Manuscript accepted on :11-10-2022
Published online on: 14-11-2022
Plagiarism Check: Yes
Reviewed by: Dr. Nagham Aljamali, Dr. Gandhi, Kushal
Second Review by: Dr. Satyajit Mohapatra
Final Approval by: Dr. Patorn Piromchai
Ehab Tousson1* , Ibrahim E. El Sayed2
, Ibrahim E. El Sayed2 , H. Abd El-Aleim2, Mervat Elabd2, Mustafa Karhib3 and Doaa T. Gebreel4
, H. Abd El-Aleim2, Mervat Elabd2, Mustafa Karhib3 and Doaa T. Gebreel4
1Zoology Department, Faculty of Science, Tanta University, Egypt.
2Chemistry Department, Faculty of Scieence, Menoufia University, Egypt.
3Department of Medical Laboratory Techniques, Al-Mustaqbal University College, 51001 Hillah, Babylon, Iraq
4Medical Equipment Department, Faculty of Allied Medical Sciences, Pharos University, Alexandria, Egypt
Corresponding Author E-mail: ehabtousson@science.tanta.edu.eg
DOI : https://dx.doi.org/10.13005/bpj/2531
Abstract
Introduction: The field of cancer nano therapeutics is quickly evolving, and it is being used to address a number of issues with traditional drug delivery techniques. The goal of this study was to find out more about the impact of novel bioactive Poria-loaded sun flowers nanoparticles (Poria Nps) as anti-carcinogenic agent for Ehrlich solid tumour (EST). Methods: A total number of 40 adult female mice were divided into 4 groups included control group, Poria Nps group, EST group, and EST treated with Poria Nps group. Results: EST induced toxicity, apoptosis and oxidative stress while treatments of EST with Poria Nps improved this alteration in kidney functions and structure. Moreover, Poria Nps could scavenge free radicals producing beneficial effects against EST induced renal toxicity through activation of oxidative stress and apoptosis. The constructed novel oral nanoparticles developed have promising features in vivo as well as a high level of safety for efficient cancer treatment. Conclusion: Poria cocos nanoparticles (Poria Nps) kill the cancer cells through apoptosis which thereby regulates the proliferation of cancer cells and inhibits its spread to other organs.
Keywords
Apoptosis; Cancer Nano therapeutics; Ehrlich solid tumor; Renal toxicity
Download this article as:| Copy the following to cite this article: Tousson E, El Sayed I. E, El-Aleim H. A, Elabd M, Karhib M, Gebreel D. T. Impact of Poria Cocos Nanoparticles Extract Against Ehrlich Solid Tumour Induced Toxicity, Oxidative Stress and Apoptosis in Female Mice Kidney. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Tousson E, El Sayed I. E, El-Aleim H. A, Elabd M, Karhib M, Gebreel D. T. Impact of Poria Cocos Nanoparticles Extract Against Ehrlich Solid Tumour Induced Toxicity, Oxidative Stress and Apoptosis in Female Mice Kidney. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3Acwr6j |
Introduction
Cancer is the disruption of the healthy cell cycle, which leads to uncontrolled cell proliferation and a lack of differentiation, which are recognized as malignant growths. The most common form of cancer, breast cancer, is also the second leading cause of mortality among women. 1,2 Ehrlich tumour was mainly characterized as a spontaneous mouse mammary adenocarcinoma that mimicked breast cancer.3-5 Ehrlich solid tumors are undifferentiated solid tumors that are widely employed in chemotherapeutic research and tumour studies.6,7
Along with causing disruptions in some tissues of the tumour host, tumour development can modify the form and function of the liver and kidneys.8,9 According to research, oxidative stress and the production of reactive oxygen species by malignancies cause tissue damage and DNA deterioration.10,11
In reality, the idea of chemoprevention using substances derived from nature is gaining more and more attention, especially because conventional cancer therapies have a poor therapeutic record and a high rate of adverse effects.12 In their simplest forms as syrups, infusions, and ointments, medicinal herbs from the plant kingdom have long been utilized as herbal remedies.13-15 The primary medical resource used in traditional or folk medicine, according to the World Health Organization, are medicinal plants.16
In many of Asian countries, Poria coco have a long history of medical use. A Poria coco contains triterpenes, steroids, polysaccharides, choline, amino acids, histidine, and other chemicals.17 In recent decades, significant progress has been achieved in chemical and bioactive research of Poria coco polysaccharides and their derivatives that exhibit many beneficial biological activities including anti-inflammatory, anticancer, antiviral and antioxidant activities.18 Natural polymeric-based nanomaterials have great potential for developing novel drug carrier designs for specific applications. Furthermore, these nanoparticles have therapeutic properties and can provoke a significant immunological response with high biological safety. Therefore; the goal of this study was to find out more about the impact of novel bioactive Poria-loaded sun flowers nanoparticles (Poria Nps) as anti-carcinogenic agent for EST induced kidney toxicity and injury.
Materials and Methods
We bought sunflower seeds at the neighbourhood market, Alexandria-Egypt. The authentic extraction of Poria cocus was purchased from Herbal House Centers Company, Cairo, Egypt.
Preparation of Sunflower lecithin and Poria cocos nanoemulsion:
The extraction of lecithin was produced according to Zorzi et al.21 method with some modifications. The husk of the crude Sunflower seeds were removed, washed with distil water and dried in oven at 40oc for one hour. The dried seeds were crushed by milling machine. Convenient volume of 5% vinegar was added to the crushed seeds and blended with homogenizer. The mixture was centrifuged for 20 min at 4000 rpm. The extraction was purified three times by adding 20 ml of vinegar to the sample and incubated at room temperature for 30 min., and the precipitate was dried for 24 hours under vacuum at room temperature. Nano-emulsions were created using a modified Solvent-emulsification evaporation technique. The nanoparticles were collected by ultracentrifugation (Beckman Coulter Life Sciences, Optima Max TL, A95761), at 3000 rpm for 20 minutes, then washed twice with distil water and dried using a lyophilizer ( Bioevopeak,Benchtop Freeze Dryer,LYO60B-1S).
Animals
A total of 40 female Swiss albino mice (Mus musculus), 8–10 weeks old, weighing 25±2 from Egypt Vaccine Establishment’s (EVC) animal house colony were provided standard mice feed and water ad libitum. All the experiments were done in compliance with the guiding principles in the care and use of laboratory animals IACUC – SCI – TU – 00179.
Experimental design and animal groups
Mice were equally divided into six groups (Gp1 – Gp4): Gp1: Control Gp; the mice did not receive any therapy. Gp2: Poria nanoparticles Gp (Poria NPs); the mice received Poria NPs orally (25mg/kg bw/2day) orally for 15 days. Gp3: EST Gp; mice were administered hypodermically with approximately 2,500,000 EAC/mouse weakened in buffer saline to begin EST.3 Gp4: Treated EST with Poria NPs (EST+Poria NPs) in which mice were administered hypodermically with 2,500,000 EAC cells per mouse to induce tumour (EST) and left for 15 days until the tumour improved, at which point they were cured with Poria NPs (25mg/kg bw/2day) orally through stomach tube for 15 days.
Blood and serum samples
Blood samples have been collected aseptically by venepuncture into a dry clean and sterile tube without anticoagulant substances and allow it to clot. Blood samples permitted to stand for 30 min at 4 o C for clotting and then centrifuged for 10 minutes at 3000 rpm. The collected serum was kept at -18° C until it was analysed to determine a blood parameter.
Electrolytes and kidney functions Biomarker
Patton and Crouch22; Bowers and Wong23 approaches were used to estimate urea and creatinine levels in sera. Estimation of electrolytes levels (Potassium, sodium, calcium and chloride ions) in sera by employing trade kits (Sensa core electrolyte, India) according to Elmasry et al.24 and Gupta et al.25
Estimations of oxidative and antioxidants parameters in kidney homogenate:
Malondialdehyde (MDA), a lipid peroxidation indicator, was measured in homogenate using the method reported by Mesbah et al.26
According to Jollow et al. 27 and Saggu et al.28; glutathione (GSH) and catalase activity in homogenate were measured.
Histopathological assessment
Processing for paraffin sectioning followed the immediate incorporation of 10% buffer neutral formalin solution for one to two days to the kidney and liver tissues. In line with Seyedalipour et al.29, the histopathological assessment was enabled by staining the sections with haematoxylin and eosin (H&E).
Apoptotic P53 and anti-apoptotic Bcl2 expressions
In accordance with the protocol designated by Tousson et al.30,31; P53 and Bcl2 immunoreactivities of the fixed kidney slices were evaluated using the avidin-biotin complex method.
Statistical analysis
An unpaired t-test to evaluate the salient variances among treatment groups enabled the undertaking of statistical investigation where data stayed articulated as average values ± SE. 0.05 served as the threshold for the biochemical data representing the criterion of statistically significant data. The SPSS – statistical version 21 software package (SPSS ® Inc., USA) was used to carry out all statistical analyses.
Results
Electrolytes and kidney functions Biomarker
Table (1) revealed that; significant (p <0.05) increase in levels of urea, creatinine, K+, Cl- and significant (p <0.05) decrease in Na+ and Ca++ in EST in comparison to control. Furthermore, levels of urea, creatinine, K+ and Cl- were significantly (p <0.05) decreased but Na+ and Ca++ were significant (p <0.05) increase in EST + Poria as contrast with the EST +.
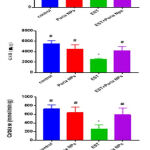
Effects of Poria Nps upon markers of oxidative stress in kidney homogenates
Figure (1) indicated a significant increase in MDA contents and significantly decreased in the GSH and activities of CAT in kidney tissues of EST as compared to control. However, EST+Poria Nps significantly decreased MDA content, while it significantly increased in the content of GSH and the activities of CAT compared to EST.
Table 1: Changes in serum electrolytes and kidney function levels.
| Control | Poria Nps | EST | EST+ Poria Nps | |
| Urea (gm/dl) | 26.7#±1.38 | 28.25# ± 1.65 | 54.5*±2.96 | 33.75#*±2.93 |
| Creatinine (gm/dl) | 0.52#±0.18 | 0.49# ± 0.03 | 0.98*±0.06 | 0.64#*±0.07 |
| Na+ (mmol/l) | 136.6# ± 8.8 | 135.7#±0.37 | 124.5*±7.1 | 130.9#*±1.4 |
| K+ (mmol/l) | 4.19#±0.24 | 4.49# ± 0.06 | 6.06*±0.54 | 5.41#* ±0.08 |
| Ca++ (mmol/l) | 1.12#±0.01 | 1.02# ± 0.01 | 0.89*±0.01 | 1.03# ± 0.03 |
| Cl– (mmol/l) | 101.5#±8.9 | 102.1# ± 0.37 | 116.4*±8.2 | 103.9# ± 1.03 |
Data are expressed as mean ± SE. (#) and (*) significant difference compared to control and EST respectively
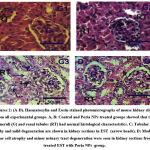
Kidney injury
Kidney structures with normal glomeruli and renal tubules were demonstrated in control (G1), and treated mice with Poria NPs (G2) (Figures 2A&2B). In contrast, marked inflammatory cellular infiltration, moderate degenerative and, atrophy in tubular cells were observed in kidney in EST (G3) (Figure 2C). Kidney sections in EST+Poria NPs (G4) revealed a moderate atrophy and moderate degenerated in tubular cells (Figure 3D).
Bcl2 immuohistochemical changes in kidney
Recognition and distribution of Bcl2 immunoreactivity (Bcl2) at kidney slices of groups under study were revealed in figures (3&5). Kidney sections in control, and Poria NPs groups show high positive responses for Bcl2 (grade 5) in glomeruli and renal tubules. While faint positive responses for Bcl2 (grade 1), were observed at kidney slices in EST group. Furthermore, mild positive responses for Bcl2 (grade 3) were observed at treated EST with Poria NPs group.
P53 immunohistochemical changes in kidney
Recognition and distribution in P53 immunoreactivity (P53) at kidney slices of groups under study were demonstrated at figures (4&5). Kidney slices of control and Poria NPs show faint positive responses for P53 (grade 1) of glomeruli and renal tubules. Moderate to strong positive responses for P53 (grade 3), were detected in kidney slices in EST group. Moreover, mild positive responses for P53 (grade 2) were detected at treated EST with Poria NPs group.
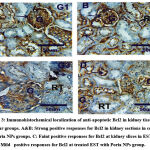 |
Figures 3: Immunohistochemical localization of anti-apoptotic Bcl2 in kidney tissues in dissimilar groups. A&B: Strong positive responses for Bcl2 in kidney sections in control, and Poria NPs groups. |
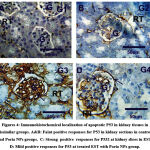 |
Figures 4: Immunohistochemical localization of apoptotic P53 in kidney tissues in dissimilar groups. A&B: Faint positive responses for P53 in kidney sections in control, and Poria NPs groups. |
Discussion
Ehrlich carcinoma has equivalence with human cancers as it is undistinguished, has a speedy advancement average and more sensitive to chemotherapy.3,32 Tumour growth in an animal’s body can affect a range of organ functions, and structure; therefore, current study aimed to study the impact of Poria NPs against EST induced kidney injury, toxicity, oxidative stress and apoptosis. Current results revealed that; EST induced defects in kidney function, which is established by increase the concentrations of creatinine, urea, chloride, potassium, and decrease in calcium and sodium levels, maybe attributable to EST induced toxicity and injury in kidney. In contrast; treatments of EST with Poria NPs improved these changes in kidney functions and alterations in electrolytes. Increased urea and creatinine levels demonstrate lower glomerular filtration rates, indicating that the kidney is less efficient at excreting waste materials.33 Furthermore, our findings were in line with Abd Eldaim et al.7,11; Mutar et al.4 who reported that; EST causes renal impairment in mice through elevation in the levels of urea and creatinine.
Oxidative stress is tightly linked to every aspect of cancer, including tumor-bearing, treatment, and prevention. The tumor-bearing condition should be under oxidative stress brought on by the tumour cells actively producing oxygen and by faulty oxidation-reduction regulation.13 Our findings showed that in kidney homogenates from EST as compared to control, MDA levels were significantly higher while GSH and catalase activity were significantly lower. According to Tousson et al.5, Aldubayan et al.3, and El-Masry et al.8, EST caused an increase in MDA levels, which was accompanied by a decrease in GSH, SOD, and catalase levels in the blood and liver. Our findings corroborate their findings.
Our study found that inducing EST in mice changed the structure of kidneys as a result of the production of (ROS), which play a crucial role in oxidative stress by disrupting cellular homeostasis and causing tissue destruction.3,33 Our biochemical outcomes were confirmed by the observations of histopathological and immuohistochemical investigation of Kidney, since Kidney section of EST bearing mice revealed renal toxicity manifested by significant deterioration and leanness of tubular cells and in glomeruli in kidney sections.
Apoptosis is an important process for keeping cell numbers in check, without affecting cell replication multiplication. P53 protein is a transcription factor responsible in cell cycle regulation, gene expression, ageing, apoptosis, and cancer suppression.16 In the current study, kidney sections in EST revealed significant increase in apoptotic P53 expressions while Bcl2 expressions were significantly decreased, indicating that EST induced apoptosis. Additionally, EST+Poria NPs adjust these changes in P53 and Bcl2emphasizing that Poria NPs has anti-apoptotic effects. Our findings agree with Khalil et al.34 who reported that E. foeminea extracts are a natural source of antioxidants that protect endothelium cells from free radical damage. Our findings revealed an unfavorable relationship amid p53 and Bcl-2 expressions. Our findings agree with Tousson et al.31 who reported the relation between p53 and Bcl-2 in cardiac tissues after treatments with amethopterin. Finally, the present work confirmed that the use of Poria NPs in treating mice bearing EST decreased P53 and increased Bcl2 expression of renal cells. Poria Nps is a potential adjuvant useful for the prevention and treatment of kidney toxicity and potentially applicable as a natural chemotherapeutic agent.
Conclusion
According to the findings of our study, the effects of Ehrlich solid tumors in mice were to induce kidney damage, oxidative stress, and modify the expression of p53 and Bcl2. Administering Poria NPs extract helped to kill the cancer cells through apoptosis which thereby regulates the proliferation of cancer cells and inhibits its spread to other organs. In comparison to chemotherapy, this novel medication has the ability to reduce the side effects significantly in normal cells in conjunction with complete spoilage for cancer cells.
References
- Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. A cancer journal for clinicians. (2014); 64(2): 104-117.
CrossRef - Oshiba RT, Touson E, Elsherbini YM, Abdraboh ME. Melatonin: A regulator of the interplay between FoxO1, miR96, and miR215 signaling to diminish the growth, survival, and metastasis of murine adenocarcinoma. BioFactors. 2021; doi.org/10.1002/biof.1758.
- Aldubayan MA, Elgharabawy RM, Ahmed AS, Tousson E. Antineoplastic activity and curative role of avenanthramides against the growth of Ehrlich solid tumors in mice. Oxidative medicine and cellular longevity. 2019; Article ID 5162687.
CrossRef - Mutar TF, Tousson E, Hafez E, Abo Gazia M, Salem SB. Ameliorative effects of vitamin B17 on the kidney against Ehrlich ascites carcinoma induced renal toxicity in mice. Environmental Toxicology. 2020; 35(4): 528-537.
CrossRef - Tousson E, Hafez E, Gazia MM, Salem SB, Mutar TF. Hepatic ameliorative role of vitamin B17 against Ehrlich ascites carcinoma–induced liver toxicity. Environmental Science and Pollution Research. 2020; 27(9): 9236–9246.
CrossRef - Abd Eldaim MA, Tousson E, Soliman MM, El Sayed IE, Abdel Aleem AA, Elsharkawy HN. Grape seed extract ameliorated Ehrlich solid tumor-induced hepatic tissue and DNA damage with reduction of PCNA and P53 protein expression in mice. Environmental Science and Pollution Research. 2021; 28(32):44226-38.
CrossRef - Abd Eldaim MA, Tousson E, El Sayed I E, Abd Elmaksoud AZ, Ahmed AA. Ameliorative effects of 9-diaminoacridine derivative against Ehrlich ascites carcinoma–induced hepatorenal injury in mice. Environmental Science and Pollution Res. 2021; 28(17): 21835-21850.
CrossRef - El-Masry TA, Al-Shaalan NH, Tousson E, Buabeid M, Alyousef AM. The therapeutic and antineoplastic effects of vitamin B17 against the growth of solid-form Ehrlich tumors and the associated changes in oxidative stress, DNA damage, apoptosis and proliferation in mice. Pak. J. Pharm. Sci. 2019; 32(6): 2801-2810.
- El-Masry T, Al-Shaalan N, Tousson E, Buabeid M, Al-Ghadeer A. Potential therapy of vitamin B17 against Ehrlich solid tumour induced changes in Interferon gamma, Nuclear factor kappa B, DNA fragmentation, p53, Bcl2, survivin, VEGF and TNF-α Expressions in mice. Pak. J. Pharm. Sci. 2020;33(1):393-401.
- Elgharabawy RM, Alhowail AH, Emara AM, Aldubayan MA, Ahmed AS. The impact of chicory (Cichoriu mintybus L.) on hemodynamic functions and oxidative stress in cardiac toxicity induced by lead oxide nanoparticles in male rats. Biomed. Pharmacother. 2021; 137: 111324.
CrossRef - Abd Eldaim MA, Tousson E, El Sayed IE, Abd El AE, Elsharkawy HN. Grape seeds proanthocyanidin extract ameliorates Ehrlich solid tumour induced renal tissue and DNA damage in mice. Biomedicine & Pharmacotherapy. 2019; 115:108908.
CrossRef - Ullah R, Rehman NU, Jamshidi-Adegani F, Bari A. Medicinal Plants and Marine-Derived Natural Products as Cancer Chemopreventive Agents. Frontiers in Pharmacology. 2022;13.
CrossRef - Dehelean CA, Marcovici I, Soica C, Mioc M, Coricovac D, Iurciuc S, Cretu OM, Pinzaru I. Plant-derived anticancer compounds as new perspectives in drug discovery and alternative therapy. Molecules. 2021 Feb 19;26(4):1109.
CrossRef - Altwaijry N, El‐Masry TA, Alotaibi B, Tousson E, Saleh A. Therapeutic effects of rocket seeds (Eruca sativa L.) against testicular toxicity and oxidative stress caused by silver nanoparticles injection in rats. Environmental Toxicology. 2020; 35(9):952-60.
CrossRef - Altwaijry N, El-Masry TA, Alotaibi BS, Tousson E, Alodhayani AA, El-Morshedy K, Elmaghed NA, Sayed AE, Saleh A. Potential therapeutic effects of avenanthramide-C against lung toxicity caused by silver nanoparticles injection in rats. Pakistan Journal of Pharmaceutical Sciences. 2021; 34(1):337-43.
CrossRef - Gali-Muhtasib H, Hmadi R, Kareh M, Tohme R, Darwiche N. Cell death mechanisms of plant-derived anticancer drugs: beyond apoptosis. Apoptosis. 2015 Dec;20(12):1531-62.
CrossRef - Bielory, L. and Lupoli, K., 1999. Herbal interventions in asthma and allergy. Journal of Asthma, 36(1), pp.1-65.
CrossRef - [18] Yichun Sun. Biological activities and potential health benefits of polysaccharides from Poria cocos and their derivatives. International Journal of Biological Macromolecules. 2014; 131-134
CrossRef - Alotaibi B, El‐Masry TA, Tousson E, Alarfaj SJ, Saleh A. Therapeutic effect of rocket seeds (Eruca sativa L.) against hydroxyapatite nanoparticles injection induced cardiac toxicity in rats. Pak. J. Pharm. Sci. 2020; 33(4): 1839-1845.
- Alotaibi B, Tousson E, El‐Masry TA, Altwaijry N, Saleh A. Ehrlich ascites carcinoma as model for studying the cardiac protective effects of curcumin nanoparticles against cardiac damage in female mice. Environmental Toxicology. 2021; 36(1):105-13.
CrossRef - Zorzi GK, Carvalho EL, von Poser GL, Teixeira HF. On the use of nanotechnology-based strategies for association of complex matrices from plant extracts. Revista Brasileira de Farmacognosia. 2015; 25:426-36.
CrossRef - Patton CJ, Crouch SR. Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia. Analytical chemistry. 1977; 49(3):464-9.
CrossRef - Bowers L.D, Wong E.T. Kinetic serum creatinine assays. II. A critical evaluation and review. Clin Chem. 1980; 26(5): 555-561.
CrossRef - Elmasry TA, Al-Shaalan NH, Tousson E, El-Morshedy K, Al-Ghadeer A. Star anise extracts modulation of reproductive parameters, fertility potential and DNA fragmentation induced by growth promoter Equigan in rat testes. Brazilian Journal of Pharmaceutical Sciences. 2018;54(1).
CrossRef - Gupta S, Gupta AK, Singh K, Verma M. Are sodium and potassium results on arterial blood gas analyzer equivalent to those on electrolyte analyzer?. Indian journal of critical care medicine: peer-reviewed, official publication of Indian Society of Critical Care Medicine. 2016; 20(4): 233
CrossRef - Mesbah L, Soraya B, Narimane S, Jean PF. Protective effect of flavonides against the toxicity of vinblastine cyclophosphamide and paracetamol by inhibition of lipid–peroxydation and increase of liver glutathione. Haematol. 2004; 7(1): 59-67.
- Jollow DJ, Mitchell JR, Zampaglione N, Gillette, JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11(3):151-169.
CrossRef - Saggu S, Sakeran MI, Zidan N, Tousson E, Mohan A, Rehman H. Ameliorating effect of chicory (Chichorium intybus L.) fruit extract against 4-tertoctylphenol induced liver injury and oxidative stress in male rats. Food and Chem. Toxicol. 2014; 72(10): 138-1.
CrossRef - Seyedalipour B, Barimani N, Dehpour Jooybari AA, Hosseini SM, Oshrieh M. Histopathological evaluation of kidney and heart tissues after exposure to copper oxide nanoparticles in Mus musculus. Journal of Babol University of Medical Sciences. 2015; 17(7): 44-50.
- Tousson E, Hafez E, Zaki S, Gad A. P53, Bcl-2 and CD68 expression in response to amethopterin-induced lung injury and ameliorating role of l-carnitine. Biomed Pharmacother. 2014; 68: 631-639.
CrossRef - Tousson E, Hafez E, Zaki S, Gad A. The cardioprotective effects of L-carnitine on rat cardiac injury, apoptosis, and oxidative stress caused by amethopterin. Environmental Science and Pollution Research. 2016;23(20):20600-8.
CrossRef - Kabel AM, Abdel-Rahman MN, El-Sisi AEDE. Effect of atorvastatin and methotrexate on solid Ehrlich tumor. Eur J Pharmacol. 2013; 713(1-3):47–53
CrossRef - Abo-Neima SE, Elsehly EM. Cancer Treatment by Laser and Electrochemical Therapy Combined with Magnetic Nanoparticles as Potent Therapy Against Ehrlich Ascites Carcinoma. BioNanoScience. 2022 May 10:1-6.
CrossRef - Khalil M, Khalifeh H, Saad F, Serale N, Salis A, Damonte G, Lupidi G, Daher A, Vergani L. Protective effects of extracts from Ephedra foeminea Forssk fruits against oxidative injury in human endothelial cells. Journal of Ethnopharmacology. 2020; 260:112976.
CrossRef