G. Kishore Kumar1 , Shanmugapriya Ramamurthy2*
, Shanmugapriya Ramamurthy2* , Arunmozhi Ulaganathan3
, Arunmozhi Ulaganathan3 Sheeja Varghese4
Sheeja Varghese4 , Arockia Antony Praveen3
, Arockia Antony Praveen3 , Saranya V5
, Saranya V5
1Sri Venkateswara Dental college, The Tamilnadu Dr. M. G. R Medical University, Chennai, India.
2Department of Periodontics, Saveetha Institute of Medicine and Technical Sciences, Sri Venkateshwara Dental College and Hospital The Tamilnadu Dr. M. G. R Medical University, Chennai, India
3Department of Periodontics, Sri Venkateshwara Dental College and Hospital The Tamilnadu Dr.M.G.R Medical University, Chennai, India.
4Department of Periodontics, Saveetha Dental College and Hospital, Saveetha Institute of Medicine and Technical Sciences, Chennai, India.
5Department of Oral Pathology, Sri Venkateshwara Dental College and Hospital, The Tamilnadu Dr.M.G.R Medical University, Chennai, India.
Corresponding Author E-mail: drshanpriya@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2542
Abstract
Moringa oleifera (MO) commonly known as drumstick plant, is recognised by modern medicine for its distinctive therapeutic benefits. One of those benefits being its antimicrobial activity. Silver nanoparticles, known for its pharmacological effects as well as synergistic action with various agents have gained popularity in recent years. The aim of this research work was to assess the antibacterial efficacy of 5% Moringa oleifera mouthwash reinforced with silver nanoparticles against oral aerobic organisms. Aqueous extract of 5% Moringa oleifera was used to synthesize silver nanoparticles and prepare the mouthwash. Characterization was done using scanning electron microscopy analysis and energy dispersive x-ray analysis. The antibacterial activity of the mouthwash against Streptococcus mutans, Staphylococcus aureus, Enterococcus faecalis, Candida albicans was investigated using agar well diffusion assay. 5% MO - silver nanoparticles mouthwash had a stronger impact on Staphylococcus aureus and a comparable effect on Streptococcus mutans. The maximum zone of inhibition was 28 mm at 100 µL for Staphylococcus aureus and minimum zone of inhibition was 16mm for Candida albicans. There was a dose dependent effect of MO - silver nanoparticles mouthwash on Staphylococcus aureus, Streptococcus mutans, Enterococcus faecalis and Candida albicans. Of these, the antimicrobial effect was more appreciable on plaque colonizers like Staphylococcus aureus and Streptococcus mutans. Thus, these characteristics of phytomedicine and nanomedicine prove to be a safer alternative in the management of Plaque associated Gingival diseases.
Keywords
Antimicrobial Activity; Moringa oleifera; Mouthwash; Silver Nanoparticles
Download this article as:| Copy the following to cite this article: Kumar G. K, Ramamurthy S, Ulaganathan A, Varghese S, Praveen A. A, Saranya V. Moringa oleifera Mouthwash Reinforced with Silver Nanoparticles - Preparation, Characterization and its Efficacy Against Oral Aerobic Microorganisms - In Vitro Study. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Kumar G. K, Ramamurthy S, Ulaganathan A, Varghese S, Praveen A. A, Saranya V. Moringa oleifera Mouthwash Reinforced with Silver Nanoparticles - Preparation, Characterization and its Efficacy Against Oral Aerobic Microorganisms - In Vitro Study. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3H4YetF |
Introduction
Microbes are ubiquitous in the oral cavity. They exist as well structured and organized communities in a polysaccharide matrix called dental biofilm. The normal habitat is maintained by adopting proper oral hygiene measures. When there is a disturbance in the homeostasis, pathogenic microflora invade the microbial habitat resulting in gingivitis, periodontitis and dental caries. 1 Gingivitis, an inflammatory condition of the gingiva affects children and adults of all age groups.2 In the initial stages of plaque formation gram positive streptococcus strains in habitat the tooth surfaces as primary colonizers. 3 Apart from this there are other atypical pathogens like Staphylococcus Aureus, Enterococcus Facecalis and Candida Albicans isolated from saliva, periodontal pockets and dental plaque. 4,5 These atypical microbes could be a reason for refractory gingival and periodontal conditions that do not respond to routine plaque control therapy. Brushing is the most widely used self-care oral hygiene technique for removing bacterial plaque, mechanically. 6
However, routine mechanical oral hygiene techniques adopted by individuals are inadequate, either because of faulty brushing techniques or the presence of multiple plaque retentive factors.6 This maintenance of oral hygiene is critical and much more demanding in patients with a history of periodontitis.6 So, chemical plaque control has emerged as an effective and obligatory alternative for all kinds of dental patients in all age groups.7
The gold standard for chemical plaque management is chlorhexidine (CHX), a broad-spectrum antimicrobial. However, long-term use of CHX mouthwashes can cause discolouration of the tooth and the tongue , alteration in taste perception and more calculus formation. These unfavourable side effects make CHX mouthwashes undesirable for long-term usage in patients. Consequently, the search for alternatives continue, with an emphasis on biogenic agents.8
Medicinal herbs are being used in Naturotherapy since thousands of years around the world. India has a very rich ancient medical system that dates back to over three thousand years, and herbal combinations have been recognized as an inevitable alternative.9 Herbal remedies originating from plant sources have long been utilized in dentistry to suppress bacteria, decrease inflammation, soothe irritation, and alleviate pain.9 A large range of herbal mouthwashes has been reported to have promising benefits in biofilm reduction. Herbal mouth rinses prepared using extracts and essential oils from phytotherapeutic plants contain active substances such as catechins, tannins, and sterols.10
Moringa oleifera found commonly in households in most regions of India has diverse medical properties. It is a versatile remedy that possesses anti-tumor, antipyretic, antispasmodic, diuretic, antiulcer, hypotensive, hypolipidemic, hepatoprotective, antifungal, and antibacterial properties. 11 It is a part of the staple diet consumed by people of different socioeconomic classes. Every part of the Moringa plant, from the leaves to the fruit, seeds, flowers, bark, and roots, offers enormous health advantages, so it is rightly known by various names like miracle tree, tree for life, wonder tree, and marvelous tree.12 The effectiveness of Moringa oleifera (MO) extracts is less explored in oral care.
Nanotechnology is a boon to drug delivery systems. It is the changes brought upon a substance at an atomic level at the nanoscale of 1-100 nm. Silver nanoparticles are rising in popularity due to their diverse biological features like anti-inflammatory and antioxidant properties. Silver has been used to heal wounds and chronic inflammation since ancient times. In Siddha medicine practice, for oral administration of any herb, a small dose of metal is given along with the preparation.13 This addition enhances the availability of the herb for rapid action at a low dosage. The aim of the present study, was to assess the antibacterial effect of 5% Moringa oleifera mouthwash with silver nanoparticles against oral aerobic organisms.
Methodology
This in-vitro study was conducted in Pharmacology department of Saveetha Dental college between May-June of 2022.
Aqueous Extract Preparation
Leaves of Moringa oleifera (drumstick) were randomly procured from local market. They were rinsed with distilled water, dried in shade, powdered and stored in airtight vials. According to the previous study report, 5% aqueous extract of MO exhibited potent anti-inflammatory and anti-oxidant activity so the same extract concentration was adopted for the present study. For aqueous preparation 100 gm of the leaf powder was added to 1 litre of distilled water, macerated, and left for three days. Following that, with sterile Whatmann filter paper the MO solution was filtered. 50 cc of the filtered extracts was heated to destroy the bacterial spores and was used for testing of antibacterial activity.14
Synthesis of silver nanoparticles
According to Espinosa-Cristóbal technique 15 silver nanoparticles were prepared. To 50 mL of deionized water, 0.0169 grams of AgNO3 was dissolved for 5 min and 50 ml of the aqueous extract of Moringa oleifera was added. For synthesis of silver nanoparticles, the solution was mixed with magnetic stirring and allowed to stand for 2 hr. The solution was boiled and observed for dissolution. Finally it was centrifuged in test tubes of 16 mm. The particles formed after centrifuging for 10 minutes at 3000 rpm.
Preparation of Mouthwash
Trituration of sucralose (0.1%), sodium benzoate (0.05%) and menthol (1%) was done. To this, 50 ml of silver nanoparticles synthesized from 5% Moringa oleifera was added. Sodium benzoate acts as a preservative and menthol as a flavouring agent (fig 1).
 |
Figure 1: Synthesis of silver nanoparticles from 5% Moringa oleifera |
Characterization of Silver Nanoparticles
The characterization of biosynthesized silver nanoparticles was done by UV-Visible spectroscopy using a double beam UV visible spectrophotometry in the wavelength range of 300-500 nm. This way the reduction of AgNO3 to AgNps was checked.
The morphology of the biosynthesized silver nanoparticles was determined using scanning electron microscopy at 10K resolution
Energy Dispersive X-Ray Analysis (EDX) is a method where x-rays are used to determine the elemental makeup of materials. Scanning Electron Microscopy (SEM) uses energy-dispersive X-ray analysis (EDAX) to study nanoparticles.
Antimicrobial Activity of Moringa oleifera Mediated Nanoparticle Mouthwash
The microbial culture of Staphylococcus aureus, Streptococcus mutans, Enterococcus faecalis, Candida albicans. used in this study were obtained from the microbiology lab of Saveetha dental college.
25μL 50μL, 100μL, 150μL of mouthwash were loaded into the wells containing the Mueller-Hinton agar plates streaked individually with Staphylococcus aureus, Streptococcus mutans, Enterococcus faecalis,and Candida albicans. The wells were incubated at 37°C for 48 hours. Amoxicillin and Fluconazole were used as the standard. For each pathogen, the zone of inhibition was measured. The tests were repeated thrice, and the mean value was determined.
Results
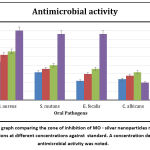
The UV visible spectra revealed a particular absorption band at 420 nm peak (fig2) for the produced silver nanoparticles. This peak was obtained after 2 hours of reaction time, and the absorption band observed was wide, suggesting the nanoparticle’s polydispersity of distribution. According to the SEM analysis, the particles were hexagonal and pentagonal shapes and the sizes were ranging between 80-150 nm (fig 3). The elemental makeup of the components evaluated by x-ray dispersion study revealed that the mouthwash comprised of 60% MO extract particles and 40% silver nanoparticles (fig 4).
Table 1 shows the zone of inhibition of the prepared mouthwash against Staphylococcus aureus, Streptococcus mutans, Enterococcus faecalis, Candida albicans. The zone of inhibition was maximum at higher concentration of the mouthwash. And at 100 µL the zone of inhibition was observed to be at a highest of 28mm for Staphylococcus aureus followed by 20mm for Streptococcus mutans (figure 5). As seen in the graphical representation (figure 6) these inhibition zones were comparatively less for the rest of the organisms.
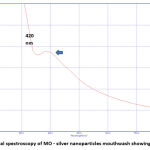 |
Figure 2: UV visual spectroscopy of MO – silver nanoparticles mouthwash showing a peak at 420nm |
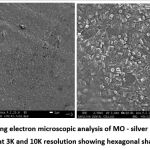 |
Figure 3: Scanning electron microscopic analysis of MO – silver nanoparticles mouthwash at 3K and 10K resolution showing hexagonal shaped AgNPs |
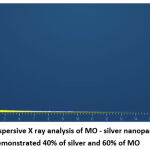 |
Figure 4: Energy dispersive X ray analysis of MO – silver nanoparticles mouthwash demonstrated 40% of silver and 60% of MO |
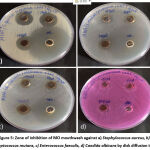 |
Figure 5: Zone of inhibition of MO mouthwash against a) Staphylococcus aureus, b) Streptococcus mutans, c) Enterococcus faecalis, d) Candida albicans by disk diffusion test |
Table 1: Extent of zone of inhibition by MO – silver nanoparticles mouthwash at different concentrations and standard on aerobic organisms.
| Organism | 25 µL | 50 µL | 100 µL | Antibiotic |
| S. aureus | 22 | 26 | 28 | 40 |
| S. mutans | 16 | 18 | 20 | 38 |
| E. faecalis | 11 | 15 | 18 | 38 |
| C. albicans | 12 | 14 | 16 | 10 |
Discussion
Plaque-induced gingivitis is a relatively prevalent oral disease caused due to the accumulation of microbial biofilms on the surfaces of teeth. This direct association between plaque and gingivitis results in a severe form of gingivitis in cases where oral care is compromised. Plaque control by mechanical and chemical means can ensure a complete reversal of the inflammation. In few instances complete resolution fail to occur which could be due to atypical infections caused by opportunistic oral microflora like S.aureus, E. faecalis, C. albicans. 16 Studies have isolated these microorganisms in saliva, subgingival plaque of gingivitis, periodontitis17 and peri-implantitis patients. 18
There are a spate of research works on the therapeutic benefits of Moringa oleifera. A systematic review analysing the anti-inflammatory, antioxidant and antiproliferative effects of MO on cell lines observed that the presence of kaempferol, quercetin, apigenin and multiflorin-B accounts for its pharmacological benefits. The extracts were selectively toxic to cancer cells and nontoxic to normal cells. Leaves had pronounced anti-inflammatory and antioxidant activities than other parts of the plant. 19
Based on these observations, in this study the effect of MO mouthwash with bio-enhancer like silver nanoparticles on opportunistic oral organisms Staphylococcus aureus, Enterococcus faecalis, Candida albicans and common plaque colonizer Streptococcus mutans was evaluated.
The antibacterial effect of MO leaf extract was studied by Singh & Tafida on Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa. Similar to the present study the leaf extract had a potential inhibitory effect on the studied aerobic organisms. 20
A study done by Peioxoto et al compared the antibacterial efficiency of various extract preparations of MO leaves. It concluded both aqueous and ethanolic extract had an significant antibacterial effect on S.aureus, E.faecalis and V.parahaemolyticus. 21 But considering the anti-inflammatory and anti-oxidant activities the aqueous extract was found to be more potent than alcoholic extract. 14 Hence for the present study, aqueous extract was chosen for mouthwash preparation.
Elagamily et al conducted an in vitro study to evaluate the effectiveness of toothpaste and mouthwash prepared with MO. The anti-microbiologial assessment against oral microbes S.aureus, S.mutans, and C.albicans was done. The formulated toothpaste displayed more antibacterial and antifungal activity than the mouthwash. And there was no antifungal activity reported for the mouthwash. In the present study the mouthwash exhibited antibacterial activity but no significant antifungal effect. The toothpaste showed highest mean inhibitory zone for Staphylococcus aureus and Streptococcus mutans. 22
The antimicrobial effect of MO is attributed to the presence of alkaloids, flavonoids, tannins, terpenoids, glycosides and saponins. The phytoconstituents cause increased permeability of bacterial cell membrane by inhibition enzyme sortase, affecting DNA replication and resulting in lysis of plasma membrane. Tannins inhibit the amino acid metabolism of the bacterium and retards multiplication. Saponins interact with the cell membrane and result in alteration of cell morphology and cell death. 23
Silver nanoparticles (AgNPs) are important metallic nanoscale materials because of their antibacterial characteristics. According to studies, the use of AgNPs in dental applications has the potential to counteract dental biofilm and diminish the prevalence of dental caries, periodontal disease, and other oral bacterial disorders.24
A study testing the efficiency of 0.2 percent curcumin nanoparticle mouthwash among periodontitis patients, demonstrated low plaque and gingival scores, decreased probing pocket depths and gain in clinical attachment levels. The outcomes were comparable to a group which used 0.2% chlorhexidine (CHX).25
Silver nanoparticles also demonstrated better wound healing effect than CHX mouthwash in palatal wounds of rabbits. 26
The development of new technologies that improve the standard of care and expand the range of applications for dentifrices and mouthwashes have accelerated the understanding of nanotechnology and its applications in the field of oral hygiene products. Given the several benefits of both silver nanoparticles and MO, our study was the first of its kind to evaluate the synergistic effects of AgNPs and MO mouthwash on oral aerobic microbes.
The Mechanism of green synthesis of silver nanoparticles with MO extract in this study, was due to the presence of alkaloid in the leaf extract. Silver ions get trapped onto the surface of the alkaloid and were reduced by proteins to produce silver nuclei. These silver nuclei clump together and expand in size, culminating in the production of silver nanoparticles. The alkaloids covering the silver nanoparticles stabilize the silver particles by preventing aggregation among them. 27 The antibacterial effect of AgNPs, is by the release of positively charged silver ions which interact with negatively charged bacterial cell wall proteins that lead to cell death by loss of cell wall integrity. 28 In the present study the UV visible spectrophotometry showed a peak at 420 nm wavelength indicating the synthesis of AgNPs. The same wavelength was also observed by Halki. 29
Herbs are employed for management and prevention of diseases. Herbal remedies are an effective alternative to conventional therapy for its minimal complications, less financial burden, and ease of availability. They possess various micronutrients and pharmacologically active constituents. And their effects are dependent on time of harvest, region of cultivation and process of preparation. With the aid of advanced technologies and well-designed studies, standardized phytotherapy protocols can be drawn employing newer drug delivery systems for the management of disease conditions.
The shortcomings of the study were the lack of assessment against other marketed mouthwashes and also leaving out testing its effect on anaerobic organisms. Since these results were obtained in a laboratory setup well designed, clinical trials are required to confirm the same. Cross over trials with longer follow-ups may be considered to extrapolate the in-vitro results clinically. In future, with the findings of this study, novel remedies in the form of local drug delivery for the management of periodontitis can be designed with MO synthesized silver nanoparticles.
Conclusion
The generation of physiologically active silver nanoparticles of size 80-150 nm was demonstrated in this study using Moringa oleifera extract. The pathogens Streptococcus mutans, Staphylococcus aureus, Enterococcus faecalis, and Candida albicans showed significant dose-dependent inhibitory activity when the nanoparticles were coated with Moringa oleifera in an aqueous media. The combined benefits of phytomedicine with nanomedicine can result in more effective treatment with fewer side effects. Clinical studies should be conducted to assess the effects of Moringa oleifera extract in the management of gingivitis and periodontitis.
Prior publication: Nil
Support: Nil
Conflicts of interest: Nil
Permissions: Nil
References
- Gurenlian, Joann. (2007). The Role of Dental Plaque Biofilm in Oral Health. Journal of Dental Hygiene. 116-116.
- Valm, A. M. (2019). The structure of dental plaque microbial communities in the transition from health to dental caries and periodontal disease. Journal of molecular biology, 431(16), 2957. https://doi.org/10.1016/j.jmb.2019.05.016
CrossRef - Bhor, K., Shetty, V., Garcha, V., Ambildhok, K., Vinay, V., & Nimbulkar, G. (2021). Effect of 0.4% Triphala and 0.12% chlorhexidine mouthwash on dental plaque, gingival inflammation, and microbial growth in 14–15-year-old schoolchildren: A randomized controlled clinical trial. Journal of Indian Society of Periodontology, 25(6), 518-524. https://doi.org/10.4103/jisp.jisp_338_20
CrossRef - Prevalence of Enterococcus faecalis in subgingival biofilm and saliva of subjects with chronic periodontal infection. Arch Oral Biol 2008; 53( 2): 155–160.
CrossRef - Cuesta AI, Jewtuchowicz V, Brusca MI, Nastri ML, Rosa AC. Prevalence of Staphylococcus spp and Candida spp in the oral cavity and periodontal pockets of periodontal disease patients. Acta Odontol Latinoam. 2010;23(1):20-6. PMID: 20645638.
- Figuero, E., Nobrega, D. F., García‐Gargallo, M., Tenuta, L. M., Herrera, D., & Carvalho, J. C. (2017). Mechanical and chemical plaque control in the simultaneous management of gingivitis and caries: a systematic review. Journal of clinical periodontology, 44, S116-S134
CrossRef - Vyas, T., Bhatt, G., Gaur, A., Sharma, C., Sharma, A., & Nagi, R. (2021). Chemical plaque control-A brief review. Journal of Family Medicine and Primary Care, 10(4), 1562.
CrossRef - Varoni, E., Tarce, M., Lodi, G., & Carrassi, A. (2012). Chlorhexidine (CHX) in dentistry: state of the art. Minerva Stomatol, 61(9), 399-419
- Eid Abdelmagyd HA, Ram Shetty DS, Musa Musleh Al-Ahmari DM. Herbal medicine as adjunct in periodontal therapies- A review of clinical trials in past decade. J Oral Biol Craniofac Res. 2019 Jul-Sep;9(3):212-217. doi: 10.1016/j.jobcr.2019.05.001. Epub 2019 May 14. PMID: 31193290; PMCID: PMC6525324
CrossRef - Moro, MG, Silveira Souto, ML, Franco, GCN, Holzhausen, M, Pannuti, CM. Efficacy of local phytotherapy in the nonsurgical treatment of periodontal disease: A systematic review. J Periodont Res. 2018; 53: 288– 297.
CrossRef - Noreen, Sana & Rizwan, Bahisht & Ijaz, Aiman & Imran, Muhammad & Nisar, Tahreem & Farooq, Sana & Iftikhar, Faiza & Sikander, Sehrish & Ahmed, Hamna. (2020). A review on Moringa oleifera-A potent medicinal herb. 10.12692/ijb/16.4.500-508
- Meena, R., Prajapati, S. K., Nagar, R., Porwal, O., Nagar, T., Tilak, V. K., … & Dhakar, R. C. (2021). Application of Moringa oleifera in Dentistry. Asian Journal of Dental and Health Sciences, 1(1), 10-13.
CrossRef - Zupancic, S., Kocbek, P., Baumgartner, S., & Kristl, J. (2015). Contribution of nanotechnology to improved treatment of periodontal disease. Current pharmaceutical design, 21(22), 3257-3271
CrossRef - Ramamurthy S, Thiagarajan K, Varghese S, et al. Assessing the In Vitro Antioxidant and Anti-inflammatory Activity of Moringa oleifera Crude Extract. J Contemp Dent Pract 2022;23(4): 437–442.
CrossRef - L. F. Espinosa-Cristóbal, C. Holguín-Meráz, E. A. ZaragozaContreras et al., “Antimicrobial and substantivity properties of silver nanoparticles against oral microbiomes clinically isolated from young and young-adult patients,” Journal of Nanomaterials, vol. 2019, 14 pages, 2019
CrossRef - Balaei-Gajan E, Shirmohammadi A, Abashov R, Agazadeh M, Faramarzie M. Detection of enterococcus faecalis in subgingival biofilm of patients with chronic refractory periodontitis. Med Oral Patol Oral Cir Bucal. 2010 Jul 1;15(4):e667-70. doi: 10.4317/medoral.15.e667. PMID: 20173722
CrossRef - Chidambar CK, Shankar SM, Raghu P, Gururaj SB, Bushan KS. Detection of Enterococcus faecalis in subgingival biofilms of healthy, gingivitis, and chronic periodontitis subjects. J Indian Soc Periodontol. 2019 Sep-Oct;23(5):416-418. doi: 10.4103/jisp.jisp_44_19. PMID: 31543613; PMCID: PMC6737857.
CrossRef - Canullo L, Rossetti PH, Penarrocha D. Identification of Enterococcus Faecalis and Pseudomonas Aeruginosa on and in Implants in Individuals with Peri-implant Disease: A Cross-Sectional Study. Int J Oral Maxillofac Implants. 2015 May-Jun;30(3):583-7. doi: 10.11607/jomi.3946. PMID: 26009909.
CrossRef - Popoola, Jacob & Aworunse, Oluwadurotimi & Oyesola, Olusola & Akinnola, Olayemi & Obembe, Olawole. (2020). A systematic review of pharmacological activities and safety of Moringa oleifera Implication for health policy/practice/research/medical education: A R T I C L E I N F O. Journal of Herbmed Pharmacology. 9. 174-190. 10.34172/jhp.2020.24.
CrossRef - Singh, K., and G. M. Tafida. “ANTIBACTERIAL ACTIVITY OF MORINGA OLEIFERA (LAM) LEAVES EXTRACTS AGAINST SOME SELECTED BACTERIA”. International Journal of Pharmacy and Pharmaceutical Sciences, vol. 6, no. 9, Sept. 2014, pp. 52-54, https://innovareacademics.in/ journals/index.php/ ijpps/article/view/3198.
- Peixoto JR, Silva GC, Costa RA, de Sousa Fontenelle JR, Vieira GH, Filho AA, dos Fernandes Vieira RH. In vitro antibacterial effect of aqueous and ethanolic Moringa leaf extracts. Asian Pac J Trop Med. 2011 Mar;4(3):201-4. doi: 10.1016/S1995-7645(11)60069-2. Epub 2011 Apr 12. PMID: 21771453.
CrossRef - Elgamily H, Moussa A, Elboraey A, El-Sayed H, Al-Moghazy M, Abdalla A. Microbiological Assessment of Moringa Oleifera Extracts and Its Incorporation in Novel Dental Remedies against Some Oral Pathogens. Open Access Maced J Med Sci. 2016 Dec 15;4(4):585-590. doi: 10.3889/oamjms.2016.132. Epub 2016 Nov 28. PMID: 28028395; PMCID: PMC5175503.
CrossRef - Felix. (2014). Mechanisms of Antimicrobial Actions of Phytochemicals against Enteric Pathogens – A Review. Journal of Pharmaceutical, Chemical and Biological Sciences. 2. 77-85.
- Prabhu, S., & Poulose, E. K. (2012). Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. International nano letters, 2(1), 1-10.
CrossRef - Farooqui Arifa Areej, Thomas Raison, Shah Rucha, & Mavinakote Gowda Triveni. (2022). Evaluation of Nanocurcumin Mouthwash as an Adjunctive Plaque Control Agent: A Clinical Pilot Study. International Journal of Ayurveda and Pharma Research, 10(4), 1-7.
CrossRef - Moaddabi A, Soltani P, Rengo C, Molaei S, Mousavi SJ, Mehdizadeh M, Spagnuolo G. Comparison of antimicrobial and wound-healing effects of silver nanoparticle and chlorhexidine mouthwashes: an in vivo study in rabbits. Odontology. 2022 Jul;110(3):577-583. doi: 10.1007/s10266-022-00690-z. Epub 2022 Feb 26. PMID: 35218448; PMCID: PMC9170635.
CrossRef - Arya, Geeta & Sharma, Nikita & Mankamna, R. & Nimesh, Surendra. (2019). Antimicrobial Silver Nanoparticles: Future of Nanomaterials. 10.1007/978-3-030-16534-5_6.
CrossRef - Zhang, X. F., Liu, Z. G., Shen, W., & Gurunathan, S. (2016). Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. International journal of molecular sciences, 17(9), 1534.
CrossRef - Halkai, K. R., Mudda, J. A., Shivanna, V., Rathod, V., & Halkai, R. (2018). Evaluation of Antibacterial Efficacy of Fungal-Derived Silver Nanoparticles against Enterococcus faecalis. Contemporary Clinical Dentistry, 9(1), 45-48. https://doi.org/10.4103/ccd.ccd_703_17
CrossRef