Manuscript accepted on :21-09-2022
Published online on: 23-09-2022
Plagiarism Check: Yes
Reviewed by: Dr. Loai Aljerf
Second Review by: Dr. Narasimha Murthy
Final Approval by: Dr. Anton R Kiselev
Shahad Turkey Mana1, Dawood Salman Mahdi1 and Mahmood Thamer Altemimi2*
1Southern Technical University/ College of Health and Medical Techniques, Basrah 61001, Iraq.
2Adult Endocrinologist, Thi Qar Specialized Diabetes Endocrine and Metabolism Center (TDEMC), Thi Qar Health Directorate, Thi Qar 64001, Iraq.
Corresponding Author E-mail: mahmoodaltimimi83@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2516
Abstract
Background: Diabetes mellitus (T2DM) is a serious health problem that affects people all over the world and places a heavy financial and social burden on individuals, families, and communities. The objectives of the study were to evaluate the risk factors of T2DM and its relationship to micro- and macrovascular complications. Methods: A cross-sectional observational study was conducted on 1189 individuals with T2DM attending A Tertiary Endocrine Center. All patients’ data were gathered from direct interviewees and the digital records of the tertiary center, which used an internal network system and Microsoft Access program. Results: The mean age was 55.9 ±11.7 years, female 58%, body mass index 31.2 ±5.5 kg/m2, waist circumference 108±11.6 cm, mean duration of T2DM 10.1 ±7 years, and glycated hemoglobin (HbA1c) 9.6 ±2.1%. The prevalence risk factors were as follows smoking 27.3%, central obesity 84.3%, history of dyslipidemia 74.6%, family history of T2DM 64.9%, hypertension 63.5%, signs of insulin resistance (IR) 61.7%, gestational Diabetes (GDM) and History of cardiovascular diseases(CVD) 20.9%. These risk factors had a statistically significant impact on both macrovascular and microvascular T2DM. Conclusion: history of dyslipidemia and GDM were the most significant independent risk factors for the prediction of macrovascular complications among T2DM, while female gender, history of dyslipidemia, and GDM were independent risk factors for the prediction of microvascular complications among T2DM. Other risk factors including: History of CVD, hypertension, central obesity, duration of T2DM more than 5 years, estimated GFR <60 ml/min/1.73 m2, and any signs of IR were significantly effect on both micro- and macrovascular complications, but as dependent risk factors to further cofounders.
Keywords
Iraq; Macrovascular; Microvascular; Risk Factors; T2DM
Download this article as:| Copy the following to cite this article: Mana S. T, Mahdi D. S, Altemimi M. T. The Prediction of Micro- and Macrovascular Complications in Individuals with T2DM with Different Risk Factors in Iraq. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Mana S. T, Mahdi D. S, Altemimi M. T. The Prediction of Micro- and Macrovascular Complications in Individuals with T2DM with Different Risk Factors in Iraq. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3C46kQr |
Introduction
Type 2 diabetes mellitus (T2DM) is a common and account for 90–95 % of all cases of diabetes. The majority of individuals with T2DM were either overweight or obese due to the high prevalence of IR among them and or relative (rather than absolute) insulin insufficiency
In the early stages of hyperglycemia, T2DM may developed gradually with mask symptoms of hyperglycemia and it usually stays untreated for years. Those undiagnosed individuals are at a higher risk for developing macro and microvascular problemsDespite the fact that patients with T2DM have normal or raised insulin levels, the inability to correct blood glucose reflects a relative deficiency in glucose-stimulated insulin production. As a result, insulin secretion in those people is impaired, and it is insufficient to compensate for IRAlthough IR may improve with different dietary, exercise, and bariatric interventions, have resulted in remission of diabetes in some cases[4, 5].
Furthermore, T2DM risk rises with age, obesity, lack of physical activity, hypertension or dyslipidemia, a family history of diabetes among first-degree relatives (more than type 1 diabetes), women with a history of gestational diabetes (GDM), and polycystic ovary syndrome (PCOS)[2]. Specific racial/ethnic subgroups (African American, American Indian, Hispanic/Latino, and Asian American) are more likely to develop T2DM. It’s frequently linked to a high hereditary predisposition [6].
Variable information exists regarding the association between these risk factors and glycemic control Also some risk variables can predict the likelihood of specific diabetic complications. Our objectives were to assess the risk factors of adults with T2DM in Thi-Qar province and to determine which risk factor can predict either micro- or macrovascular complications.
Material and methods
This was a cross-sectional observational study, conducted on 1189 individuals with T2DM attending Thi-Qar Specialized Diabetes Endocrine and Metabolism Center (TDEMC) in Thi-Qar, Southern Iraq from October 2021 through June 2022. The official agreement was approved by the ethical committee of the participating institute by the number (65/2021 at 24th-October-2021) and an informed consent was taken from every patient before enrollment. The present study was including any patient with aged 18-year-old and above and diagnosed with T2DM for more than 6 months according to American diabetes association (ADA) criteria with a fasting blood glucose of 126 mg/dl or more, post prandial blood glucose 200 mg/dl or more, HbA1c 6.5 % or more. Any patients with Type-1 diabetes mellitus, and any patients with diabetes who are aged < 18 years were excluded from the study.
All patients’ data were gathered from direct interviewees and the digital records of TDEMC, which used an internal network system and Microsoft Access program to keep track of all patients’ information and examinations. In order to determine sample size, the following equation was used: Sample size (N) = P (1-P) Z2 /d2 where N = the minimum required size of the sample, p = proportion of (T2DM) in the population which was (196 per 1000) according to prior study[8], z = is standard normal variate (at 5% type I error (p <0.05) which is 1.96, d = is the desired margin of absolute error. (=0.05). so that the minimum sample size required to conduct this study was 246, the actual count of cases in this research was (1189).
Questionnaire and Study Variables
Demographic and Behavioral Characteristic Data
Direct in-person interviews utilizing an interviewer-administered questionnaire to collect demographic and risk factors like age, gender, marital status, address, occupation type, duration of T2DM, history of hypertension, family history of first degree relatives with T2DM, previous history of CVD, and smoking habits.
Physical Measurements
All individuals were examined for weight in kilogram, height in meter and body mass index (BMI) was calculated by dividing weight on square height in meters’ kg/m2. The degree of obesity was assessed according to International Diabetes Federation (IDF)[9] as: underweight < 18.5 kg/m2, normal (18.5-24.9) kg/m2, overweight (25-29.9) kg/m2, class 1 obesity (30-34.9) kg/m2, class II obesity (35-39.9) kg/m2, and class III obesity >40 kg/m2. A flexible plastic tape measure was used to calculate the waist circumference (WC) at the approximate halfway between the lower border of the last palpable rib and the top of the iliac crest. WC value 99 cm or more in women and 97 cm or more in men were defining for central obesity[10].
A digital sphygmomanometer was used to take blood pressure in a sitting position from the right arm. The mean of two blood pressure readings obtained five minutes apart was used as the final BP result. Prehypertension is defined as a systolic blood pressure of 120-139 mm Hg and diastolic blood pressure of 80-89 mm Hg. A systolic blood pressure of 140 mm Hg or more and diastolic blood pressure of 90 mm Hg or more were considered hypertension[2].
Biochemical Measurements
Every individual was sent for plasma glucose measurement, renal function test, and lipid profile after an overnight fasting for at least 8-10 hours. Microvascular complications included the presence of any one of: nephropathy, clinical Neuropathy, and retinopathy. Nephropathy among T2DM confirmed as albuminuria 30 mg/mol or more, decreased estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2, or both. The calculated e GFR < 60 ml/min/1.73 m2 by the NKF-ASN Task Force was the solid method that had been used to define CKD among those individuals[11].
Clinical neuropathy in individuals with T2DM was assessed subjectively by a well-designed questionnaire including slipping foot, loss of sensation, numbness, paresthesia, etc. Retinopathy was determined by qualified ophthalmologists during interviewing session using the international diabetic retinopathy and macular edema disease scales[12] with slight modification: stage 0, no retinopathy; stage 1, hemorrhage and hard exudates; stage 2, soft exudates; stage 3, intraretinal microvascular abnormalities and venous changes, including beading, loop, and duplication; and stage 4, new vessels, vitreous hemorrhage, fibrous proliferation, and retinal detachment. A retinopathy was confirmed to stage 3 or 4.
Macrovascular complications included the occurrence of any one of: CVD events (nonfatal definite coronary heart disease) like angina pectoris or myocardial infarction), stroke (evidence of ischemic or hemorrhagic stroke or intracerebral hemorrhage), and clinical peripheral arteria disease (PAD).
Statistical Analysis
Parametric variables were normally distributed by using the one-sample Kolmogorov–Smirnoff test, and presented as mean and standard deviation (SD). The data were analyzed using statistical SPSS (Statistics Package of Socio Science version 23). For independent variables, chi-square cross tab descriptive statistics were utilized. The selected risk factors were screened through a backward variable selection with the critical value of P =0.1: age, gender, BMI, WC, duration of T2DM, History of hypertension, dyslipidemia, CVD, family history of T2DM, GDM, PCOS, and signs of IR. a binary logistic regression analysis was performed to investigate the independence of the important factors. A p-value of 0.05 or below was deemed significant and warranted further examination.
Results
The baseline characteristics of patients with T2DM were presented in (mean ±SD) as follows: age 56.0 ±11.6 years, BMI 31.2 ±5.5 kg/m2, HbA1c 9.6 ±2.1 %, WC 108 ±11.6 cm, with 687 (57.8%) of them were women. T2DM rated among self-employer, employee, housewife, student and retired as 17.0%, 13.1%, 51.3%, 0.5% and 17.6 %, respectively. One thousand 83.9% of the individuals were married, 152(12.8%) widows, 20 (1.7%) single, and 20 (1.7%) divorced. T2DM prevalence was higher in the urban group (83.1%) compared to rural humanity (16.9%), and 76.5 % of the individuals had T2DM for five years or longer (P <0.001) when the average duration 10.1 ± 7 years.
Table 1: Baseline information of patients with type 2 diabetes mellitus.
| Variable
|
Frequency (%)\ Range | |
| Gender | Men | 502(42.0) |
| Women | 687(58.0) | |
| Age (years) | (M ±SD) = 55.9±11.7
18 – 30 years 31-40 years 41-50 years 51-60 years 60 years |
21 (1.85) 107 (9) 227 (19.1) 419 (35.2) 415 (34.9) |
| Body mass index (Kg/m2) | (M ±SD) = 31.2±5.5
Underweight Normal weight Overweight class I Obesity class II Obesity class III obesity |
Range (17-55)
4(0.3) 116(9.8) 345(29.0) 437(36.8) 196(16.5) 91(7.7) |
| HbA1c % | (M ±SD)= 9.6±2.1 | Range (3.5-17) |
| Waist circumference (centimeter) | (M ±SD)= 108±11.6 | Range (71-152) |
| Duration of diabetes mellitus (years)
|
(M ±SD) = 10.1 ± 7 | Range (0-40) |
| Less than 5 years | 280 (23.5) | |
| qual or more than 5 years | 909 (76.5) | |
| Marital status
|
Single | 20(1.7) |
| Married | 1000(83.9) | |
| Divorced | 20(1.7) | |
| Widow | 152(12.8) | |
| Occupation | Self-employer | 202(17.0) |
| Employee | 162(13.1) | |
| Housewife | 610(51.3) | |
| Student | 6(0.5) | |
| Retired | 214(18.0) | |
| Address
|
Urban | 988(83.1) |
| Rural | 201(16.9) | |
| Smoking | 325(27.3) | |
Three hundred and twenty-five (27.35%) individuals were smoking. According to BMI, our participants were subdivided into underweight, normal weight, overweight, class I obesity, class II obesity, and class III obesity in a relative frequency (0.3%, 9.8%, 29.0%, 36.8%, 16.5%, 7.7% respectively). Housewives were constituting 51.0% of individual with T2DM and other occupations were distributed as retired 17.6%, self-employer 16.9%, employee 13.3% and student 1.1%.
According to the Figure-1, the risk factors for T2DM were presented in a descending manner as central obesity 84.3%, history of dyslipidemia 74.6%, family history of DM 64.9%, hypertension 63.5%, signs of IR 61.7%, history of cardiovascular disease 20.9%, GDM 10.3%, history of PCOS among women with T2DM 7.6% and pregnant women was representing 2.4% of the enrolled sample.
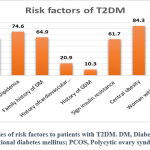 |
Figure 1: Frequencies of risk factors to patients with T2DM. DM, Diabetes Mellitus; GDM, Gestational diabetes mellitus; PCOS, Polycytic ovary syndrome. |
Table 2 shows the prevalence of different outcomes of macrovascular complications in relations to risk factors among T2DM.
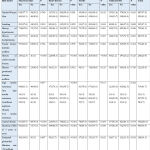 |
Table 2: the effect of risk factors on macrovascular complications among patients with T2DM. |
The following risk factors were significantly affecting the overall macrovascular complications including: History of cardiovascular disease, hypertension, dyslipidaemia, central obesity, history of GDM, duration of T2DM more than 5 years, estimated glomerular filtration rate <60 ml/min/1.73 m2, and any signs of IR by influencing any parameter of the macrovascular complications like (CVD, stroke or clinical PAD).
Table 3 shows the prevalence of different outcomes of microvascular complications in relations to risk factors among T2DM.
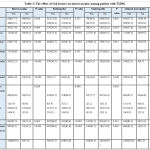 |
Table 3: The effect of risk factors on microvascular among patient with T2DM. |
Table 4: Regression of risk factor to macrovascular complication
| Variables | B | S.E. | Wald | df | Sig | Exp(B) |
| Gender(women) | -1.573- | 1.238 | 1.615 | 1 | .204 | .207 |
| Smoking | -.934- | .764 | 1.494 | 1 | .222 | .393 |
| History of hypertension | -.248- | .326 | .578 | 1 | .447 | .780 |
| History of dyslipidemia | -1.463- | .332 | 19.412 | 1 | <0.001 | .232 |
| Family history diabetes mellitus | -.410- | .314 | 1.711 | 1 | .191 | .664 |
| history of cardiovascular disease | -1.950- | 1.032 | 3.571 | 1 | .059 | .142 |
| History of gestational diabetes mellitus | 1.010 | .321 | 9.922 | 1 | .002 | 2.746 |
| Any sign of insulin resistance | -.219- | -.219- | .392 | 1 | .531 | .804 |
| Central obesity | .044 | .413 | .011 | 1 | .916 | 1.045 |
| Constant | -1.049- | 1.652 | .403 | 1 | .525 | .350 |
Duration of T2DM more than five years, any sign of IR, history cardiovascular disease, hypertension, dyslipidaemia, history of GDM and central obesity were significantly increase the chance for developing microvascular complications (retinopathy, nephropathy and clinical neuropathy) among individuals with T2DM.
Table 5: regression of risk factor to microvascular complication
| Variables | B | S.E. | Wald | df | Sig | Exp(B) |
| Gender(women) | -2.034- | 1.303 | 6.429 | 1 | .011 | .041 |
| Smoking | -1.525- | 1.054 | 2.477 | 1 | .116 | .182 |
| History of hypertension | -.248- | .364 | 1.264 | 1 | .261 | .673 |
| History of dyslipidemia | -1.791- | .375 | 22.085 | 1 | <0.001 | .171 |
| Family history diabetes mellitus | -.469- | .344 | 2.761 | 1 | .097 | .569 |
| history of cardiovascular disease | -1.583- | 1.044 | 2.316 | 1 | .128 | .204 |
| History of gestational diabetes mellitus | 1.241 | .346 | 11.508 | 1 | .001 | 3.191 |
| Any sign of insulin resistance | -.388- | .376 | 1.065 | 3 | .302 | .678 |
| Central obesity | -.186- | .455 | .167 | 1 | .683 | .830 |
| Constant | 2.259 | 1.338 | 2.850 | 1 | .091 | 9.569 |
Discussions
In this study, the risk factors for T2DM and their influencing on both micro- and macrovascular outcome were discussed in detailed as
Age
Increasing age considered a major non-modifiable risk factor for developing T2DM and we found an increment in the prevalence of T2DM among individuals over the age of fifty. This was consistent with that registered in Saudi Arabia at 2004 where the risk of developing diabetes rises with age[13].
Gender
T2DM was having a higher prevalence rate among women than men (58.0% and 42.0% respectively) which was consistent with a local study done in Basrah 2020[14] and researches done in Saudi Arabia [13] and Iran[15] and was incongruent with other studies carried out in Saudi Arabia [16]. This genders’ prevalence discrepancy may be due to racial differences, community distribution of gender .
There was a significant gender difference (men 20.7%, women 15.6%) for developing a clinical PAD. PAD is a common symptom of atherosclerosis, and it has historically been thought to be more common in men than in women which was also evident in of Kautzky-Willer et al [17]. In other studies, the differences and similarities between women and men in T2DM with symptomatic PAD were variable. Recent research, however, has found that men and women have the same frequency of PAD [18].
More than one-fifth (22.5%) of the participants were having an established IHD without significant gender-specific differences (men 20.5%, women=18.4%, p = 0.381) as same as a Nigerian study [19].
Stroke was non-significantly presented in both genders (men 6.5%, women=8.7%, p = 0.152) among individuals with T2DM. These findings were similar to Kolawole et al [20], but it was different from a local study done in Basrah 2019 where men were having a high case mortality rates due to stroke than woman with T2DM [21]. This could be explained by the fact that women tend to live longer than men, and these women were exposed to the combination of obesity, dyslipidemia, and hypertension as cardiovascular risk factors as documented in this study.
Regarding microvascular problems, both genders had similar rates of overall microvascular events among T2DM, with a woman: men ratio of 1.3:1. The lack of statistically significant gender differences in microvascular events was in line with the results of Kautzky-Willer et al, who found gender offers some protection against the onset and progression of non-diabetic kidney disease especially in premenopausal women [17].
Diabetic retinopathy was statistically non-significant common in women than men in this cohort, other studies have shown men were more associated with the existence and severity of DR [22]. While Sparrow et al found that women’s gender was associated with an increased risk of developing and/or worsening diabetic retinopathy[23]. This discrepancy could be brought about by variations in study designs, patient characteristics such diabetes duration and comorbidity, and features of communities sampled such as race, region, and economic level.
Diabetic nephropathy was found in 14% of individuals in this cohort which was considered as lower than that occurred globally in 20-40% of patients with diabetes [24], As the number of individuals with diabetic nephropathy increased, there was a strong link between the male gender and the evidence of nephropathy (p=0.001).
Also there was no statistically significant difference in the prevalence of clinical neuropathy among both genders, but little evidence might predict the more severe form of clinical neuropathy among men than women [25].
BMI
Diabetes incidence was considerably enhanced by having a high BMI, which may be because obesity increases IR [26]. A prior study done on Saudi patients showed a direct link between high BMI and T2DM, which was consistent with our findings [27]. The promotion of fast food, alteration of the conventional diet especially high carbohydrate food including rice, wheat bread, and sweetly tea consumption in terms of both amount and quality, and lack of exercise had a greater effect on new incidence of T2DM among our societies
Central obesity and IR
Through the current study, there was a strong relationship between central obesity with the risk of T2DM and WC is a reliable physical measure of visceral fat, as most patient with T2DM have an excess of visceral fat which may greatly lead to excess IR and consequence increasing the risk of T2DM [29].
Less than 2/3 (61.7%) of our participants were having at least one sign of IR and more insulin is therefore required to convince fat and muscle cells to take up glucose and the liver to keep storing it so that overweight or obesity increase the risk of IR [9].
Particularly in women, central obesity (85.5 % ) has increased recently and became more prevalent than the whole body obesity (56.6%) as seen in other study[30]. Central obesity was a significant contributing factor to the risk of CVD and other related morbidities. and it has significant effects on developing heart disease, clinical PAD, and stroke (86.5%/P<0.001, 85.4% /P 0.039, 82.9%/P 0.014, respectively).
Central obesity was significantly increasing the risk of microvascular complications (retinopathy, clinical neuropathy, and nephropathy) in this study as the number of those possessing diabetic retinopathy (88.6/P=<0.001) was consistent with Chinese study [31]. Many hypotheses and processes, such as increased oxidative stress in people with central obesity and DR, as well as links between DR and metabolic syndrome, have been put out to explain and account for this association.
Duration of T2DM
Duration of T2DM has an important effect on the outcome of individuals with T2DM . In this cohort, the mean duration of T2DM was 10.1 ± 7 years which was comparable to ( 9.7 years) of a large cohort study done in Basrah [14] and the majority of our participants 76.5% were having T2DM for more than five years. A systematic review done in the Middle East and North Africa found inadequate glycemic control and high risk of diabetic complications among T2DM with a long history of the disease [33].
A prolonged duration of T2DM of more than five years was significantly increase the risk of each element of macrovascular events and later on the overall macrovascular complications (81.8 /P<0.001). This was in agreement with a local and national cross-sectional researches that showed macrovascular complications are more common in people having longer durations of T2DM [34, 35].
All modalities of microvascular complications affected significantly by the long duration of T2DM in comparable rate ranging from the commonest one retinopathy then nephropathy and later on clinical neuropathy. These was in consistent with another cross sectional study done in Ethiopia [36].
Smoking
Smoking was a known modifiable risk factor for T2DM and it was presenting and causing an established macrovascular complications in more than one quarter (27.3%) of our participants due to ccurrent smoking linked to glucose intolerance, impaired fasting glucose, and ultimately T2DM [37]. History of smoking considered a significant risk factor for developing stroke (37.1/P 0.032), and IHD (34.8 /P 0.005) while it was having statistically non-significant effect on both clinical PAD (28.4/P 0.682) and overall macrovascular disease, these results were as same as to Chang et al [38].
Regarding microvascular complications, smoking significantly increased the development of retinopathy and nephropathy among our patients (p=0.030, 0.024 respectively), but it was not statistically affecting clinical neuropathy (p=0.529). The harmful mechanism of smoking has been clearly reported that smoking was identified as a risk factor for the development of the three modalities of microvascular complications[39].
Hypertension
In this cohort, hypertension presented in around two-thirds (63.5%) of T2DM. The prevalence of T2DM tends to be higher among hypertensive patients and its relationship to T2DM is significant and well known[40]. In this cohort, those patients were either known hypertensive on medical treatment or newly diagnosed during surveillance. Both T2DM and hypertension were interrelated conditions with overlapping clinical consequences and complications. , and this risk rises further when T2DM is present. Controlling HTN especially by Renin angiotensin system can prevent or delay the consequences of T2DM, according to the findings of
Hypertension was common (65.6%) among T2DM with microvascular complications and the presence of hypertension increased the risk of retinopathy (75.4% P/<0.001), clinical neuropathy (65.4% /P<0.001), and nephropathy (75.6%<0.001) of the patient with T2DM. This high rate of hypertension with microvascular complication among T2DM was also observed in Saudi Arabia (69) were a study reported hypertension among only 71% of the patients with T2DM and was lower than 89.6% among large cohort study done in Basrah 2012 [43].
Dyslipidemia
Dyslipidemia was predominant associated condition in most T2DM as documented in our three quarter of respondents (74.6%). The overall prevalence of dyslipidemia obtained in this study was comparable with many studies and it seemed to be due to IR which is the most common cause of lipid abnormalities in people with diabetes Peripheral IR increases the release of free fatty acids from adipose tissue, which the liver absorbs; increased hepatic uptake of free fatty acids leads to more triglyceride synthesis [45].
Dyslipidaemia in T2DM was a common metabolic derangement 77.6% and it increased the risk of all elements of macrovascular complication at a rate (87.8% P/<0.001) heart disease, (85.4% P/0.015) stroke and (79.6% P/ 0.063) clinical PAD. These findings were consistent to a nationwide study in Thailand (54) and it may be due to increased levels of leptin, dysregulated adipocytes, IR, and C-reactive protein which all contribute to the mechanism causing the increased cardiovascular morbidity and mortality [46].
Also, dyslipidemia was the primary cause of the emergence of diabetes microvascular complications. Abnormal lipid parameters significantly increased the risk of clinical neuropathy (76.6%, P/<0.001), nephropathy (82.1%, P/0.015), and retinopathy (82.7%, P/<0.001). These finding were matched with many evidences and it might attributed to different pathophysiological mechanisms [47]. Thus, it was postulated that lipid-induced renal injury may occur by stimulating transforming growth factor-beta (TGF-β), thereby inducing the production of reactive oxygen species causing damage to the glomeruli and glomerular glycocalyx [48]. The effect of adiposity and low physical activity on the incidence of T2DM is clear, but there was no satisfied evidence that being physically active could completely compensate for the adverse outcome of adiposity on diabetes risk Clinical research conducted in the past has produced inconsistent findings about the role of aberrant lipid levels in the development or progression of DR, according to the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR), total cholesterol did not significantly influence the severity of DR
Family history of diabetes
Family history of diabetes is a culprit risk factor for the development of T2DM as it was represented in less than two-third (64.9%) of the participants and similar data were observed in a study done by
Despite the great effect of positive family history of T2DM on the new incidence of T2DM, it was statistically not effecting the occurrence of both overall micro- and macrovascular complications. Apart from nephropathy was significantly increased among our participants with positive family history of T2DM. The link between diabetic microvascular complications and familial history of T2DM is unclear and some evidence found it was an independent risk factor for the development of retinopathy [53] while others have shown that family history was only related to clinical neuropathy [54].
History of previous cardiovascular diseases
One fifth (20.9%) of this cohort were having a history of established atherosclerotic cardiovascular disease which was in agreement with a study done on immigrants from the Middle East compared to native Swedes [55]. This risk factor was significantly predisposing the patient at a high risk for developing all events of both micro- and macrovascular complications. The burden of cardiovascular disease (CVD), the leading cause of morbidity and mortality worldwide, is disproportionately high in patients with T2DM, with the proportion of CVD caused by diabetes rising in the general population as seen in the Framingham heart study [56].
Gestational diabetes mellitus (GDM)
In this study, the prevalence of GDM was 10.3% in a comparable ratio to 8.3% of that reported by Anzaku et al among Nigerians [57]. The exact pathophysiological mechanism of GDM is complex, but Catalano et al. studied it minutely by using hyperinsulinemic-euglycemic clamp studies, and discovered significantly decreased insulin sensitivity in GDM prior to conception and persisted across pregnancy . GDM was thought to impact 14% of all pregnancies worldwide and it was an important risk factor for developing later on T2DM and Ischemic heart diseases
Microvascular dysfunction was documented in (15.9%) of the participants (100 out of 122 women with previous GDM). GDM was significantly associated with retinopathy (12.7%, P/0.001), clinical neuropathy (15.9%, P/<0.001), and statistically non-significant with nephropathy (12.8%, P/0.206). Our results were consistent with other studies especially for the prevalence of proliferative retinopathy in patients with GDM [60]. Data on this association are relatively limited and the progression of GDM is influenced by many factors, including obesity, family history, and physical activity . One study, tracked 72 women for five to eight years after the last GDM occurrence and found that women with a history of GDM had a higher risk for microalbuminuria than control group . The results from the Kidney Early Evaluation Program (KEEP) used self-report data and involved a large cohort (571 women with GDM vs. 25, 045 women without GDM). The development of microalbuminuria in the future was revealed to be at risk from GDM alone (without eventual T2DM). The authors noted that patients with a This discrepancy may be due to racial differences, community distribution of gender,
In this study, 2.4% of a pregnant lady was having T2DM and they were representing 8.62% of reproductive-age women. These findings were consistent with an IDF meta-analysis of studies published during 2010–2020 discussing the prevalence of pre-existing diabetes among pregnancies and pregnancy itself, especially the second half of it, had a higher chance for developing GDM due to high occurrence of IR [64].
History of PCOS
Women with history of PCOS considered an important risk factor for the development T2DM and we found 7.6% of T2DM individuals were diagnosed as PCOS earlier, which was supported by a previous local study[65]. PCOS is considered a hallmark trigger for the development of dysglycemia due to an insulin secretory defect, and the high risk for glucose intolerance [65].
Low e GFR
Both of T2DM and CKD increased the risk of CVD and CKD considered as an independent risk factor for developing macrovascular complications even in patients without T2DM. Around 40% of people with T2DM developed CKD, which manifests as albuminuria, decreased estimated glomerular filtration rate (eGFR) < 60 ml/min/1.73 m2, or both [2]. Our results showed a statistically significant relationship between heart disease and eGFR < 60 ml/min/1.73 m2 (p=<0.001). Although various studies have looked into the link between eGFR and CVD, the majority of them have concentrated on individuals who seem to be in good health, who already have CVD, or who are at high risk for CVD. According to the total epidemiological data, people with pre-existing CVD or those at high risk for CVD who had an eGFR of 60 ml/min/1.73 m2 or less were at an increased risk of CVD outcomes [66], But not all studies have found a strong inverse association between eGFR and the risk of CVD [67].
In reverse, there was no significant effect of low eGFR on both stroke and clinical PAD (p=0.162, 0.181 respectively) which was similar to other study [68]. where they discovered that incident coronary artery disease and stroke risk increased at eGFR <60 mL/min/1.73 m2, when compared with eGFR ≥90 mL/min/1.73 m2 at baseline, which is consistent with the results of Ninomiya et al [69].
Additionally, there was a higher risk of CHD and stroke even among persons with somewhat lower baseline eGFR (60-74 ml/min/1.73 m2) and mean eGFR (60-89 ml/min/1.73 m2) throughout follow-up. This may more accurately reflect the strength of the relationship between renal function and the risk of incident CVD because kidney function might change over time [70].
After doing a logistic regression analysis, history of dyslipidemia, and history of GDM were considered as independent risk factors for the prediction of macrovascular complications (p<0.0001, <0.001 respectively). While others risk factors including smoking, hypertension, history of CVD, signs of IR, central obesity, estimated GFR <60 ml/min/1.73 m2, and duration T2DM of five years or more required another cofounder that could be dependent on them to predict these complications.
For microvascular complications, female gender, history of dyslipidemia, and history of GDM were independent risk factors for the prediction of microvascular complications among T2DM. Other risk factors (smoking, hypertension, history of CVD, signs of IR, central obesity, and duration T2DM of five years or more were dependent risk factors.
There are some limitations to our study including, firstly these data represent a single tertiary center that is receiving the complicated with prolonged history duration of T2DM. Secondly, each subgroup of microvascular complications like types of clinical neuropathy, and degree of retinopathy were not studied, Other metabolic and hormonal abnormalities like beta cell function, insulin resistance index, fasting/serum amino acid, and selected acylcarnitine were not discussed here so further studies are required to judge in the future,
In conclusions, history of dyslipidemia and history of GDM are the most significant independent risk factor for the prediction of macrovascular complications among individuals with T2DM, while female gender, history of dyslipidemia, and history of GDM were independent risk factors for the prediction of microvascular complications among T2DM. Other risk factors including: History of CVD, hypertension, central obesity, duration of T2DM more than 5 years, estimated GFR <60 ml/min/1.73 m2, and any signs of IR were significantly effect on both micro- and macrovascular complications, but as dependent risk factors to further cofounders.
Acknowledgement
we would like to thanks the participants for their acceptance and cooperation to recruit in this study.
Conflict of Interest
There is no conflict of interest.
Funding Source
There is no funding source.
References
- Aljerf, L. and I. Alhaffar, Salivary Distinctiveness and Modifications in Males with Diabetes and Behcet’s Disease. Biochem Res Int, 2017. 2017: p. 9596202.
- American Diabetes, A., 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care, 2021. 44(Suppl 1): p. S15-S33.
- Surampudi, P.N., J. John-Kalarickal, and V.A. Fonseca, Emerging Concepts in the Pathophysiology of Type 2 Diabetes Mellitus. Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine, 2009. 76(3): p. 216-226.
- Cresci, B., et al., Metabolic surgery for the treatment of type 2 diabetes: A network meta-analysis of randomized controlled trials. Diabetes Obes Metab, 2020. 22(8): p. 1378-1387.
- Lean, M.E., et al., Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet, 2018. 391(10120): p. 541-551.
- Chung, W.K., et al., Precision medicine in diabetes: a Consensus Report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia, 2020. 63(9): p. 1671-1693.
- Tsai, C., C. Hayes, and G.W. Taylor, Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol, 2002. 30(3): p. 182-92.
- Mansour, A.A., et al., Diabetes screening in Basrah, Iraq: a population-based cross-sectional study. Diabetes Res Clin Pract, 2008. 79(1): p. 147-50.
- Alberti , K.e.a., IDF Epidemiology Task Force Consensus Group: The metabolic syndrome: a new worldwide definition. Lancet, 2005. 366: p. 1059-1062.
- Mansour, A.A., A.A. Al-Hassan, and M.I. Al-Jazairi, Cut-off values for waist circumference in rural Iraqi adults for the diagnosis of metabolic syndrome. Rural Remote Health, 2007. 7(4): p. 765.
- Delgado, C., et al., A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. J Am Soc Nephrol, 2021.
- Wilkinson, C.P., et al., Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology, 2003. 110(9): p. 1677-82.
- Al-Nozha, M.M., et al., Diabetes mellitus in Saudi Arabia. Saudi Med J, 2004. 25(11): p. 1603-10.
- Mansour, A.A., et al., Prevalence and correlation of glycemic control achievement in patients with type 2 diabetes in Iraq: A retrospective analysis of a tertiary care database over a 9-year period. Diabetes Metab Syndr, 2020. 14(3): p. 265-272.
- Azimi-Nezhad, M., et al., Prevalence of type 2 diabetes mellitus in Iran and its relationship with gender, urbanisation, education, marital status and occupation. Singapore Med J, 2008. 49(7): p. 571-6.
- Al Mansour, M.A., The Prevalence and Risk Factors of Type 2 Diabetes Mellitus (DMT2) in a Semi-Urban Saudi Population. Int J Environ Res Public Health, 2019. 17(1).
- Kautzky-Willer, A., et al., Sex-specific differences in metabolic control, cardiovascular risk, and interventions in patients with type 2 diabetes mellitus. Gend Med, 2010. 7(6): p. 571-83.
- Al-Zoubi, N.A. and N.J. Shatnawi, Gender variation in symptomatic peripheral arterial occlusive disease among type-2 diabetic patients. SAGE Open Med, 2019. 7: p. 2050312119840198.
- Enikuomehin, A., et al., Influence of gender on the distribution of type 2 diabetic complications at the obafemi awolowo teaching hospital, Ile-Ife, Nigeria. Afr Health Sci, 2020. 20(1): p. 294-307.
- Kolawole, B.A. and A.A. Ajayi, Prognostic indices for intra-hospital mortality in Nigerian diabetic NIDDM patients. Role of gender and hypertension. J Diabetes Complications, 2000. 14(2): p. 84-9.
- Altemimi, M.T. and A.R. Hashim, Acute Stroke in Diabetes Mellitus: A Prospective Observational Study Evaluating the Course and Short-Term Outcome in Basrah, Southern Iraq. Cureus, 2019. 11(10): p. e6017.
- Nittala, M.G., et al., Risk factors for proliferative diabetic retinopathy in a Latino American population. Retina, 2014. 34(8): p. 1594-9.
- Sparrow, J.M., et al., The prevalence of diabetic retinopathy and maculopathy and their risk factors in the non-insulin-treated diabetic patients of an English town. Eye (Lond), 1993. 7 ( Pt 1): p. 158-63.
- Hussain, S., et al., Diabetic kidney disease: An overview of prevalence, risk factors, and biomarkers. Clinical Epidemiology and Global Health, 2021. 9: p. 2-6.
- Ugoya, S.O., et al., Clinically diagnosed diabetic neuropathy: frequency, types and severity. J Natl Med Assoc, 2006. 98(11): p. 1763-6.
- Meigs, J.B., et al., Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab, 2006. 91(8): p. 2906-12.
- Al-Daghri, N.M., et al., Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): a decade of an epidemic. BMC Med, 2011. 9: p. 76.
- The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care, 2002. 25(12): p. 2165-71.
- Phillips, L.K. and J.B. Prins, The link between abdominal obesity and the metabolic syndrome. Curr Hypertens Rep, 2008. 10(2): p. 156-64.
- Barroso, T.A., et al., Association of Central Obesity with The Incidence of Cardiovascular Diseases and Risk Factors. International Journal of Cardiovascular Sciences, 2017. 30(5): p. 416-424.
- Zhou, J.B., et al., Is central obesity associated with diabetic retinopathy in Chinese individuals? An exploratory study. J Int Med Res, 2019. 47(11): p. 5601-5612.
- Currie, C.J., et al., Survival as a function of HbA(1c) in people with type 2 diabetes: a retrospective cohort study. Lancet, 2010. 375(9713): p. 481-9.
- Ghouse, J., et al., Effect of diabetes duration on the relationship between glycaemic control and risk of death in older adults with type 2 diabetes. Diabetes Obes Metab, 2020. 22(2): p. 231-242.
- Khuwaja, A.K., et al., Macrovascular complications and their associated factors among persons with type 2 diabetes in Karachi, Pakistan–a multi-center study. J Pak Med Assoc, 2004. 54(2): p. 60-6.
- Khawaja, N., et al., The prevalence and risk factors of peripheral neuropathy among patients with type 2 diabetes mellitus; the case of Jordan. Diabetol Metab Syndr, 2018. 10: p. 8.
- Seid, M.A., et al., Microvascular complications and its predictors among type 2 diabetes mellitus patients at Dessie town hospitals, Ethiopia. Diabetol Metab Syndr, 2021. 13(1): p. 86.
- Maddatu, J., E. Anderson-Baucum, and C. Evans-Molina, Smoking and the risk of type 2 diabetes. Transl Res, 2017. 184: p. 101-107.
- Chang, S.A., Smoking and type 2 diabetes mellitus. Diabetes Metab J, 2012. 36(6): p. 399-403.
- Tracey, M.L., et al., Risk Factors for Macro- and Microvascular Complications among Older Adults with Diagnosed Type 2 Diabetes: Findings from The Irish Longitudinal Study on Ageing. J Diabetes Res, 2016. 2016: p. 5975903.
- El-Hazmi, M.A. and A.S. Warsy, Association of hypertension and non-insulin-dependent diabetes mellitus in the Saudi population. Ann Saudi Med, 2001. 21(1-2): p. 5-8.
- Ji, L., et al., Primacy of the 3B approach to control risk factors for cardiovascular disease in type 2 diabetes patients. Am J Med, 2013. 126(10): p. 925 e11-22.
- James, P.A., et al., 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA, 2014. 311(5): p. 507-20.
- Mansour, A.A., Prevalence and control of hypertension in iraqi diabetic patients: a prospective cohort study. Open Cardiovasc Med J, 2012. 6: p. 68-71.
- S., B., Lipid links inflammation, immunity and insulinresistance to cause epidemic diabetes. CURRENT SCIENCE, VOL. 110, NO. 10, 25 MAY 201, 2016. 110(10).
- Bello-Ovosi, B.O., et al., Prevalence and pattern of dyslipidemia in patients with type 2 diabetes mellitus in Zaria, Northwestern Nigeria. Pan Afr Med J, 2019. 34: p. 123.
- Narindrarangkura, P., et al., Prevalence of dyslipidemia associated with complications in diabetic patients: a nationwide study in Thailand. Lipids Health Dis, 2019. 18(1): p. 90.
- Rutledge, J.C., et al., Role of triglyceride-rich lipoproteins in diabetic nephropathy. Nat Rev Nephrol, 2010. 6(6): p. 361-70.
- Raz, I., Guideline approach to therapy in patients with newly diagnosed type 2 diabetes. Diabetes Care, 2013. 36 Suppl 2: p. S139-44.
- Hjerkind, K.V., J.S. Stenehjem, and T.I. Nilsen, Adiposity, physical activity and risk of diabetes mellitus: prospective data from the population-based HUNT study, Norway. BMJ Open, 2017. 7(1): p. e013142.
- Klein, B.E., et al., The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XIII. Relationship of serum cholesterol to retinopathy and hard exudate. Ophthalmology, 1991. 98(8): p. 1261-5.
- Morton, J., et al., Low HDL cholesterol and the risk of diabetic nephropathy and retinopathy: results of the ADVANCE study. Diabetes Care, 2012. 35(11): p. 2201-6.
- A, G., G. S, and U. R, Study on the impact of family history of diabetes among type 2 diabetes mellitus patients in an urban area of Kancheepuram district, Tamil Nadu. International Journal Of Community Medicine And Public Health, 2017. 4(11).
- Maghbooli, Z., et al., Predictive factors of diabetic complications: a possible link between family history of diabetes and diabetic retinopathy. J Diabetes Metab Disord, 2014. 13: p. 55.
- Amelia, R., A.S. Wahyuni, and Y. Yunanda, Diabetic Neuropathy among Type 2 Diabetes Mellitus Patients at Amplas Primary Health Care in Medan City. Open Access Maced J Med Sci, 2019. 7(20): p. 3400-3403.
- Bennet, L., C.D. Agardh, and U. Lindblad, Cardiovascular disease in relation to diabetes status in immigrants from the Middle East compared to native Swedes: a cross-sectional study. BMC Public Health, 2013. 13: p. 1133.
- Fox, C.S., et al., Lifetime risk of cardiovascular disease among individuals with and without diabetes stratified by obesity status in the Framingham heart study. Diabetes Care, 2008. 31(8): p. 1582-4.
- Anzaku, A.S. and J. Musa, Prevalence and associated risk factors for gestational diabetes in Jos, North-central, Nigeria. Arch Gynecol Obstet, 2013. 287(5): p. 859-63.
- Johns, E.C., et al., Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol Metab, 2018. 29(11): p. 743-754.
- Li, G., et al., Incidence and Risk Factors of Gestational Diabetes Mellitus: A Prospective Cohort Study in Qingdao, China. Front Endocrinol (Lausanne), 2020. 11: p. 636.
- Axer-Siegel, R., et al., Diabetic retinopathy during pregnancy. Ophthalmology, 1996. 103(11): p. 1815-9.
- Bao, W., et al., Physical activity and sedentary behaviors associated with risk of progression from gestational diabetes mellitus to type 2 diabetes mellitus: a prospective cohort study. JAMA Intern Med, 2014. 174(7): p. 1047-55.
- Friedman, S., et al., Microalbuminuria following gestational diabetes. Acta Obstet Gynecol Scand, 1995. 74(5): p. 356-60.
- Bomback, A.S., et al., Gestational diabetes mellitus alone in the absence of subsequent diabetes is associated with microalbuminuria: results from the Kidney Early Evaluation Program (KEEP). Diabetes Care, 2010. 33(12): p. 2586-91.
- Chivese, T., et al., IDF Diabetes Atlas: The prevalence of pre-existing diabetes in pregnancy – A systematic reviewand meta-analysis of studies published during 2010-2020. Diabetes Res Clin Pract, 2022. 183: p. 109049.
- Altemimi, M.T., A.K. Musa, and A.A. Mansour, The Performance of Glycated Hemoglobin vs. Oral Glucose Tolerance Test in the Diagnosis of Glycemic Disorders among Women with Polycystic Ovary Syndrome in Southern Iraq. The Indonesian Biomedical Journal, 2021. 13(2): p. 178-85.
- Gansevoort, R.T., et al., Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int, 2011. 80(1): p. 93-104.
- Kurth, T., et al., Kidney function and risk of cardiovascular disease and mortality in women: a prospective cohort study. BMJ, 2009. 338: p. b2392.
- Sarfo, F.S., et al., Estimated glomerular filtration rate predicts incident stroke among Ghanaians with diabetes and hypertension. J Neurol Sci, 2019. 396: p. 140-147.
- Ninomiya, T., et al., Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol, 2009. 20(8): p. 1813-21.
- Wang, Y., et al., Kidney function and the risk of cardiovascular disease in patients with type 2 diabetes. Kidney Int, 2014. 85(5): p. 1192-9.







