amplification took place under the following conditions: initiated denaturation at 95° C – 10 min; then 50 cycles: denaturation – 95° С, 15 sec., primer annealing – 58-63° С, 30 sec., elongation 72° С, 30 sec. The registration of the fluorescence intensity took place automatically at the end of the elongation stage of each cycle along the SybrGreen channel. The actin beta (Actb) gene was used as a reference gene to determine the relative value of the change in the expression level of the studied genes.
The significance of differences between groups was performed using the Mann-Whitney nonparametric U test. Differences with a significance level of more than 95% (p<0.05) were considered significant. The research results were processed using the statistical package of the licensed program STATISTICA 13.0 TIBCO Software inc. (StatSoftInc., No. JPZ804I382130ARCN10-J), as well as “SPSS16.0”, “MicrosoftExcel 2010”.
Results
Analyzing the data presented in table2, the following was established by characterizing the expression of eNOS and iNOS mRNA (Mean±SEM) in the soft tissues of the joint with a polypropylene implant. The indicators characterizing the expression of eNOS mRNA in Group A have higher numerical values(1.53±0.66)relative to the comparison group (0.14±0.12 (p<0.05)). In comparison, in Group B, the expression values are lower than the corresponding values of the reference group (1.00±0.58 (p<0.05)). The expression pattern of iNOS mRNA in the comparison group has a high value (3.61± 0.57) about Group C (1.00±0,28 (p<0.05))and Group A (1,82±0,43 (p<0.05)). Thus, it can be concluded that using a polypropylene implant in the area of the articular capsule changes the character of NOS mRNA expression in the soft tissues surrounding the joint – the expression of eNOS mRNA is reduced and the expression of iNOS mRNA is significantly increased.
Table 2: The results of iNOS and eNOS mRNA expression in the soft tissues(Mean±SEM)
| Data Set | Target | Sample | Expression | Expression SEM | Corrected Expression SEM | Mean Cq | Cq SEM |
| 1-SYBR | eNOS | Group C | 1.00000 | 0.58849 | 0.58849 | 40.43 | 0.77750 |
| 1-SYBR | eNOS | Group A | 1.53093 | 0.66011 | 0.66011 | 41.30 | 0.61357 |
| 1-SYBR | eNOS | Group B | 0.14842 | 0.12219 | 0.12219 | 45.26 | 1.18282 |
| 1-SYBR | iNOS | Group C | 1.00000 | 0.28280 | 0.28280 | 35.10 | 0.22394 |
| 1-SYBR | iNOS | Group B | 3.61097 | 0.57258 | 0.57258 | 34.73 | 0.20453 |
| 1-SYBR | iNOS | Group A | 1.82239 | 0.43965 | 0.43965 | 36.32 | 0.31287 |
Microscopic study of the joint capsule on the 7th day after the operation in Group B showed the phenomena of tissue edema, and disorganization of the fibers was noted in the fibrous membrane. The blood vessels of the capsule are dilated. They account for 8.3±0.4% of the relative area, capillaries and venules are full-blooded, imbibition of the capsule tissue by erythrocytes is observed, hemorrhages occupy 12.6±0.5% of the relative area of the capsule membrane of the joint. The distribution of fibers of the synovial membrane of the capsule is 21.4±2.3%, and the intercellular substance due to edema occupies 49.6±2.6% of the relative area; cells, including perivascular leukocyte-lymphocytic, infiltrates, occupy 8.1±0.4% of the relative area of the synovial membrane of the knee capsule (Table 3).
In animals of Group A, after 7 days, an accumulation of connective tissue cells around the filaments is noted in the area of PM implantation. Among the cells are macrophagocytes, leukocytes, lymphocytes, fibroblasts, and fibrocytes. Short, thin collagen fibers are between the PM cells (Figure 3).
Table 3: Morphological indicators of changes in the joint capsule after arthrotomy and closure of the defect in both groups of animals (relative area, percentage, Mean±SEM)
| Day p/o | Group A (n=10) | Group B (n=10) | p, value |
| Haematoma | |||
| 7th | 13,3±0,7 | 12,6±0,5 | p<0.05 |
| 14th | 12,7±0,7 | 11,7±0,7 | p<0.05 |
| Fibers of the synovial membrane | |||
| 7th | 29,6±1,4 | 21,4±2,3 | p<0.05 |
| 14th | 35,6±2,2 | 33,6±2,2 | p<0.05 |
| Intercellular substance | |||
| 7th | 50,3±2,0 | 49,6±2,6 | p>0.05 |
| 14th | 34,8±2,3 | 32,8±1,3 | p>0.05 |
| Perivascular infiltrate | |||
| 7th | 10,1±0,6 | 8,1±0,4 | p<0.05 |
| 14th | 12,1±0,5 | 13,1±0,5 | p>0.05 |
| Blood vessels | |||
| 7th | 9,7±0,5 | 8,3±0,4 | p<0.05 |
| 14th | 10,3±0,6 | 8,8±0,6 | p<0.05 |
In the sub-mesh space, fibrin-blood clots are formed, which initiate the formation of granulation tissue. In general, foci of diapedetic hemorrhages are observed in the capsule tissue, especially pronounced in the marginal section. The blood vessels are dilated and full-blooded, and the lymphatic vessels of the capsule are also dilated. The tissue of both synovial and fibrous membranes of the articular capsule is edematous, and fibers are disorganized. Short, thin fibers that do not have a general direction of location prevail among the fibers in the defect area. Within the synovial membrane, the phenomena of edema are more pronounced than in the fibrous membrane.
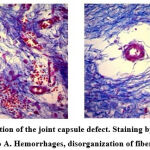 |
Figure 3: 7th day after simulation of the joint capsule defect. Staining by Mason. Magnification x100. a) Group B, b) Group A. Hemorrhages, disorganization of fibers, blood vessels. |
Hemorrhage foci occupy 13.3 ± 0.7% of the relative area of the synovial membrane of the joint capsule, blood vessels – 9.7 ± 0.5%, fibers account for 29.6 ± 1.4%, and the intercellular substance due to edema prevails and occupies 50.3 ± 2.0% of the relative area; cells, including perivascular, infiltrates, occupy 10.1 ± 0.6% of the relative area of the synovial membrane of the capsule. Thus, examining the capsule of the knee joint in Group A, there was a statistically significant (p<0.05) gradual decrease in the relative area of the hematoma and intercellular substance (Figure 4).
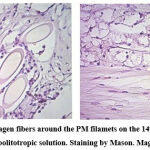 |
Figure 4: Formation of collagen fibers around the PM filamets on the 14th day after the implantation of the PM with meabolitotropic solution. Staining by Mason. Magnificationx100, |
Discussion
It is known that nitric oxide (NO) is one of the most important biological mediators involved in many physiological and pathophysiological processes. Inducible NOS, represented by NOS-2 and functioning under physiological conditions in the “background” mode, exhibits increased cell activity upon its induction by bacterial endotoxins and some inflammatory mediators 4,5. In particular, this process can be triggered by bacterial lipopolysaccharides, some endotoxins, and cytokines such as interleukin-1, interleukin-2, etc. INOS is usually not detected in resting cells but can be detected in macrophages and neutrophils after induction. It is iNOS and the NO formed under its influence that plays a major role in the cytotoxic effect, as well as the functioning of the immune system. It has been proven that implantation of all materials causes an aseptic inflammatory process, including ischemia in soft tissues. Ischemia leads to an increase in NOS activity, mainly due to the expression of iNOS. iNOS plays the main role in the formation of nitrosative stress – an important link in inflammation, neuron destruction, arthritis, tumor growth, etc. [6]. It is known that NO in target cells forms active derivatives such as nitrosonium (NO+), nitroxyl (NO–), and peroxynitrite (ONOO–). Recent studies have established that NO, and especially its conversion products, such as peroxynitrite (ONOO–), nitrosonium ion (NO+), nitroxyl (NO–), and diazotrioxide (N2O3), are the main factors in the implementation of nitrosating stress, as a result of which there is a direct interaction of NO with metals (heme iron of hemoglobin, myoglobin, iron protein, as well as non-heme iron of iron-sulfur proteins and DNA, copper and zinc active centers of enzymes), as well as indirect interaction of NO+ (S, N, O nitrosation) with thiol, phenolic, hydroxyl and amino groups of proteins and DNA 6. Peroxynitrite causes T-cell dysfunction, as well as IL-1b/ROS-dependent osteoresorption and oxidative modification of hyaluronic acid 7,8. Thus, the expression and activity of a particular isoform can determine the ability of NO to act as a physiological regulator or toxic agent. Considering the anatomical and physiological features of the structure of the joint capsule, the degree of oxygenation of the structures, as well as the immune balance, especially during its contact with implants, is of great importance 9,10. Currently, there are practically no works on the character of iNOS and eNOS mRNA expression and methods of their correction during the implantation of a polypropylene mesh in the area of the joint capsule.
The choice of solution components is due to their pharmacological properties. L-arginine is a substrate for forming NO in vascular endothelial cells and exhibits vasodilating, anti-ischemic and anti-inflammatory properties 11. Thiotriazoline is a metabolitotropic agent with a pronounced antioxidant mechanism of action, showing wound healing, anti-inflammatory, cardioprotective and anti-ischemic properties 10,11. Thiotriazoline, due to the physicochemical properties of the molecule, can act as a “trap” for superoxide radicals (O-2) and peroxynitrite (ONOO–). Thiotriazoline, due to its antioxidant and membrane stabilizing properties, can reduce the disruption of the membrane bilayer by free radicals, affecting the exchange of arachidonic acid, and may have an inhibitory effect on the biosynthesis of pro-inflammatory prostaglandins. It has been proven that Thiotriazoline limits the free-radical site of the cyclooxygenase pathway for the synthesis of pro-inflammatory prostaglandins. Thiotriazoline inhibits endothelial damage in the alteration zone and induces the development of adaptation reactions, in particular, the conversion of plasminogen into plasmin, followed by an increase in fibrinolysis, which ensures thrombolysis and normalization of blood circulation in the inflammatory zone. Thiotriazoline, reducing ROS-dependent endothelial damage in the alteration zone, indirectly reduces the transformation of prostaglandin G2 into thromboxane A2 under the influence of platelet thromboxane synthetase. One of the most important pro-inflammatory mediators in the pathogenesis of arthritis is NO, which causes T-cell dysfunction. Thiotriazoline, a “trap” of NO, can inhibit ROS- and IL-1b/ROS-dependent stimulation of chondro- and osteoresorption, oxidative modification of hyaluronic acid and chondroitin sulfate 11.
The point of impregnation and coating of the PM with a metabolitotropic solution is the bioavailability of the drug against the background of aseptic inflammation caused by the implant. Given the rheological properties of the metabolite and its content in the pores of the mesh, absorption into soft tissues continues during the first 2 days after implantation. It should be noted that the use of PM in orthopedics is due to the possibility of mechanical strengthening of soft tissue structures and creating a “plateau” for connective tissue integration. In this scenario, the metabolitotrope creates favorable conditions for better tissue integration. The safety of introducing Thiotriazoline and L-arginine solution into the trigger zone has been proven without using a mesh. Still, the bioavailability of the drug is significantly reduced, reducing the time of its exposure and effect. Moreover, given the anatomical features, it is not always possible to access the drug and its accumulation in the tissues.
We have established for the first time that the use of PM without metabolitotropic correction leads to a significant decrease in the expression of eNOS mRNA relative to the values of the experimental group by 10.8 times and an increase in the expression of iNOS mRNA by 3.6 times relative to the values of the reference group. This fact indicates the participation of iNOS in inflammatory processes accompanying implants and using a polypropylene mesh. A decrease in eNOS mRNA expression may indirectly indicate the development of secondary ischemic tissue damage. An increase in the expression of iNOS mRNA leads to overproduction of NO, and against the background of ischemia and its cytotoxic derivative – peroxynitrite, which triggers and enhances many ROS and IL-1b – dependent links of inflammation.
In this study, for the first time, we obtained data using a polypropylene mesh preoperative coated with a solution of L-arginine and Thiotriazoline, and stimulation of eNOS mRNA expression was observed. The pharmacological effect of a solution of L-arginine and Thiotriazoline is due to a positive effect on the synthesis, transport, and bioavailability of NO and the physiological functions of this molecular messenger. NO is an unstable, short-lived radical. For its stabilization and subsequent transportation, mechanisms such as forming stable S-nitrosole complexes with thiol-containing low molecular weight compounds are envisaged. Under a deficiency of thiol compounds (oxidative stress, ischemia, intoxication, hypertension, etc.), NO transport is impaired because it is attacked by such ROS as superoxide radical and hydroxyl radical with transformation into a cytotoxic product – peroxynitrite 6,9,11. Thiotriazoline, part of the solution, increases the level of reduced thiols, particularly glutathione, by activating glutathione reductase and direct reduction of the oxidized thiol group. In addition, due to the antioxidant properties of L-arginine and Thiotriazoline, the solution prevents NO’s an oxidative modification by oxygen radicals 12. Thiotriazoline can act as a transport molecule of NO, formed from L-arginine, forming nitrosothiols. The combined use of L-arginine and Thiotriazoline also directly stimulates NO synthase activity and NO production. Therefore, the combined use of a solution of L-arginine and Thiotriazoline has unique properties of a protective effect concerning the synthesis and transport of NO, its bioavailability, which underlies the mechanism of such properties as an anti-inflammatory and wound healing 13.
The data obtained do not contradict the results of a morphological study. Pronounced inflammatory response, incl. pronounced lymphocytic-macrophage reaction compared to the comparison group, develops, which leads to an increase in the expression of mRNA eNOS and iNOS.
However, a similar tendency was observed in Group B animals, to a lesser extent due to the lower severity. In comparison with the attenuation of the exudative phase, the proliferative phase was activated, which was expressed in an increase in the relative area of the fibers of the synovial membrane and vascular anastomoses, which is explained by the compromise of the mesh and its adhesive properties, which cause stimulation of the proliferative function against the background of a prolonged inflammatory reaction. In addition, the data obtained align with the concept of the participation of iNOS in the development of ischemia, inflammation, neurodegeneration, and neurotoxicity 14.
Further research regarding the proposed method of using a mesh with a metabolitotropic solution is going on. We have received a lot of data that will be displayed in future manuscripts.
Conclusions
The use of a PM with an adhered solution of metabolitotropic correction based on Thiotriazoline and L-Arginine leads to the expression in the soft tissues of iNOS mRNA and eNOS mRNA, which indicate a decrease in the severity of inflammation and ischemia (mainly due to the growth of the network of capillaries and an increase in the number of anastomoses) of the soft tissues in direct contact with the mesh, which contributes to the improvement of regenerative mechanisms. The data obtained indicate the potential of using the polypropylene mesh impregnated and coated with the Thiotriazoline and L-arginine solution in clinical practice.
Conflict of interest
: Pat. 118166 Ukraine, IPC A61F 2/02 (2006.01), A61L 27/00, A61L 31/00. Polypropylene medical mesh / Maslennikov SO; Belenichev IF; Chairman ML; Kucherenko LI; Mazur IA; applicant and patent owner of LLC “Scientific and Production Association “PHARMATRON”; publ. 08/27/2018.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 118166 Ukraine, IPC A61F 2/02 (2006.01), A61L 27/00, A61L 31/00. Polypropylene medical mesh / Maslennikov SO; Belenichev IF; Chairman ML; Kucherenko LI; Mazur IA; applicant and patent owner of LLC “Scientific and Production Association” PHARMATRON “; application. 08/27/2018; publ.
- Hiren Patel, Donald R. Ostergard, Gina Sternschuss. Polypropylene mesh and the host response.Int Urogynecol J, 2012; 23:669–679.
CrossRef - Lei-Ming Zhu, Schuster P, Klinge U.Mesh implants: An overview of crucial mesh parameters.World J Gastrointest Surg, 2015;7(10): 226-236.
CrossRef - Belenichev I, Kucherenko L, Pavlov S, Bukhtiyarova N, Popazova O, Derevianko N, Nimenko G Therapy of post-COVID-19 syndrome: improving the efficiency and safety of basic metabolic drug treatment with tiazotic acid (thiotriazoline). Pharmacia,2022; 69(2): 509-516.https://doi.org/10.3897/pharmacia.69.e82596
CrossRef - FörstermannU., SessaW.C.Nitric oxide synthases: regulation and function. Heart J., 2012; 33(7): 829-837.
CrossRef - Belenichev I, Gorbachova S, Pavlov S, Bukhtiyarova N, Puzyrenko A, Brek O. Neurochemical status of nitric oxide in the setting of the norm, ishemic event of central nervous system, and pharmacological intervention. Georgian Med News, 2021; 315:169-176. PMID: 34365445.
- F. Bukhtiyrova N.V.,Kolesnik Yu.M.,Pavlov S.V., Sokolik E.P. Malate-aspartate shunt in neuronal adaptation to ishemic conditions: molecular-biochrmical mechanisms of activation and regulation.Neurochemical Journal, 2012; 29: 28-34.
CrossRef - Maslennikov S.O Rationale for the use of polypropylene mesh for the treatment of dislocations of the hip arthroplasty: dis. .Doctor of Philosophy: 21.09.21 / Maslennikov Serhii Olehovych – Z., 2021. – 178 p.
- Gunina LM, Shustov YeB, Belenichev IF, Vysochina NL, Golovashchenko RV, Morozova O Specialized nutrition for athletes: evaluation of ergogenic action using the principles of evidence-based medicine. Pharmacia, 2022; 69(1): 37-44.https://doi.org/10.3897/pharmacia.69.e76599
CrossRef - F.,BukhtiyarovaN.V.,KolesnikYu.M., PavlovS.V., SokolikE.P. Disturbance of HSP70 Chaperone Activity is a possible mechanism of Mitochodrial Dysfunction.Neurochem. Journal, 2011; 5(4): 251-256.
CrossRef - Cadenas E., Davies K. J. A. Mitochondrial Free Radical Generation Oxidative Stress and Aging. Free Radic. Biol. Med., 2000; 29 (3-4): 222-230.
CrossRef - Kumar M, Bansal N. Effects of Chronic Lithium Chloride and L-Arginine Treatment on Prevention of Streptozotocin Induced Cognitive Deficits by Ellagic Acid. Biomedical and Pharmacology Journal. 2018;11(1):53-65.
CrossRef - El-Laithy NA, Mahdy EM, Youness ER, Shafee N, Mowafy MS, Mabrouk MM. Effect of Co Enzyme Q10 Alone or in Combination with Vitamin C on Lipopolysaccharide-Induced Brain Injury in Rats. Biomedical and Pharmacology Journal. 2018 Sep 21;11(3):1215-26.
CrossRef - Al-kuraishy HM, Al-Gareeb AI, Alkhuriji AF, Al-Megrin WAI, Elekhnawy E, Negm WA, et al. Investigation of the impact of rosuvastatin and telmisartan in doxorubicin-induced acute cardiotoxicity. Biomedicine & Pharmacotherapy. 2022;154:113673: DOI:10.1016/j.biopha.2022.113673
CrossRef