Manuscript accepted on :23-11-2022
Published online on: 07-12-2022
Plagiarism Check: Yes
Reviewed by: Dr. Yogendra Singh
Second Review by: Dr. Kartik Salwe
Final Approval by: Dr. Patorn Promchai
Burhan Ma’arif1 , Iffatul Abada1, Anisah Mahardiani1
, Iffatul Abada1, Anisah Mahardiani1 , Abdul Hakim1*
, Abdul Hakim1* , Novia Maulina1, Neny Purwitasari2
, Novia Maulina1, Neny Purwitasari2 , Khoirul Hidayah3
, Khoirul Hidayah3  and Seow Lay Jing4
and Seow Lay Jing4
1Department of Pharmacy, Faculty of Medical and Health Science, Maulana Malik Ibrahim State Islamic University, Malang, Indonesia
2Department of Pharmaceutical Science, Faculty of Pharmacy, Universitas Airlangga, Surabaya, Indonesia
3Faculty of Sharia, Maulana Malik Ibrahim State Islamic University, Malang, Indonesia
4Department of Pharmaceutical Chemistry, Faculty of Pharmacy and Health Sciences, Universiti Kuala Lumpur Royal College of Medicine, Perak, Malaysia
Corresponding Author E-mail: ahakim@farmasi.uin-malang.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2523
Abstract
Osteoporosis is a bone disorder characterized by the decrease of bone mass along with bone micro-architecture damage and has a risk become a fracture. One of the causes of osteoporosis is estrogen deficiency. Genistein is a phytoestrogen compound in the isoflavone group containing a similar structure compared to 17β-estradiol, thus it can bind to estrogen receptors and produce an estrogenic effect. Genistein induction can stimulate bone formation and promote the increase of alkaline phosphate (ALP) activities in osteoblast cells which can be observed by immunocytochemistry or Enzyme-linked Immunosorbent Assay (ELISA) or Western blot method. Using the PRISMA guideline technique, choose and strategize article searches by reading the title, abstract, and then the whole text of the article. Articles with the keywords "genistein or osteoblast cells or alkaline phosphate or immunocytochemistry or immunofluorescence or ELISA or western blot" were retrieved from databases including Google Scholar, PubMed, Researchgate, and Sciencedirect. 24 relevant research articles were uncovered as a result of this systematic review. Comparison of immunocytochemistry and ELISA methods in order to analyze the activities of ALP in osteoblast induced by genistein includes selectivity, sensitivity, processing time, and cost efficiency parameters. The immunocytochemistry method has a higher level of sensitivity and a faster processing time, whereas the ELISA method has a higher level of selectivity and less cost efficiency. The western blot method has selectivity for detecting complex-level protein expression.
Keywords
Cost Efficiency; ELISA; Immunocytochemistry; Selectivity; Sensitivity, Processing Time; Western Blot
Download this article as:| Copy the following to cite this article: Ma’arif B, Abada I, Mahardiani A, Hakim A, Maulina N, Purwitasari N, Hidayah K, Jing S. L. A Systematic Review: Comparison of Immunocytochemistry, ELISA, and Western Blot Methods in Alkaline phosphatase Measurement at Genistein-induced Osteoblast Cell. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Ma’arif B, Abada I, Mahardiani A, Hakim A, Maulina N, Purwitasari N, Hidayah K, Jing S. L. A Systematic Review: Comparison of Immunocytochemistry, ELISA, and Western Blot Methods in Alkaline phosphatase Measurement at Genistein-induced Osteoblast Cell. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3VEBEwe |
Introduction
Osteoporosis prevalence increases with increasing age in women. Over 30% of women aged 60-70 years suffer from osteoporosis, this data can increase to 70% at 80 years.1 Elderly woman will face the postmenopausal phase due to estrogen deficiency, which is one of the factors causing osteoporosis.2-5 Osteoporosis is a bone disorder characterized by a decrease in bone mass and bone micro-architecture damage, leading to an increased risk of fractures.6
Treating estrogen deficiency in postmenopausal women can be treated using hormone replacement therapy (HRT).7 Long-term use of HRT can cause side effects such as endometrium cancer, breast cancer, and stroke.8,9 Research on the use of natural ingredients to replace estrogen function with minimal side effects has been done and the results have led to the use of phytoestrogen compound.10-12
Genistein is a phytoestrogen compound in the isoflavone group, it has estrogenic activities because of the presence of phenolic rings that bind to estrogen receptors to treat target cells.13,14 In vitro, genistein can suppress osteoclast function and bone resorption, furthermore, induce the apoptosis of mature osteoclast cells. In vivo, genistein can prevent trabecular bone loss in the male rats’ model.15-19 In silico, a proof that genistein has an active compound identity range like 17β-estradiol. 20,21
Administration of genistein causes a direct effect on the growth and the differentiation of osteoblast and expresses a protein marker for bone formation, one of them is ALP. 2,22-24 Immunocytochemistry and enzyme-linked immunosorbent assay (ELISA) methods can do the measurement of ALP expression.25-28 These methods have their advantages, several parameters to analyze both methods are selectivity, sensitivity, processing time, and cost efficiency parameters.
Important parameters to be used in analyzing an observational method, when doing observation must pay attention to how much the ability of a method to recognize and measure a protein in a sample affects the success of a study. Also, short observational duration and less cost efficiency are parameters that must be considered in research, thus producing effective and efficient research. This systematic review is expected to summarize the decision of the selection of an appropriate method for in vitro observation, especially to observe bone formation activity in vitro.
Materials and Methods
Materials
Criteria for collecting data
The inclusion criteria in this review article were (i) studies related to the effect of genistein on increasing ALP expression in osteoblast with immunocytochemistry or ELISA or western blot method; (ii) studies related to genistein compound; (iii) English articles; (iv) full text and open access articles that published in 2010-2020.
The exclusion criteria in this review article were (i) studies related to other than the effect of genistein on increasing ALP expression in osteoblast cells with immunocytochemistry or ELISA or western blot method; (ii) studies related to other than genistein; (iii) articles in foreign languages other than English; (iv) articles were not full text and open access that published before 2011.
Strategy for article selection and search
The data used in the systematic review was primer data by collecting published articles from Google Scholar, PubMed, Researchgate, and Sciencedirect databases with keywords “genistein or osteoblasts or alkaline phosphatase or immunocytochemistry or immunofluorescence or ELISA or western blot.” Afterward, those articles were screened and selected based on their title and abstract. Then, a feasibility test was conducted by reading the entire article according to inclusion and exclusion criteria.
Methods
The method used in the systematic review uses the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.29,30
Data analysis
Data analysis according to PRISMA guideline, which describes the results of research articles and provides exposure to relevant results in the form of article summary tables.
Results
Strategy for article selection and search
After selecting a specific and systematic article search strategy using the PRISMA guideline method with four databases, namely Google Scholar, PubMed, Researchgate, and Sciencedirect, 24 research articles were relevant to the topic of discussion, including 8 research articles from GoogleScholar, 7 from PubMed, 4 from ResearchGate, and 5 from ScienceDirect (Figure 1). Then every research article found was published in less than the last 10 years (2010-2020) (Figure 2).
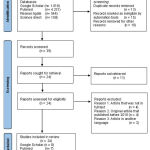 |
Figure 1: PRISMA Guideline diagram |
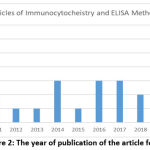 |
Figure 2: The year of publication of the article found |
In Vitro Analysis
The marker factor observed in this literature study was ALP. ALP protein is produced in the early stage of osteoblast differentiation and is one of the specific marker factors, also plays an important role in bone formation and mineralization.18,29-33 Type of osteoblast cell can be primary cell cultures from cells, tissues, and organs obtained directly from its origin organism, however, cell lines culture is obtained from the first subculture of primary culture. Primary cell culture refers to the culture starting primary cell culture has several weaknesses, for instance, needs an experimental animal as a raw material for culture and it most likely occurs virus or microbe contamination. This contamination can infect experimental animals used as culture stocks.34 The increase of ALP activity as a marker of bone formation can occur through various types of osteoblast cells obtained from primary culture or cell line with the process of bone formation around >10% to >50%.35
The process of osteoblast cell differentiation can be divided into several stages, namely proliferation, matrix maturation (early differentiation), and mineralization (late differentiation). The main marker protein in osteoblast cells differentiation is ALP, collagen type 1, osteopontin (OPN), and osteocalcin (OSC), with runt-related transcription factor 2 (Runx2) and osterix (OSX) as transcription factors.18,36-39 Besides, differentiated osteoblasts will express receptor activators of nuclear factor kβ-ligand (RANKL) and osteoprotegerin (OPG). RANKL and OPG can be used as markers to observe osteoclast cell function.37
Measurement of ALP marker factor shows osteoblastic activity from genistein compound administration. Genistein is an isoflavone group and has structural similarities with 17β-estradiol (Figure 3). Genistein can bind to ER, thus it shows as a phytoestrogen that produces the estrogenic effect.18,37-43 Administration of genistein causes a direct effect on the growth and differentiation of osteoblast cells and the release a protein.18,31,41,43 Therefore, increasing the production of mRNA from several markers of osteoblast cell differentiation, namely ALP.36,44
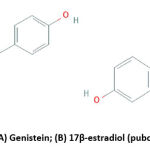 |
Figure 3: Structure of (A) Genistein; (B) 17β-estradiol (pubchem.ncbi.nlm.nih.gov). |
Genistein can affect several molecular pathways that stimulate bone formation and inhibit bone resorption. Administration of genistein can increase the production of ALP, OPN, and OSC and activate the Runx2 transcription factor, hence causing osteogenesis. Genistein can stimulate the occurrence of proliferation and differentiation through nitric oxide (NO) or cyclic guanosine monophosphate (cGMP) pathway and can inhibit osteoclastic activity. Also, genistein can affect the nuclear factor kappa–light–chain-enhancer of activated B cells (NF-ĸB) pathway, it stimulates the occurrence of osteoclastogenesis through RANKL and RANK bonds. RANKL is expressed by osteoblast cell surface and RANK is expressed by osteoclast cell surface.45 Osteoclastogenesis can be inhibited by OPG, as a result, RANKL binds to OPG and it will maintain the balance of bone formation and bone absorption in the process of bone remodeling. RANKL is a cytokine and plays a role in recruitment, differentiation, and osteoclast cell activation, meanwhile, OPG is a cytokine and plays a role in the inhibition of osteoclastogenesis through its bonds to RANKL.15,31,36,37,46,47
Genistein compound induced in osteoblast cells will enter the cell membrane and then translocate into the nucleus and binds to ER or activated estrogen receptor (ER*). The bond between genistein with ER will cause transcription by transcription factor Runx2 and encourages osteogenesis through osteoblast cell differentiation and produce ALP.2,3,38,44,48,49 Measurement of ALP by induction of genistein compound in osteoblast cells was performed by immunocytochemistry or ELISA method.
Characteristics regarding the potential genistein increasing or decreasing ALP activities in various types of osteoblasts by measuring immunocytochemistry or ELISA methods and the mechanism of osteogenesis and osteoclastogenesis was summarized in (Table 1 and Figure 4).
Table 1: Result of Genistein-induced.
| Type of Osteoblast cells | ALP Activity | Condition of Bone Formation Process | Methods | |
| Increase | Decrease | |||
| Baboon primary cell | ✓ | Decrease RANKL expression and increase OPG expression | ICC 34 | |
| MC3T3-E1 cell line | ✓ | Increases production of bone γ-carboxyglutamate (BGLAP) or OSC | ICC 38 | |
| Calvaria female Wistar rats primary cell | ✓ | Increase proliferation and differentiation and produce ALP | ICC 39 | |
| MC3T3-E1 cell line | ✓ | Increase cell proliferation and ALP activity in preosteoblast MC3T3-E1 cells by binding with ERβ | ICC 17 | |
| MC3T3-E1 cell line | ✓ | Decrease OPN expression | ICC | |
| Ishikawa cell line | ✓ | Increases ALP activity by binding to ER | ELISA 48 | |
| Calvaria Wistar rats primary cell | ✓ | Increase osteoblast cell differentiation by stimulating OSX as a transcription factor | ELISA 15 | |
| Rat osteoblast-like UMR106 cell line | ✓ | Increases ALP, RANKL, and OPG expressions, but does not increasing of Runx2, OSX, and OSC | ELISA 35 | |
| Human bone marrow mesenchymal stem cell cultures (hBMSC) cell line |
✓ | Increases proliferation and differentiation by binding to ER | ELISA 42 | |
| MC3T3-E1 cell line | ✓ | Increases differentiation and maturation of MC3T3-E1 osteoblasts and controls osteoclasts by regulating the expression and secretion of OPG and RANKL mediated by ERα | ELISA 44 | |
| MC3T3-E1 cell line | ✓ | Increase MC3T3-E1 osteoblast cell differentiation and ALP activity | ELISA 39 | |
| Rat primary cell | ✓ | Increase osteoblast cell differentiation and increase ALP activity by binding to ER | ELISA 37 | |
| Sales-2 human cell line | ✓ | Enhances Runx2 and OSX expressions and is responsible for controlling RANKL and OPG expressions | ELISA 49 | |
| Bone marrow mesenchymal stem cell (BMSCs) cell line |
✓ | Stimulates adipogenic differentiation and stimulates osteogenic by regulating PPARγ expression, thereby decreasing the production of Runx2, ALP, type 1 collagen, and OSC | ELISA 50 | |
| MC3T3-E1 cell line | ✓ | Increased ALP activity, marked by a decrease in Ereg and Efcab2 and an increase in Lif, Mmp13, and Pde4b | ELISA 36 | |
| MC3T3-E1 cell line | ✓ | Increase ALP production through transcription in ERα | ELISA 46 | |
| Calvaria female Wistar rats primary cell | ✓ | Increase ALP activity, expression of OSC, collagen, and Runx2 as transcription factors in ERα | ELISA 37 | |
| Calvaria rat’s primary cell | ✓ | Increase osteoblast cell differentiation by increasing ALP and Runx2 expression, and inhibiting osteoclast cell differentiation by inhibiting RANK expression | ELISA 18 | |
| Calvaria Wistar rats primary cell | ✓ | Stimulate osteogenesis by increasing production of ALP, Runx2, and OSC through transcription in ERα | ELISA 45 | |
| Human osteosarcoma Saos-2 cell line | ✓ | Increase the expression of RANKL, OPG, and Runx2 as transcription factors, as well as increase ALP activity | ELISA 43 | |
| hBMSC cell line | ✓ | Increases ALP activity by binding to ERα | ELISA 19 | |
| MC3T3-E1 cell line | ✓ | Increasing Runx2 protein | Western blot 68 | |
| Mesenchymal stem cells (MSCs) | ✓ | Increasing PPARγ, p‐PPARγ, Runx2, bone
morphogenetic receptor IA (BMPR IA) |
Western blot 69 | |
| Bone marrow macrophage cells | ✓ | Reduction of osteoclast differentiation (c-Fos, NFATc1, and TRAP) | Western blot 71 | |
| MC3T3-E1 osteoblastic cells (Xu et al., 2011) | ✓ | Increases ALP activity and decreased reactive oxygen species
Production |
Western blot 72 | |
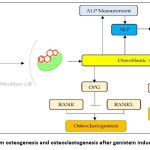 |
Figure 4: Mechanism osteogenesis and osteoclastogenesis after genistein induced osteoblast cell. |
Comparative Analysis Method
The most used method in ALP analysis as a bone formation biomarker is immunocytochemistry and ELISA methods. The immunocytochemistry method is a common method used in laboratories (in vitro) to visualize the location or place of a specific protein or antigen in the cells using primary antibodies which specifically bind to these proteins or antigens. Primary antigens allow visualization of protein under fluorescence microscopy after extending binding with secondary antibodies that have conjugated with fluorophore groups.24 The ELISA method is a serological method based on the specific reaction between antigen and antibody. The method has high selectivity and sensitivity owing to the change of substrate color (colorimetric) as an indicator for enzyme measurement.52-54
Immunocytochemistry Method
The immunocytochemistry method is divided into 2 types, direct immunocytochemistry, and indirect immunocytochemistry55 (Figure 5). Direct immunocytochemistry using primary antibody only, this antibody acts as specific antibodies that have conjugated with the fluorophore group and localize the antigen precisely and specifically, therefore it can be observed on fluorescence microscopy. While indirect immunocytochemistry uses primary and secondary antibodies, the secondary antibody acts as specific antibodies that have conjugated with fluorophore groups and localize antigens precisely and specifically, as a result, it can be observed under fluorescence microscopy.56,57
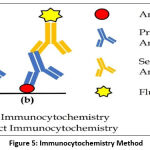 |
Figure 5: Immunocytochemistry Method. |
ELISA Method
There are four types of ELISA methods, for instance, direct ELISA, indirect ELISA, sandwich ELISA, and competitive ELISA (Figure 6). Direct ELISA using a conjugated primary antibody, can bind directly to the antigen. Indirect ELISA uses a primary antibody that binds to the antigen and then ca conjugated secondary antibody is added. Sandwich ELISA using a suitable pair of antibodies, each specific antibody will bind to a different epitope of the antigen. The first antibody is known as the trapping antibody and the second antibody is known as the detection antibody. Meanwhile, competitive ELISA occurs through a competitive process among antigens in the microplate. After the antibodies are incubated in the sample, next, it is inserted into a micro-pcontainingtains the antigens and then rinsed to remove specific antibodies that do not bind to the antigen. The more antigens in the sample, the more antigens, and antibodies bound are formed, thus fewer antibodies that do not bound will cause those antibodies to bind to the antigens in the micro-plate.58,59
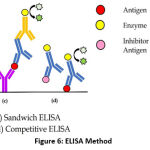 |
Figure 6: ELISA Method. |
Western Blot Method
Western blot or immunoblotting is a qualitative technique used to identify specific antibodies from complex protein samples with a certain molecular weight that have been separated. This method can show proteins by reacting antibodies and antigens that are used to detect cross-reactions (Figure 7).60 Western blot is very effective in detecting antigens that have small sizes in solutions that contain a lot of protein. The stages begin with sample preparation (protein purification), measurement of the concentration and amount of protein used, separation of proteins by polyacrylamide gel electrophoresis, transfer of proteins from gel to a nitrocellulose membrane, membrane blocking, incubation of primary and secondary antibodies, detection of target proteins, and calculation of protein bands. Even though the working technique is simple, specific, and precise in implementation, sometimes there are difficulties with unclear, non-specific, and bubble protein bands.
Discussion
Parameter Analysis
Parameters used in the analysis of Immunocytochemistry, ELISA, and Western blot methods are selectivity, sensitivity, processing time, and cost efficiency parameters. The following is an explanation of each parameter and a summary can be seen in (Table 2).
Table 2: Parameter Analysis Results
| No | Parameters | Method | Source | ||
| Immunocytochemistry | ELISA | Western – Blot | |||
| 1. | Selectivity | ü | ü | 59,64,66 | |
| 2. | Sensitivity | ü | 59,66 | ||
| 3. | Processing Time | ü | 62,72 | ||
| 4. | Cost Efficiency | ü | 77 | ||
Description: sign “✓” is a method that has advantages according to each parameter
Selectivity
The selectivity parameter is used to describe the ability of a method to observe protein in the complex sample accurately.58 ELISA method a has higher selectivity than immunocytochemistry because the antigen can be detected by using antibodies marked with an enzyme.59,66 In addition, the western blot method has selectivity in detecting targets at endogenous levels in complex samples and can optimize antibody and assay performance.64 The results in detecting antigens and antibodies bound to detect autoimmune disorders in humans like a pemphigus foliaceous, bullous pemphigoid, dermatitis herpetiformis, myositis, autoimmune hepatitis, etc, when most studies have used Hep2 cells from Ali et al., 2016, Karumanchi and Oommen, 2018, and Menezes et al., 2020 were performed by immunocytochemistry/immunofluorescence and ELISA methods.67,73-74 Result of selectivity analysis in immunocytochemistry/immunofluorescence and ELISA methods used kappa analysis and cohen’s kappa coefficient. Showed ELISA method had a higher selectivity around 84-99%, whereas the immunocytochemistry/immunofluorescence method about 40-95%.
Sensitivity
The sensitivity parameter is used to describe the ability of a method to measure a protein in the cells that can be affected by changing situations.65 The immunocytochemistry method has a higher sensitivity than the ELISA method because the observation is performed by using fluorescence microscopy which can detect an extremely sensitive antigen.59,66 The results in the detection of antigen and antibody bound were formed to detect autoimmune disorders in humans like a pemphigus foliaceous, bullous pemphigoid, dermatitis herpetiformis, myositis, autoimmune hepatitis, etc when most studies have used Hep2 cells from Ali et al., 2016, Karumanchi and Oommen, 2018, and Menezes et al., 2020 were performed by immunocytochemistry/immunofluorescence and ELISA methods.67,73-74 Result of sensitivity analysis in immunocytochemistry/immunofluorescence and ELISA methods used kappa analysis and cohen’s kappa coefficient. It was shown that the immunocytochemistry/immunofluorescence method had a higher sensitivity of about 50-100%, while the ELISA method was only about 30-97%.
Then, from several studies, the western blot method is ideal to be combined with the ELISA or ICC methods. In the study of Marycz et al. (2021), Western blot was used in observing to detect protein level expression (increased expression of RUNX-2) protein and ICC identified osteopontin (OPN) and fluorescence imaging (reduced OPN expression) with p<0.05.68 The same thing was also done in the study of Donoso et al. (2014), using mesenchymal stem cells (MSCs). The western blot method was used to detect protein content such as transcription factors PPARγ, p‐PPARγ, Runx2, bone morphogenetic receptor IA, and the ICC method for cell localization. derived from pSmad1/5/8.69 Then in the research of Wang et al. (2017), the ELISA method was used in the analysis of alkaline phosphatase (ALP) and osteocalcin (OC) as well as the western blot method to see protein expressions such as Sirtuin 1 (SIRT1), nuclear factor (NF)-κB and NF-B inhibitor (IkB) with p< 0.01 70.
Processing Time
The processing time parameter describes how long it takes to process the ALP observation in each method. The results in the detection of antigen-antibody bound were formed to detect autoimmune disorders in humans like a pemphigus foliaceous, bullous pemphigoid, dermatitis herpetiformis from Ali et al., 2016 and Tayde et al., 2018.67,76 The research was performed by using immunocytochemistry/immunofluorescence and ELISA methods, explained that the immunocytochemistry/immunofluorescence method could be run in faster processing time than the ELISA method. The western blot method has a long time starting from preparation to protein calculation, which takes approximately 45 minutes for pretreatment and 1 hour for incubation time.62,72
Cost Efficiency
The cost-efficiency parameter describes the amount of cost used in the observation of each method. Observation using the immunocytochemistry method or fluorescence is more expensive than the ELISA method. It is because in immunocytochemistry method requires fluorescence microscopy that can observe up to the molecular level.77 In the western blot method, using high-quality antibodies to detect the desired protein such as polyacrylamide SDS, and polyvinylidene difluoride (PVDF).60 Observation using the ELISA method costs less than immunocytochemistry and western blot method because observation with ELISA methods without fluorescence microscopy and is also easier to apply in the environment, especially in environments that are limited by resources like an operator and equipment.76,78
Conclusion
The immunocytochemistry and ELISA methods can measure the ALP after genistein induction, which is characterized by an increase or decrease in ALP activities in osteoblast cells. While western blot is used to detect protein expression levels. Both methods have their respective advantages, the immunocytochemistry method has higher sensitivity with faster processing time, meanwhile, the ELISA method has a higher selectivity with less cost efficiency dan the metode western blot has selectivity in detecting targets at the endogenous level of complex samples. This article is expected to be used as a reference by researchers to select suitable methods for the measurement of bone formation markers in vitro.
Acknowledgment
This research is supported and funded by the International Collaborative Development Research Maulana Malik Ibrahim State Islamic University, Malang, Indonesia, in 2022-2023.
Conflict of Interest
There is no conflict of interest.
References
- Dimyati F. Pengaruh Antara Aktivitas Fisik, Kebiasaan Merokok, dan Sikap Lansia Terhadap Kejadian Osteoporosis., J Berk Epide. 2017; 5(1). doi:10.20473/jbe.v5i1
- Kini U and Nandeesh N. Physiology of bone formation, remodeling, and metabolism. Radio and Hyb Bo Im., 2012; 29-57. doi.org/10.1007/978-3-642-02400-92
- Lee R. R and Phillips P. Role of Estrogen Receptors in Male Reproductive Physiology. Rev Inter Des Sci de La Santé – Inter Jou of Hea Sci., 2016; 3(1): 40–45. doi.org/10.18192/riss-ijhs.v3i1.1452
- Ji M-X and Yu Q. Primary osteoporosis in postmenopausal women. Chronic Dis Transl Med., 2015; 1(1): 9-13. doi:10.1016/j.cdtm.2015.02.006
- Villa A, Vegeto E, Poletti A, and Maggi A. Estrogens, neuroinflammation, and neurodegeneration. Endocr Rev., 2016; 37(4): 372-402. doi:10.1210/er.2016-1007
- Ramadani Faktor-Faktor Resiko Osteoporosis dan Upaya Pencegahannya. J Kesehat Masy Andalas., 2010; 4(2): 111-115. doi.org/10.24893/jkma.v4i2.78
- Djamal Z, Gunawan H. A, Dewi F, Saru A, Ferry G and Utami S. Pengaruh terapi sulih hormon estrogen, preparat kalsium dan kombinasinya pada tulang mandibula. Jou of Dent Ind., 2003; 10: 321-328.
- Jantaratnotai N, Utaisincharoen P, Sanvarinda P, Thampithak A, and Sanvarinda Y. Phytoestrogens mediated anti-inflammatory effect through suppression of IRF-1 and pSTAT1 expressions in lipopolysaccharide-activated microglia. Int Immunopharmacol., 2013; 17(2): 483-488. doi:10.1016/j.intimp.2013.07.013
- Lee L, Tsui K. H, Seow K. M, Cheng M. H, Su W. H, Chen C. P and Wang P. H. Hormone therapy for postmenopausal women-An unanswered issue. Gynecol Minim Invasive Ther., 2013; 2(1): 13-17. doi:10.1016/j.gmit.2012.12.003
- Ososki L and Kennelly E. J. Phytoestrogens: A review of the present state of research. Phyther Res., 2003; 17(8): 845-869. doi:10.1002/ptr.1364
- Constantine D and Pickar J. H. Estrogens in postmenopausal women: Recent insights. Curr Opin Pharmacol., 2003; 3(6): 626-634. doi:10.1016/j.coph.2003.07.003
- Yang S, Wang S. Y, Yang Y. C, Su C. H, Lee F. K, Chen S. C, Tseng C. Y, Jou H. J, Huang J. P and Huang K. E. Effects of standardized phytoestrogen on Taiwanese menopausal women. Taiwan J Obstet Gynecol., 2012; 51(2): 229-235. doi:10.1016/j.tjog.2012.04.011
- Aguiar P. N and Nahas-Neto J. The effects of soy isoflavone in postmenopausal women. Clin Rev, Cur Drug Therapy., 2006; 1: 31-36. doi.org/10.2174/1574885268533
- Md Zin S. R, Omar S. Z, Ali Khan N. L, Musameh N. I, Das S and Kassim N. M. Effects of the phytoestrogen genistein on the development of the reproductive system of Sprague Dawley rats. Clinics., 2013; 68(2): 253-262. doi:10.6061/clinics/2013(02)OA21
- Ma P, Ming L. G, Ge B. F, Zhai Y. K, Song P, Xian C. J, and Chen K. M. Icariin is more potent than genistein in promoting osteoblast differentiation and mineralization in vitro. J Cell Biochem., 2011; 112(3): 916-923. doi:10.1002/jcb.23007
- Ming G, Chen K. M and Xian C. J. Functions and action mechanisms of flavonoids genistein and icariin in regulating bone remodeling. Jour of Cell Phy., 2012; 228(3): 513-521. doi.org/10.1002/jcp.24158
- Ho X, Poon C. C. W, Wong K. C, Qiu Z. C, and Wong M. S. Icariin, but not genistein, exerts osteogenic and anti-apoptotic effects in osteoblastic cells by selective activation of non-genomic ERα signaling. Front Pharmacol., 2018; 9(MAY): 1-17. doi:10.3389/fphar.2018.00474
- Siddiqui S, Mahdi A. A and Arshad M. Genistein contributes to cell cycle progression and regulates oxidative stress in the primary culture of osteoblasts along with osteoclasts attenuation. BMC Complement Med Ther., 2020; 20(1): 1-11. doi:10.1186/s12906-020-03065-5
- Qiu C, Zhang Y, Xiao H. H, Poon C. C. W, Li X. L, Cui J. F, Wong M. K, Yao X. S and Wong M. S. 8-prenylgenistein Exerts Osteogenic Effects Via ERα and Wnt-dependent Signaling Pathway, Exper Cell Research., 2020; 395: 112186. doi.org/10.1016/j.yexcr.2020.112186
- Dalal K and Agarwal M. Postmenopausal syndrome. Indian J Psychiatry., 2015; 57: 222-232. doi:10.4103/0019-5545.161483
- Ganai A and Farooqi H. Bioactivity of genistein: a review in vitro and in vivo studies. Bio and Pharm., 2015; 76: 30-38. doi.org/10.1016/j.biopha.2015.10.026
- Wang W, Olson D, Cheng B, Guo X, and Wang K. Sanguis Draconis resin stimulates osteoblast alkaline phosphatase activity and mineralization in MC3T3-E1 cells. J Ethnopharmacol., 2012; 142(1): 168-174. doi:10.1016/j.jep.2012.04.033
- Aisha D, Nor-Ashikin M. N. K, Sharaniza A. B. R, Nawawi H, and Froemming G. R. A. Orbital fluid shear stress promotes osteoblast metabolism, proliferation, and alkaline phosphates activity in vitro. Exp Cell Res., 2015; 337(1). doi:10.1016/j.yexcr.2015.07.002
- Ma’arif B, Agil M, and Laswati H. Alkaline phosphatase activity of Marsilea crenata Presl. extract and fractions as marker of MC3T3-E1 osteoblast cell differentiation. J App Pharm Sci., 2018; 8(3): 55-59. doi:10.7324/JAPS.2018.8308
- Chen L. L, Lei L. H, Ding P. H, Tang Q, and Wu Y. M. Osteogenic effect of Drynariae rhizoma extracts and Naringin on MC3T3-E1 cells and an induced rat alveolar bone resorption model. Arch Oral Biol., 2011; 56(12): 1655-1662. doi:10.1016/j.archoralbio.2011.06.008
- Fujiwara K, Shin M, Hougaard D. M, and Saita T. Distribution study of peplomycin in rat kidney revealed by immunocytochemistry using monoclonal antibodies. Histochem Cell Biol., 2011; 135(1): 93-101. doi:10.1007/s00418-010-0768-9
- Li L, Zeng Z, and Cai G. Comparison of neoeriocitrin and naringin on proliferation and osteogenic differentiation in MC3T3-E1. , 2011; 18(11): 985-989. doi:10.1016/j.phymed.2011.03.002
- Taylor R and Rudbeck L. Immunohistochemical staining methods. Dako Denmark: IHC Handbook. 2013.
- Selçuk A.A. A Guide for Systematic Reviews: PRISMA. Turk Arch Otorhinolaryngol., 2019; 57(1):57-8. doi: 0.5152/tao.2019.4058, PMID 31049257.22
- Snyder H. Literature Review As A Research Methodology: An Overview And Guidelines. J Bus Res., 2019; 104: 333-339. doi: 10.1016/j.jbusres.2019.07.039.jbusres.2019.07.039.
- Cepeda B, Sandoval M. J, Rauschemberger M. B, and Massheimer V. L. Beneficial role of the phytoestrogen genistein on vascular calcification. J Nutr Biochem., 2017; 50: 26-37. doi:10.1016/j.jnutbio.2017.08.009
- Jafary F, Hanachi P, and Gorjipour K. Osteoblast differentiation on collagen scaffold with immobilized alkaline phosphatase. Int J Organ Transplant Med., 2017; 8(4): 195-202.
- Chen H, Li J, and Wang Q. Associations between bone-alkaline phosphatase and bone mineral density in adults with and without diabetes. Med (United States)., 2018; 97(17). doi:10.1097/MD.0000000000010432
- Andiana M, Rachmawati Y, and Andayani S. S. Kultur Sel Baby Hamster Kidney (BHK) Menggunakan Media Dulbeccos Modified Eagle Medium (DMEM). BIOTROPIC The Jou of Trop Biology., 2007; 1(1).org/10.29080/biotropic.2017.1.1.1-8
- Xu M. L, Bi C. W. C, Kong A. Y. Y, Dong T. T. X, Wong Y. H, and Tsim K. W. Flavonoids induce the expression of acetylcholinesterase in cultured osteoblasts. Chem Biol Interact., 2016; 259: 295-300. doi:10.1016/j.cbi.2016.03.025
- Tiyasatkulkovit W, Malaivijitnond S, Charoenphandhu N, Havill L. M, Ford A. L, and Vandeberg J. L. Pueraria mirifica extract and puerarin enhance proliferation and expression of alkaline phosphatase and type i collagen in primary baboon osteoblasts. , 2014; 21(12): 1498-1503. doi:10.1016/j.phymed.2014.06.019
- Tiyasatkulkovit W, Charoenphandhu N, Wongdee K, Thongbunchoo J, Krishnamra N, and Malaivijitnond Upregulation of osteoblastic differentiation marker mRNA expression in osteoblast-like UMR106 cells by puerarin and phytoestrogens from Pueraria mirifica. Phytomedicine., 2012; 19(13): 1147–1155.doi.org/10.1016/j.phymed.2012.07.010
- Kim M, Jisun L, Jung-Hee L, Kyung-Mi L, Suji K, Kye W. P, Chu W. N, and Yoon S. C. Understanding the functional role of genistein in the bone differentiation in mouse osteoblastic cell line MC3T3-E1 by RNA-seq analysis. Sci Rep., 2018; 8(1): 1-12. doi:10.1038/s41598-018-21601-9
- Cepeda S. B, Sandoval M. J, Crescitelli M. C, Rauschemberger M. B, and Massheimer V. L. The isoflavone genistein enhances osteoblastogenesis: signaling pathways involved. J Physiol Biochem., 2020; 76(1): 99-110. doi:10.1007/s13105-019-00722-3
- Katsuyama M, Demura M, Katsuyama H, Tanii H, and Saijoh K. Genistein and menaquinone-4 treatment-induced alterations in the expression of mRNAs and their products are beneficial to osteoblastic MC3T3-E1 cell functions. Mol Med Rep., 2017; 16(1): 873-880. doi:10.3892/mmr.2017.6632
- Nishide Y, Tousen Y, Tadaishi M, Inada M, Miyaura C, Kruger M. C, and Ishimi Y. Combined Effects of Soy Isoflavones and β-Carotene Osteoblast Differentiation, Int J Environ Res Public, 2015; 12: 13750-13761. doi.org/10.3390/ijerph121113750
- Donzelli A, Braida D, Finardi A, Capurro V, Valsecchi A. E, Colleoni M, and Sala, M. Neuroprotective effects of genistein in mongolian gerbils: Estrogen receptor-β involvement. J Pharmacol Sci., 2010; 114(2): 158-167. doi:10.1254/jphs.10164FP
- Yu J, Bi X, Yu B, and Chen D. Isoflavones: Anti-inflammatory benefit and possible caveats. Nutrients. 2016; 8(6): 1-16. doi:10.3390/nu8060361
- Dai J, Li Y, Zhou H, Chen J, Chen M, and Xiao Z. Genistein promotion of osteogenic differentiation through BMP2/SMAD5/RUNX2 signaling. Int J Biol Sci., 2013; 9(10): 1089-1098. doi:10.7150/ijbs.7367
- Zakłos-Szyda M, Budryn G, Grzelczyk J, Perez-Sanchez H, and Zyzelewicz D. Evaluation of isoflavones as bone resorption inhibitors upon interactions with receptor activator of nuclear factor-κB ligand (RANKL). Molecules., 2020; 25(1). doi:10.3390/molecules25010206
- Luo D, Kang L, Ma Y, Chen H, Kuang H, Huang Q, He M, and Peng W. Effects and mechanisms of 8‐prenylnaringenin on osteoblast MC 3T3‐E1 and osteoclast‐like cells RAW 264.7. Food Sci Nutr., 2014; 2(4): 341-350. doi:10.1002/fsn3.109
- WuJ, Chen J.T, Cherng Y.G, Chang C.C, Liu S.H, and Chen R.M. Genistein improves bone healing via triggering estrogen receptor alpha-mediated expressions of osteogenesis-associated genes and consequent maturation of osteoblasts. J Agric Food Chem., 2020; 68(39): 10639-10650. doi:10.1021/acs.jafc.0c02830
- Fokialakis N, Alexi X, Aligiannis N, Boulaka A, Meligova A. K, Lambrinidis G, Kalpoutzakis E, Pratsinis H, Cheilari A, Mitsiou D. J, Mitakou S, and Alexis M. N. Biological evaluation of isoflavonoids from Genista halacsyi using estrogen-target cells: Activities of glucosides compared to aglycones. PLoS One., 2019; 14(1): 1-29. doi:10.1371/journal.pone.0210247
- Golub E and Boesze-Battaglia K. The role of alkaline phosphatase in mineralization. Curr Opin Orthop., 2007; 18(5): 444-448. doi:10.1097/BCO.0b013e3282630851
- Kretzschmar G, Zierau O, Wober J, Tischer S, Metz P, and Vollmer G. Prenylation has a compound specific effect on the estrogenicity of naringenin and genistein. J Steroid Biochem Mol Biol., 2010; 118(1-2): 1-6. doi:10.1016/j.jsbmb.2009.08.005
- Karieb S, Jawad M. M, Al-shmgani H, and Kadri Z. H. M. The effect of the combination of vitamin K2 and genistein, coumestrol and daidzein on the osteoblast differentiation and bone matrix formation. J Biotechnol Res Cent., 2016; 10: 12-19
- Zhang Y, Xue H. G, Chen J. Y, Chai W, and Ni M. Genistein induces adipogenic differentiation in human bone marrow mesenchymal stem cells and suppresses their osteogenic potential by upregulating PPARγ. Exp Ther Med., 2016; 11(5): 1853-1858. doi:10.3892/etm.2016.3120
- Murkati N, Supargiyono T. S, Hnes M, Artama W. T, and Prayitno A. Perbedaan metode ELISA sandwich A dan B dalam deteksi antigen membran toxoplasma gondii. Bioteknologi Journal., 2004; 1: 54-57. http://biosains.mipa.uns.ac.id/C/C0102/C010205.pdf
- Xie X, Wang C, Xie Y, Wang X, Chen G, Yan X, Cui J, Chen F, Li H, and Jin B. Development and evaluation of a sandwich ELISA method for the detection of human CD306. Journal of Immunological Methods., 2013; 396: 65-73. org/10.1016/j.jim.2013.07.013
- Ghanadan A, Saghazadeh A, Jahanzad I, and Rezaei N. Clinical aspects of indirect immunofluorescence for autoimmune diseases. Expert Rev Clin Immunol., 2015; 11(5): 597-616. doi:10.1586/1744666X.2015.1027152
- Friis T, Pedersen K.B, Hougaard D, and Houen G. Immunocytochemical and immunohistochemical staining with peptide antibodies. Methods Mol Biol., 2015; 1348: 311-325. doi:10.1007/978-1-4939-2999-3_27
- Im K, Marenino S, Diaz M. F. P, and Yong W. H. Chapter 26. Cary Grant, Mak a Hollywood Legend. 2019; 1897: 358-375. doi:10.1093/oso/9780190053130.003.0027
- Aydin A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides., 2015; 72: 4-15. doi:10.1016/j.peptides.2015.04.012
- Sakamoto S, Putalun W, Vimolmangkang S, Phoolcharoen W, Shoyama Y, Tanaka H, and Morimotot S. Enzyme-linked Immunosorbent Assay for the Quantitative / Qualitative Analysis of Plant Secondary Metabolites. J of Nat Medic., 2018; 72(1): 32-42. https://10.1007/s11418-017-1144-z
- Robert Hnasko. ELISA: Methods and Protocols, Methods in Molecular Biology: ELISA Handbook. 2015
- Mishra M, Tiwari S, and Gomes A.V. Protein Purification And Analysis: Next Generation Western Blotting Techniques. Expert Rev Proteomics., 2017; 14: 1037–1053. doi: 10.1080/14789450.2017.1388167
- Mahmood T, Yang P.C. Western blot: Technique, theory, and trouble shooting. North Am J Med Sci., 2012; 4: 429– 434. doi: 10.4103/1947-2714.100998
- Huang YT, van H.D, Ledahawsky L.M, Motyl A.A.L, Jordan C.Y, Gillingwater T.H, and Groen E.J.N. Robust comparison of protein levels across tissues and throughout development using standardized quantitative western blotting. J Vis Exp., 2019; 146 doi:10.3791/59438
- Kastoori L.M, Heaton S, Shiflett S.D, Roberts A.C, Solache A, and Schutz-Geschwender A.R. Antibody validation for western blot: by the user, for the user. Biol.Chem., 2020; 295(4): 929-939. doi: 10.1074/jbc.RA119.010472.
- UNODC. Guidance for the validation of analytical methodology and caliberation of equipment used for testing illicit drugs in seized materials and biological specimens. New York: United Nations. 2009
- Dobrucky W. Fluorescence microscopy. USA: Wiley-VCH Verlag GmbH & Co KgaA., 2013; 97-142
- Ali S, Lauren N. S, Olayemi S, and Kiran M. Comparison of histopathology immunofluorescence, and serology for the diagnosis of autoimmune bullous disorder: an update. Glob Dermatology., 2016; 3(4): 343-351. doi.org/10.15761/GOD.1000S1005
- Marycz K, Śmieszek A, Kornicka-Garbowska, Pielok A, Janeczek M, Lipińska A, Nikodem A, Filipiak J, Sobierajska P, Nedelec J.M, Wiglusz R.J. Novel Nanohydroxyapatite (nHAp)-Based Scaffold Doped with Iron Oxide Nanoparticles (IO), Functionalized with Small Non-Coding RNA (miR-21/ 124) Modulates Expression of Runt-Related Transcriptional Factor 2 and Osteopontin, Promoting Regeneration of Osteoporotic Bone in Bilateral Cranial Defects in a Senescence-Accelerated Mouse Model (SAM/P6). Jour of Nanomed., 2021; 16: 6049-6055. doi: 10.2147/IJN.S316240
- Donoso O, Pino A.M, Osses N, and Rodríguez J.P. Osteoporosis‐associated alteration in the signalling status of BMP‐2 in human MSCs under adipogenic conditions doi: 10.1002/jcb.25082
- Wang X, Chen L, and Peng W. Protective effects of resveratrol on osteoporosis via activation of the SIRT1 NF κB signaling pathway in rats. Expe And Therapeu Medic., 2017; 14: 5032-5038. doi: 10.3892/etm.2017.5147.
- He L, Lee J, Jang J.J, Sakchaisri K, Hwang J, Cha-Molstad H.J.C, Kim K.A, Lee I.J, Kim S.O, Soung N.K, Lee K.S, Kwon Y.T, Erikson R.L, Ahn J.S, and Kim B.Y. Regulation by salubrinal through eIF2α mediated differentiation of osteoclast and osteoblast. Cellular Sig., 2013; 25: 552–560 doi: 10.1016/j.cellsig.2012.11.015.
- Xu Z.S, Wang X.Y, Xiao D.M, Hu M, Wu Z.Y, and Bian J.S. Hydrogen sulfide protects MC3T3-E1 osteoblastic cells against H2O2-induced oxidative damage—implications for the treatment of osteoporosis. Free Rad Bio and Medic., 2011; 50: 1314–1323. doi: 10.1016/j.freeradbiomed.2011.02.016
- Karumanchi D and Oommen S. Evaluation of Diagnostic Significance and Cost Effectiveness of ELISA and IFA for the Diagnosis of Autoimmune Disorders. Immunome Res., 2018; 14(2). doi:10.4172/1745-7580.1000155
- Menezes M, Rossener R, da Silva A. P. M. A, Rodrigues S. C, and Mangueira C. L. P. Comparison between enzyme-linked immunosorbent assay and indirect immunofluorescence for detection of antineutrophil cytoplasmic antibodies. Einstein (Sao Paulo)., 2020; 18: 1-6. doi.org/10.31744/einstein_journal/2020ao5132
- Mullins M. Overview of fluorochrome in immunocytochemical methods and protocols. Meth in Mole Bio., 2015; 15.
- Tayde A, Agrawal C, and Deshmukh A. T. Comparison of immunofluorescence assay (IF) with ELISA in detection of antinuclear antibodies. Indian J Pathol Oncol., 2018; 5(3): 418-420. doi:10.18231/2394-6792.2018.0081
- Sarkari B, Ashrafmansouri M, Hatam G. R, Habibi P, and Khabisi S. A. Performance of an ELISA and indirect immunofluorescence assay in serological diagnosis of zoonotic cutaneous leishmaniasis in Iran. Interdiscip Perspect Infect Dis., 2014; 2014. doi:10.1155/2014/505134
- Thiha A and Ibrahim F. A. colorimetric enzyme-linked immunosorbent assay (ELISA) detection platform for a poin-of-care dengue detection system on a lab-oncompact-disc. Sesors., 2015; 15(5): 11431-11441. doi.org/10.3390/s150511431







