Manuscript accepted on :10-06-2022
Published online on: 25-07-2022
Plagiarism Check: Yes
Reviewed by: Dr. Sumayah Faruq
Second Review by: Dr. Salman Ahmed Pharmacognosy
Final Approval by: Dr. H Fai Poon
Satish Arunrao Polshettiwar , Dipali Hiralal Sawant
, Dipali Hiralal Sawant , Neeta Bhausaheb Abhale
, Neeta Bhausaheb Abhale , Neeta Bhagvat Chavan
, Neeta Bhagvat Chavan , Akshay Motilal Baheti
, Akshay Motilal Baheti , Manish Shivdas Wani
, Manish Shivdas Wani , Amol Ambadas Tagalpallewar
, Amol Ambadas Tagalpallewar , Chinamay Devidas Deshmukh and Abhishek Pramod Polshettiwar
, Chinamay Devidas Deshmukh and Abhishek Pramod Polshettiwar
School of Pharmacy, Dr. Vishwanath Karad, MIT World Peace University Pune, India
Corresponding Author E-mail: drsatishpolshettiwar@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2458
Abstract
The classification of drugs varies from country to country, with active foods, dietary supplements and traditional medicines being included in certain categories. The stability of those products is also unknown and complex to the critical problem in the analysis of herbal products that this is a complex ingredient combination, as well as the elements responsible for the treatment effects. In order to identify the changes to the newly introduced regulations or regulations, detailed literary searches and online searches for herbal medicinal products regulations have been made in South-east Asia and European countries. Curcumin is an important pharmaceutical compound derived from turmeric. Curcumin is extracted from dried curcuma longa rhizomes. The demand for curcumin grows daily due to its use in the treatment of a number of diseases. Curcumin has long established challenges with its health benefits, such as poor uptake and poor bioavailability.
Keywords
Case study on Curcumin; Herbs; Herbal Drugs; Regulation
Download this article as:| Copy the following to cite this article: Polshettiwar S. A, Sawant D. H, Abhale N. B, Chavan N. B, Baheti A. M, Wani M. S, Tagalpallewar A. A, Deshmukh C. D, Polshettiwar A. P. Review on Regulation of Herbal Products Used as a Medicine Across the Globe: A Case Study on Turmeric - Golden Medicine. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Polshettiwar S. A, Sawant D. H, Abhale N. B, Chavan N. B, Baheti A. M, Wani M. S, Tagalpallewar A. A, Deshmukh C. D, Polshettiwar A. P. Review on Regulation of Herbal Products Used as a Medicine Across the Globe: A Case Study on Turmeric - Golden Medicine. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3J3oKTa |
Introduction
Herbs are raw plant matters which are leaves, flowers, fruits, seeds, stems etc, which may be entirely powdered form.
Simple processes involve harvesting, drying of herbal drug plant parts for preparation of phytochemicals.
As we know that some allopathic drugs undergo some serious toxic effects, so in order to meet these effects we go for herbal drugs, most of the chronic ailments such as HIV, TB, Malaria, etcthey lack medicines so herbal medicines from ancient times its been prescribed to treat the no of chronic diseases example Garlic supplements & Ginseng for HIV. Therefore popular in expanding countries and other developed countries because of its indigenous origin and adverse results.
However, in current time, modern medicine is highly developed over the generations in the larger part of the globe, now the world has turned to alternative natural methods like the indigenous system of medicine (also known as the traditional system of medicine) which plays a vital role for public interest as they are considered safer than conventional medicines. The contribution of many researchers has led to the identification on goodness of many herbs. World Health Organization determines indigenous herbal medicine as a natural, herbal extract with little or no industrial processing used to treat disorders within the local or regional use. The WHO contributes to promote the use, sale of herbal medicine practice, as it is culturally acceptable, effective and accessible. Several practices that come under traditional medicines are Ayurveda, UNANI, and Siddha medicine along with many other formulations. Ayurveda system has made us believe that many products can be used for therapeutic and supplementary use. India, the pioneer of herbal medicines has an abundance of well-experienced and recorded rich source knowledge of traditional herbal medicine. Knowledge of herbal medicines has become important in many areas and the regulatory standards are being strengthened. Traditional medicine is the medicines which occur normally and derived from the plant and with very little or without industrial processing that have been used to treat different illness this is the definition of traditional herbal medicine as per WHO. Herbal medicine isan central component of the world population of about 75-80%, generally in the countries who are developing.
The market for herbal products is divided into health and personal care products, which are estimated to grow faster at the time of prediction than medical products. At Rs 30,000 crore per year, the size of the Indian dairy industry is well compared to Rs 85,000 crore, the size of the Indian health sector.
Regulatory Control and Sales of Herbal and Traditional Medicines
In addition to improving general health and well-being, many phytochemical chemicals and herbal remedies appear to be useful in treating disease. With the success of several herbal remedies in the local market, there is concern, for there is no scientific basis for believing that the adverse effects can outweigh its potential benefits. With the rapid growth of Internet use worldwide to the point of aggressive online marketing by manufacturers and retailers, the demand and popularity of these drugs is growing in modern times.
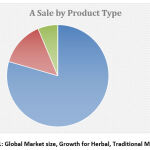 |
Figure 1: Global Market size, Growth for Herbal, Traditional Medicine. |
It is difficult to calculate the data related to the sale of use of herbs across the world. The global market is near about US $83 billion annually estimates by WHO. In some countries the main reason for sale and marketing of herbs is to earn profit, however, in some countries they are considered as a major element to treat the disease.[2]It is difficult to evaluate the market size for products because of different regulations and regulatory categories for traditional medicine product. EU, USA, Canada, Australia, Singapore, Japan comes under the global expert herbal market while new emerging markets are Mexico, China, Argentina, Brazil, Indonesia. Approximately 70% of modern drugs are discovered from natural resources in India. The American botanical council, September 2017 reported the sale of herbal supplement rise to 7.7% in 2016. Tsumura, Madaus, Schwabe, Yunnan Baiyao, Tongrentang and TASLY are the main market player. The sale of herbal medicine was 636621 MT in 2013 and increase to 763382 MT in 2018. The Annual growth rate of Ayurvedic medicine in the field of traditional medicine is 6.6%.
Table 1: Globle Herbal Medicine Market.
| Global Herbal Medicine Market by section analysis (Income, USD Billion, 2015-2026) | Global Pharmaceutical Market Review Form (Money, USD, Billion, 2015-2026) | Global Pharmaceutical Marketplace by Source Analysis (Income, USD Billion, 2015-2026) | Herbal Medicine Market Distribution Channel Analysis (Revenue, USD Billion, 2015-2026) | Global Herbal Medicine Market Competitive Landscape (Revenue, USD Billion, 2015-2026) |
| • Remedies
|
• Tablets and tablets
|
• Fruits
|
•Commercial
|
•Schaper&Brümmer GmbH & Co KG
|
| •Herbal cosmetics
|
• Powders
|
• Leave
|
•Hospitals and Commercial Pharmacies | •Hishimo Pharmaceuticals Pvt. Ltd.
|
| • The use of herbs Food
|
• Quotes
|
• Roots & Bark
|
• Arkopharma Laboratories Co. Ltd.
|
|
| • Herbal supplements | • syrups
|
• All Plants
|
The Himalayas Global Holdings Ltd.
|
|
| • Others | • Others
|
• IBeovita, Vital GmbH
|
||
| • PatanjaliAyurved Ltd.
|
||||
| • IVenusPharma GmbHLaboratories .
|
||||
| Dasherb Corp Arkopharma Co. Ltd.
|
||||
| • Bayer AG
|
||||
| Dabur India Ltd.
IBlackmores Ltd |
||||
| Arizona Natural Products |
In addition to improving overall health and well-being, many phytochemicals and herbal remedies are seen as helpful in cure diseases. With the prosperity of several herbal cures in the local market, there is concern, as there is no scientific basis for belief, that catastrophic side effects could outweigh its potential advantages. With the rapid growth of Internet usage worldwide as far as online aggressive marketing by producers and retailers is concerned, the demand and popularity of these medicines is growing in modern times.
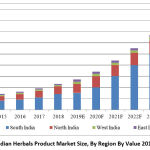 |
Figure 2: Indian Herbals Product Market Size, By Region By Value 2014- 2024F |
Regulations of Herbal Medicines in Southeast Asia and European Countries
Herbal regulationare defined as a concept, power or legislation designed to regulate or regulate the manufacturers and manufacturers of herbal medicines. For example, the regulation would mean that herbal remedies should be shown to be safe, effective and appropriate before reaching the public. The Drug and cosmetic Act 1940 and the laws of 1945 regulate the herbal medicines in India. [2] It is important ensuring safety, efficacy and quality of herbal medicines. There are a variety of requirements available to deliver clinical data and toxic data, and its size in the market and its environment.
The US FDA has provided the Industrial Model Guide to “Complementary and Alternative Medicine Products and Regulation”.
India
Herbal medicine in India is regulated under the Drugs and Cosmetics Act 1940 and drug and cosmetics laws of 1945, where Ayurveda, Siddha, and Unani prescribe AYUSH drugs. Clinical trials that require use of herbal medicines are mandate to follow the AYUSH GCP guidelines released on March 28, 2013. These GCP guidelines have to be followed during a clinical trial, if not followed than clinical trial gets suspended by regulatory authorities. Also, it is now mandateto have manufacturing and expiry date on the product label from 2017 onwards. For the sale of herbal products form 24 D or for the renewal of license for manufacturing herbal medicines is submitted to regulatory authorities.Schedule “T” of the Act sets out the best production practices (GMP) that must be followed when making pharmaceuticals. The first version of the D&C Act drafted authorized documents, which must be followed to license any herbal product under these two categories: Ayurvedic, Siddha or Unani drugs , Patent or proprietary medicines
The laws concerning Ayurveda, siddha, that price under Drugs and Cosmetics 1945 are set out below
Part XVI: Defines the conduct guidelines for Ayurvedic sales (including Siddha) or Unani drugs.
Section XVII: Discusses the placement, implantation and restriction of alcohol in Ayurvedic (including Siddha) or Unani drugs.
Section XVIII: Provide details of government analysts and Ayurvedic examiners (including Siddha) or Unani drugs.
D&C action improves licensing management, construction, manufacturing, labeling, packaging, quality and export.
In India to obtain a permit to manufacture or sell Ayurvedic drugs, Siddha, Unani, the manufacturer must purchase a GMP certificate. According to D and C act rule 157,To obtain a certificate for ASU’s drug manufacturing practices, the applicant must apply to an uneducated paper with full knowledge of the existing manufacturing unit’s infrastructure including instruments available, equipment and technical staff name with qualification. After full verification by licensing authority as per Schedule T requirements licensing authority has to issue a certificate within three months of Form 26-E-I.
Germany
The German parliament that came into force on 1 January 1978 enacts the national laws and regulations on herbal medicines in Germany in 1976. The last amendment in German medicines law was done in 2009.It controls over all the issues for the production, quality control and trade of medicinal drugs in Germany. The purpose of this law is to ensure safety, quality, and efficacy in while supplying the medicines for human and animal use.
On January 1, 1978, a second drug law enacted a new standard of licensing in accordance with the European Medicines Regulation Framework. Under the new law, quality, safety and efficacy became an important factor in the registration of medicines.
To manufacture any herbal medicines the regulatory requirements include sticking to the pharmacopoeias or if there is any absence of pharmacopoeias , monographs and GMP rules for generic medicines, German pharmaceutical practice and Europeanunion laws regarding medical products.
Canada
In order to get the license in Canada GMP must be followed for labelingand packaging along with that safety and efficacy evidence must be provided.Data related to an herbal product like stability, standardization, safety, efficacy, product composition, tolerance limit, chemical containment testing methods are submitted to Natural Health Product Directorate (NHPD).
USA
According to this legal supplement “anything that adds to food”. Vitamins, minerals, herbs, enzymes, metabolites fall under the supplements. In addition to demonstrating the safety and efficacy of food additives it is produced and sold.
Europe
There are two ways for registration of herbal medicine product as per the European Medicine Agency 1) to get market authorization dossier is submitted which contain data related to quality, safety, the efficacy of medicinal product, including biological, microbiological test, physicochemical, toxicological, pharmacological, clinical trial data under directive 2001/83/EC2) the medicinal product who have long traditional use and do not require medical supervision, An adequate scientific textbook on effective medical practice cannot be provided, a simple procedure performed under the direction of 2004/24 / EC exists.
Herbal remedies are defined as a concept, power or law designed to control or regulate the manufacturers and producers of herbal medicines. For example, the regulation would mean that antiretroviral drugs must be shown to be safe, effective and appropriate before reaching the public.
Case Study on Curcuminlongum
 |
Figure 3: Turmeric ( Curcuma Longa L). |
Turmeric (Curcuma longa) is widely used as a spice, dyeing, cosmetic and food preservative in India, China and South East Asia. It contains a yellow chemical known as curcumin. It has been used in traditional medicine as a home remedy for many ailments, including Fever, Inflammation biliary disorders, cough, foot anorexia, diabetic, rheumatism, hepatic diseases and sinusitis. For decades, important work has been done to initiate the therapeutic action and the natural functions of turmeric and its extraction. Curcumin (diferuloylmethane), a yellow bioactive component of turmeric has been shown to have many forms of biological action. These include antioxidant, antiviral, anticarcinogenic, antimutagenic, antiulcer, antifertility, antidiabetic, antibacterial, antifungal, antiprotozoal, antifibrotic, antivenom, anticoagulant, hypothermic and hypotensive. Its anticancer action is most effective with apoptosis. Antioxidant, anticancer roles can be used clinically to control carcinogenesis and rheumatism, a pathogenesis related to oxidative stress-related stress. Therapeutically, curcumin has already been used to minimize postoperative inflammation. Safety tests show that both turmeric and curcumin tolerate very high doses without toxic side effects. Therefore, both turmeric and curcumin have the potential for modern therapies for the treatment of various ailments.
Production
Production of curcuma longa is home-grown. India contributes 90% of global turmeric production.Asia and India. Mainly curcumin is obtained from turmeric because it is a major source of curcurmin. Its insolubility is found in dimethysulphoxide, ethanol, acetone and methanol. 6mg per day dose of curcumin is used to improve symptoms of oral lichen planus. A Mixture of curcuminoid (mainly curcumin), demethoxycurcumin, and bisdemethoxycurcumin are present in rhizomes of turmeric. Curcumin is mainly produced from feruloyldiketide-CoA with the help of CURS1, 2, and 3. Feruloyldiketide-CoA formed when phenylalanine and malonyl-CoA get condensed.
Extraction of Curcumin from Turmeric
Turmeric or Indian saffron is dried rhizomes of curcuma longa, family zingiberaceae. It has a natural yellow pigment which dissolves in alcohol to form a deep yellow solution. Turmeric rhizome contains about 5%of the curcumins and its derivative, which are the diarylheptanoid compounds of a dark yellow colour which is extracted from rhizome of turmeric. Turmeric hails mostly from tropical and subtropical regions in India, Southeast Asia and China. Curcumin extract is used from the dried root of the rhizome curcuma longa. There are many ways in which curcumin is extracted – a common extract using soxhlet, microwave to help extract, curcumin, – ultrasound to help curcumin extract, -enzyme to help curcumin extraction. The first step in the process of removing the dried rhizomes in the oven at 105 degrees Celsius for 3h. It is then ground into a fine powder using trituration using mud and tested with a sieve mesh 80 to obtain the same particle size of 0.18mm, which is then stored to cool in the refrigerator to prevent moisture absorption. It was then placed under an axle device used as a reference. After this 15 g of ground powder was weighed and added to two soxhlet soils that were filled with acetone as a solvent and made at 60 degrees Celsius within 8h. After completion of extraction the acetone was separated and extracted using a rotary evaporator (rotovap) .The remaining oil oleoresin was measured and dissolved in a 10ml methanol. This process results in purified yellow, enriched with more than 90% curcumin and a very small amount of volatiles and natural substances. There are various solvents used in the extraction process such as isopropanol, ethyl acetate, acetone, methanol and the rest are hexane, ethanol, co2. Later, the curcumin content obtained is calculated using HPLC. In the whole experiment acetone was used due to its highly solubilizing capacity.
Curcumin yield (%)=Mextractedcurcumin/Mpristine turmeric ×100
FSSAI Standard and Regulations for Turmeric Powder
Turmeric or Haldi best known as a golden spice has been cultivated in India since ancient times. At that time it was used as a spice in cooking and had some sacred significance. Turmeric is homegrown to tropical South Asia. Turmeric holds a key bioactive compound called curcumin. Commercially the rhizomes are called bulbs or a finger Curcumin present in turmeric has both anti-bacterial and anti-inflammatory properties.
For Food Market Amendment and Fraud Control, Food Safety and Standards Authority of India appear with Food Safety Regulations and Standards (Standards for Food Products and Food Additives), 2011
The regulation consists of following specifications for Turmeric whole and Turmeric powder:
Turmeric is the result of the Curca longa, which is a primary or secondary herbaceous perennial that belongs to the Zingiberaceae family. All the rhizomes of Turmeric have been properly refined. Rhizomes boiled or boiled to remove the green smell, starch, then drying them to steer clear of regeneration. Whether natural or machine polished, the rhizomes must produce a uniform characteristic color, odor, and flavor.
Depending on the standards, turmeric powder (Haldi) refers to a powder made by grinding dried roots of Curcuma Longa L. The powder will have a distinct aroma and taste of turmeric.
The standards of Turmeric (Haldi) powder
According to the standard, moisture content within turmeric powder should be NMT 10.0% by weight, Ash insoluble in dil. HCL on a dry basis should NMT 2.0% by weight and Total ash on drying basis, Total starch should NMT that 9.0%, 60.0% respectively.
The standards of Turmeric (Haldi) whole:
| 1. | Extraneous matter | NMT 1.0% by weight |
| 2. | Moisture | NMT 12.0% by weight |
| 3. | Defective rhizomes | NMT 5.0 % by weight |
| 4. | Insect damaged matter | NMT 1.0 % by weight |
| 5. | Test for lead chromate | Negative |
Curcumin Market Size, Share and Trends
Curcumin is an active compound commonly extracted from turmeric. Turmeric is widely used in Southeast Asian countries for food and medical products considering its medicinal properties. Increased awareness among people regarding the health benefits seen in the use of curcumin and its popular therapeutic properties of Ayurvedic medicine should be active in other parts of the world during the forecast period.
The market size of curcumin worldwide was priced at USD 58.4 Million in 2019 and is looking forward to witnessing a CAGR of 12.7% in the forecast period. This expansion of demand is being addressed in the natural areas of anti-cancer, anti-inflammatory and anti-oxidant, which is improving its use in all end user industries. Curcumin has also found widespread use in ringworm protection, including protection against ring worm skin applications, leech bites, eye infections, painful skin, bruising and inflammation, which should also facilitate product stability in the prognosis.
Curcumin has been shown to have significant health and fitness benefits as well as the ability to prevent various disorders, including cancer, and coronary heart disease. Transfers noted in consumer trends and increased awareness of the benefits of using natural and organic ingredients in medical, cosmetic, and food applications should increase demand over the projected 2020 to 2027.
Increasing demand for Ayurvedic skin care products and herbs, due to consumer tenders to use natural ingredients to complement the product range, should boost market growth. Curcumin is gaining ground in applications in cosmetics, food, pharmaceuticals, PV-sensitive dye technology, and textiles. This, in turn, is expected to continue the curcumin market during the planned period.
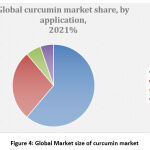 |
Figure 4: Global Market size of curcumin market. |
Application Perception
Part of the cosmetic application is exploring to extended USD 7.9 Million by 2027. Its usefulness in the use of cosmetics can be on the whole assigned to its anti-inflammatory, anti-oxidant and anti-aging property. Subjection to UV radiations causes the rise in the development of free radical species, which reacts with DNA, protein, and fatty acids and perhaps produces irritation on the epidermis.
These free radicals are also involved to damage the skin’s regulatory process, resulting in visible image aging effects such as wrinkles, blood clots and loss of skin firmness. Curcumin incorporated make-up can reduce these effects associated with providing better skin colour. Owners of the product with personal care and vertical cosmetics continue to use this product as a supplement.
A growing customer awareness regarding the basic benefits of using curcumin and the consumer’s tendency for synthetic products should underpin its market size in the prediction period. This increase in demand for food claims may be due to its widespread use as a dietary supplement due to its ability to inhibit arachidonic acid metabolism. 11.7% CAGR over time.
Turmeric has been tested as a widely used spice in the world and dates back more than 2000 years. Usefulness and no clear evidence of environmental protection should increase its use in the coming years. Turmeric has gained widespread popularity worldwide due to its therapeutic efficacy and low levels of toxicity.
Inventive Steps of Curcumin
Curcumin, which is the active ingredient of turmeric is being used and consumed as a dietary spice and functional food due to its long history. Due to the popularity of its health benefits, it continues to rise in its innovation and as the market demand grows. It has a multitude of health benefits, especially its antioxidant and anti-inflammatory actions. Recent research has shown due to its anti-inflammatory property, has the potential to forbid the activity of nuclear factor (NF) -kB which is a potent antagonist of the tumor necrosis factor alpha (TNF-α), which is a major cause of inflammation in various diseases. Osteoarthritis in which curcumin represents a new standard as its treatment. Although the authors have asked for its well-designed clinical trials to determine how they work on their own as the report from meta-analysis concluded that 8-14 weeks of standardized turmeric extract (1000mg/day) of curcumin can alleviate arthritis symptoms and are hence recommended. A research published in 2018 talks about how it is being used to treat metabolic syndrome, Hence curcumin + piperine diet is effective as it reduces body fat and suppresses high fat diet inflammation. Curcumin has numerous health benefits due to which it has received a new paradigm on the gut microbiota. It has a special role and effect on the gut microbiome as the curcuminoid found in turmeric has a larger role in influencing the bacterial population as they not only metabolize to useful products like tetrahydrocurcumin and demethylatedcurcuminoids, but also expand the population of several species of gut flora. Adding to its new potential researches show that it helps in cognitive preservation due to its fluorescent effects. In a 2016 publication curcumin offers a unique benefit for athletes as longvida, an administration of 400mg/day two and four days before following exercise that produced muscle damage went on to reduce biological inflammation except for quadriceps muscle soreness. Hence, these observed advances in inflammation may render to early recovery and enhanced functional capacity in the course of the exercise drill which in turn reduces muscle soreness and myoglobin concentration in athletes following a marathon run.
Innovation and Efficacy
In spite of various health benefits of curcumin, it has long established a challenge due to its inherent poor absorption. It has poor bioavailability in the human intestine, in water and its absorption is poor and is easily removed from the circulatory system. However, this has led to the formation of soluble curcumin formulation with new delivery options and utilizing technologies that will enhance its absorption. One such case is, ‘’Theracurmin’’ which was prepared of 10w/w % curcumin, 2% of the curcuminoid like demethoxycurcumin and is demethoxycurcumin, 46% glycerin 4% gum ghatti and 38% water. Hence, it proved to be an innovative approach that developed its bioavailability 30 times more than that of curcumin powder in both rats and humans, thus setting an ideal candidate for development of Nutraceuticals. Another innovation includes enhancement of the blood barrier crossing of curcumin efficaciously. Another formulation that has shown increased bioavailability of ‘’Stratum NutritionsBiocurc’’ which is a curcumin liquid droplet micromicellar formulation, which means offering curcumin in liquid micelle has shown improved absorption. The aqueous combination of curcumin and fenugreek fiber, such as “CurQfen ” has proven adequate clinical efficacy and improved blood brain inhibition.
Turmeric as a traditional medicine in modern medicine
Traditional medicine is used to treat various ailments and is quite safe and effective. It’s working is not well understood.
In vitro studies with turmeric
Turmeric has various properties and is used to treat and prevent various diseases. The release of 5% turmeric inhibits the growth of histamine-producing bacteria. Turmeric inhibits the production of histamine in Morganellamorganii. The release of turmeric also inhibits the growth of food pathogen V. parahaemolyticus.
Ethanolic Release of C. longa there is good work against Trichophytonlongifusus. Antifungal activity was obtained using the agar disc diffusion method which showed that an ethanolic release killed approximately 29 tested therapeutic dermatophytes
In Vivo studies with turmeric
In vivo study it was found that this yellow spice shows anti-cancer, cardioprotective, hypoglycaemic, hepatoprotective and antiarthritic drugs.
In various models, it has been reported that turmeric shows activity in the fight against the growth of skin cancer, oral cancer, and stomach cancer. In the study, Daloma’s lymphoma cells were inserted into mice internally and medically and treated with turmeric extract for 10 days. It was found that mice that were not treated with turmeric in that, lump formation was reduced with 80% after 30 days.
Clinical study using turmeric
In clinical studies turmeric was examined in 16 chronic smokers for its antimutagenic effect. Turmeric was given for 30 days to chronic smokers at the dose of 1.5 g/day and it was found that urinary excretion of mutagens in these smokers was reduced significantly. These show that dietary turmeric shows the antimutagenic effect.
It was found that turmeric was useful in the treatment of peptic ulcer. . In the second phase, turmeric-filled capsules were administered to 42 patients with peptic ulcers at a dose of 2 capsules five times daily. After four weeks, it was found to be successful in 48% of cases. After 12 weeks, the ulcer-free cases raised to 76%.
Future perspective
The herbal medicine’s uses for treating various ailments will continue to be used in the future. The Source of modern medicine is natural products about 70% of modern medicines are obtained from a natural product.
Most medicinal plants have not yet been studied in medicinal practice, and they may be effective in treating diseases. One of the challenges that medicinal plants face is that most types of medicinal plants are decreasing day by day due to improper use of resources. According to the International Union for Conservation of Nature, 50,000-80000 species of flowering plants are used for pharmaceutical purposes. Of that, 15,000 species are endangered due to habitat destruction and high harvest.
The use of herbal medicine is on the rise in developed and developing countries of the world. People and health care professionals are expressing their concerns about the safety, efficacy, care, and quality of herbal medicine. To alleviate their anxiety, research on herbal medicine is needed not only for great health care but also for commercial purposes. The production and sale of herbal medicine should be regulated formally and legally to ensure its safety, efficacy, and quality.
Herbal medicines are increasing in popularity not only in the cosmetic industry, but also in everyday life for personal health care and well-being. Herbal plants play a major role in the growth process. Undoubtedly, the demand for herbal medicines has increased worldwide.
The bioavailability of curcumin can be increased by formulating the curcumin in two new formulations such as liposome, polyethylene glycol, biopolymer, cellulose, corn oil, and hydrogel.
The aim of improving curcumin’s anti-cancer activity by preparing formulations in which curcumin binds to novel metallic and oxide nanoparticles for easy manipulation to improve activity, conductivity and specificity.
Conclusion
Curcumin is an important pharmaceutical compound derived from turmeric. The demand for curcumin grows daily due to its use in the treatment of a number of diseases. The WHO contributes to promoting the use and sale of herbal medicine practice, as it is culturally acceptable, effective, and accessible. Curcumin is gaining ground in applications in cosmetics, food, pharmaceuticals, PV-sensitive dye technology, and textiles. Herbal medicine is a central component of the world population of about 75-80%. Turmeric (Curcuma longa) is widely used as a spice, coloring agent, cosmetic, and food preservative. It contains a yellow chemical known as curcumin. It has been used in traditional medicine as a home remedy for many ailments, including fever, inflammation, biliary disorders, cough, foot anorexia, diabetes, rheumatism, hepatic diseases, and sinusitis. Curcumin (diferuloylmethane), a yellow bioactive component of turmeric, has been shown to have many forms of biological action. The market size of curcumin worldwide was priced at USD 58.4 million in 2019 and is looking forward to witnessing a CAGR of 12.7% in the forecast period. Research published in 2018 talks about how it is being used to treat metabolic syndrome. It has a special role and effect on the gut microbiome as the curcuminoid found in turmeric has a larger role in influencing the bacterial population.
Acknowledgement
The authors would like to express heartfelt gratitude to Dr. Vishwanath Karad’s management at MIT World Peace University for providing database access and assisting us in writing this review.
Conflict of Interest
The authors declared no conflict of interest in the manuscript.
Funding Sources
There is no funding Source.
References
- Manish Gunjan, Thein Win Naing, Rahul Singh Saini, Dr.Amaluddin Bin Ahmad , Dr.Jegathambigairameshwar Naidu , Ishab Kumar, Marketing Trends & Future Prospects Of Herbal Medicine In The Treatment Of Various Disease, World Journal Of Pharmaceutical Research,2015; Volume 4, No.9, 132-155.
- Guan Wang, 2019, Market Analysis Of “Herbal, Traditional & Alternative Medicine, Asian Journal of Plant Science & Research, 2019; Volume 9, No.5.
- Sanjay Sharma, Current Status of Herbal Product: Regulatory Overview, J Pharm Bioalliedsci 2015, 293–296. Doi: 10.4103/0975-7406.168030
CrossRef - Bodhisattwamaiti, Nagori B.P., Rambir Singh, Pragati Kumar and Nishantupadhyay, Jsjadoun Science, Recent Trends In Herbal Drugs: A Review, Int. J. Drug Res. Tech. 2011, Volume 1 (1), Pp.17-25.
- Kanalajagadeeswara Reddy, Maggie Jo Alex, Alex Thomas, 2020, Regulations For Herbal Medicine-Worldwide, Pp. 44-48
- Stephen Bent,Herbal Medicine In The United States: Review Of Efficacy, Safety, And Regulation, J Gen Intern Med. 2008 Jun; 23(6): 854–859.Doi: 10.1007/S11606-008-0632-Y
CrossRef - jay Sharma,Current Status Of Herbal Product: Regulatory Overview, Journal Of Pharmacy &Bioallied Sciences, 2015; Vol-7, Issue.4, Pp. 293-296.
CrossRef - Yoheikatsuyama, Tomoko Kita, Sueharuhorinouchi., “Identification And Characterization Of Multiple Curcumin Synthases From The Herb Curcuma Longa” , Elsevier, 2009; Volume 583, No.17, Pp. 2799-2803. Doi:10.1016/J.Febslet.2009.07.029
CrossRef - Sahdeo Prasad and Bharat B. Aggarwal, Herbal Medicine: Biomolecular and Clinical Aspects, 2nd Edition.
- Dr Abdul Ghani, Herbal Medicines: Present Status, Future Prospects,Http://Www.Pharmabiz.Com/Newsdetails.Aspx?Aid=78355&Sid=21
- Gauravsharma, Nitikathakur,Curcumin – The Healing Herb: Properties And Future Prospective, Asian Journal Of Pharmaceutical And Clinical Research, 2020; Volume 13, Issue. 2. Doi Https://Doi.Org/10.22159/Ajpcr.2020.V13i2.28929
CrossRef - Shi‑Lin Chen, Hua Yu2,, Hong‑Mei Luo , Qiong Wu2,, Chun‑Fang Li And André Steinmetz, Conservation And Sustainable Use Of Medicinal Plants: Problems, Progress, And Prospects, Chen Et Al. Chin Med (2016) 11:37, Doi 10.1186/S13020-016-0108-7
CrossRef - Rudra Prasad Giri1, Dr.Ajit K Gangawane2, Dr.Suchetaghorai Giri3, Regulation on Herbal Product Used As Medicine around the World: A Review, 2018; Volume: 05 Issue: 10.
- Jadhavpriyanka M1 * and Kshirsagarnilima A2. 2015. Innovative Approach for Classification Of Traditional System Of Medicine. Http://Dx.Doi.Org/10.4172/2329-6836.1000191
CrossRef - Harshal Ashok Pawar*, Amitjagannathgavasane and Pritam Dinesh Choudhary. A Novel And Simple Approach For Extraction And Isolation Of Curcuminoids From Turmeric Rhizomes, 2018; Doi: 10.4172/2329-6836.1000300
- India Herbals Products Market by Category (Personal Care & Cosmetics and Healthcare), By Distribution Channel (Traditional & Departmental Stores, Supermarkets/Hypermarkets, Online, and Others), By Region, Competition, Forecast & Opportunities. 2019.
- Https://Www.Techsciresearch.Com/Report/India-Herbals-Products-Market/4368.Html (Accessed 4 February 2021).
- Herbal Medicines: Overview on Regulations in India and South Africa, 2017; Volume 6, Issue 8, 690-698. Doi: 10.20959/Wjpr20178-9091.
CrossRef - Kanala Jagdeeshwara Reddy, Alex Thomas (Ed), Maggie Jo Alex, Regulations For Herbal Medicines- Worldwide, Lap Lambert Academic Publication, July 2020 Doi: : Https://Www.Researchgate.Net/Publication/342945459
- Frank Xiaoqing Liu, Ms, Phd, And J. Warren Salmon, Phd, Herbal Medicine Regulation In China, Germany, And The United States, Tk Publications, Vol. 9, No. 5, Oct/Nov 2010
- Anupama Singh , Vikas Anand Saharan , Vandanakharb And Anil Bhandari, Current Status Of Regulations Of Herbal Medicines In Europe, United States And India, Journal Of Naturaconscientia, Vol.2, Issue 3, May 2011doi: Http://Www.Jonc.In/
- Dr Xiaorui Zhang, Regulatory Situation of Herbal Medicines A Worldwide Review Guidelines for the Appropriate Use of Herbal Medicines. Who Regional Publications, Western Pacific Series No. World Health Organization, Regional Office for the Western Pacific, Manila. 1998.
- General Guidelines for Methodologies on Research and Evaluation of Traditional Medicine. Who/Edm/Trm/2000.1., World Health Organization, Geneva. 2000.
- Traditional Medicine. Fact Sheet N°134 December 2008 Accessed On 20th April Available At Http://Www.Who.Int/ Mediacentre/Factsheet S/Fs134/En/ (Accessed On 20th April 2021). World Health Organization, Geneva, 2008.
- Kumar V. Herbal Medicines: Overview on Regulations in India And South Africa. World Journal of Pharmaceutical Research.2017.6 (8):690-698.
CrossRef - Liu Fx, Salmon J W. Herbal Medicine Regulation In China, Germany, And The United States. Integrative Medicine.2010.9(5)
- World Health Organization. Regulatory Situation of Herbal Medicines—A Worldwide Review. Who Traditional Medicine Programme; 1998.







