Manuscript accepted on :12-09-2022
Published online on: 20-09-2022
Plagiarism Check: Yes
Reviewed by: Dr. Francisco Solano, Dr. Daya Shankar Gautam
Second Review by: Dr. Grigorios Kyriakopoulos
Final Approval by: Dr. Anton R Kiselev
Risha Catra Pradhany1 , Ferbian Milas Siswanto2*
, Ferbian Milas Siswanto2* , Hendro Sukoco3
, Hendro Sukoco3 , I Nyoman Suarsana4
, I Nyoman Suarsana4 and I Gusti Ayu Agung Suartini4
and I Gusti Ayu Agung Suartini4
1Department of Animal Husbandry, Politeknik Pertanian Negeri Pangkajene Kepulauan, Pangkep 90652, Sulawesi Selatan, Indonesia.
2Department of Biotechnology, Dhyana Pura University, Badung 80361, Bali, Indonesia
3Department of Animal Husbandry and Fisheries, Universitas Sulawesi Barat, Majene 91412, Sulawesi Barat, Indonesia.
4Biochemical Laboratory, Faculty of Veterinary Medicine, Udayana University, Denpasar 80234, Bali, Indonesia.
Corresponding Author Email: ferbianms@undhirabali.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2514
Abstract
Deep-frying oil is a source of free radicals that causes oxidative stress in the body and lead to chronic and degenerative diseases, including non-alcoholic fatty liver (NAFLD). Steatosis, or fatty liver, is one of NAFLD and is a common result of moderate to severe hepatocellular insult. L-Carnitine involves in the metabolism of fat and has a potential role as an antioxidant. In the present study, we aimed to elucidate the role of L-Carnitine in deep-frying oil-treated rats. We equally divided thirty-six male Wistar albino rats into three groups: the group of rats without any treatment (control group), the group of rats treated with deep-frying oil of 0.42 ml/rat/day (negative control group), and the group of rats co-treated with deep-frying oil of 0.42 ml/rat/day and L-Carnitine of 30 mg/kg/day (treatment group). After three weeks of experimental treatment, we found that the deep-frying oil treatment in negative control group caused a significant elevation in the number of hepatic steatosis, hepatic TG content and serum ALT and AST accompanied by increased MDA levels, suggesting the oxidative stress-induced fatty liver. The treatment of L-Carnitine reduced the number of hepatic steatosis and TG content, as well as serum ALT and AST levels. To test the involvement of antioxidant activity of L-Carnitine to its beneficial effects on the development of fatty liver, we observed the expression of endogenous antioxidant. We found that the expression of hepatic SOD, CAT, and GPx were up-regulated by L-Carnitine, followed by a concomitant depletion of MDA levels. In general, this study suggests that L-Carnitine prevents the development of hepatic steatosis and oxidative damage, as well as improves the hepatic antioxidant defenses.
Keywords
Antioxidant; Deep-Frying Oil; L-Carnitine; Oxidative Stress; Steatosis
Download this article as:| Copy the following to cite this article: Pradhany R. C, Siswanto F. M, Sukoco H, Suarsana I. N, Suartini I. G. A. A. L-carnitine Prevents Hepatic Steatosis in Deep-Frying Oil-Treated Rat. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Pradhany R. C, Siswanto F. M, Sukoco H, Suarsana I. N, Suartini I. G. A. A. L-carnitine Prevents Hepatic Steatosis in Deep-Frying Oil-Treated Rat. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3UpVs6i |
Introduction
Indonesian people in general, are fond of fried foods using a deep-frying process 1. During the deep-frying process, various degradation reactions (hydrolysis, thermal oxidation, polymerization, etc) of cooking oil take place and produce trans fatty acids 2. Studies in experimental animals and humans indicated that trans fatty acids raise blood low-density lipoproteins (LDL), triglycerides, and insulin and decrease high-density lipoproteins (HDL) 3. In addition, deep-frying oil can form free radicals 4. It is well known that the free radicals lead to oxidative stress, which causes damage to DNA, protein, lipid peroxidation, and cell membrane damage. Oxidative stress can accelerate aging and cause arising chronic and degenerative diseases 5. Nonalcoholic fatty liver disease (NAFLD) is one of the degenerative diseases with the risk increased by aging and frequent intake of deep-fried food. NAFLD is a spectrum of conditions caused by a build-up of fat in the liver, including non-alcoholic steatohepatitis (NASH) and steatosis 6. The prevalence of steatosis increases with aging, with the highest prevalence in those over 40 years in males and over 60 years in females 7. In addition, the pathogenesis of steatosis is worsened by an unhealthy lifestyle, such as a high-fat diet and a high-deep-frying oil-containing diet 1,8,9.
Chronic insult of the free radicals leads to impairment of antioxidant defense and decreases endogenous antioxidant activity such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) 10,11. In addition, free radicals caused by deep-frying oil, directly stimulate lipid peroxidation on the cell membrane of hepatocytes which can be detected by the elevation of malondialdehyde (MDA), thereby causing intracellular enzymes such as plasma aspartate transaminase (AST) and aminotransaminase alanine (ALT) to be secreted into the bloodstream 12,13.
In order to ameliorate the negative effects of high consumption of deep-fried foods, various strategies have been extensively studied, one of which is by the additional consumption of antioxidants. L-carnitine is an amino acid derivative produced in the brain, liver, and kidneys with a potent antioxidant and protects various tissues including liver from oxidative damage 14,15. It also involves in the metabolism of fat and facilitates the oxidation of fat by regulating the mitochondrial matrix sequestration of long-chain fatty acids to be processed in beta-oxidation pathway 16. Study found that the treatment of L-Carnitine alongside nicotinamide riboside increases fat metabolism; hence, reducing body weight, total body fat content, and abdominal fat mass 17. Furthermore, the research found that L-Carnitine reduces plasma lipoprotein concentrations as a risk factor for cardiovascular diseases 18. However, the biological role and physiological implication of L-Carnitine supplementation to prevent the development and progression of steatosis-induced deep-frying oil, as well as in enhancing hepatic antioxidant defense, is yet to be proved.
Materials and methods
Experimental design
Thirty-six experimentally naïve rats, male, Wistar strain, 3-4 months old, weighing 193 ± 12 g at the beginning of the experiment, were used as subjects. Rats were housed under with ad libitum source of food and water throughout the experiment. Rats were randomly divided into three groups: control untreated (C) group (n= 12, without any treatment), negative placebo-control (NC) group (n= 12, deep-frying oil of 0.42 ml/rat/day for three weeks) and L-Carnitine treatment (T) group (n= 12, deep-frying oil of 0.42 ml/rat/day, and L-Carnitine dose of 30 mg/kg/day for three weeks). The deep-frying oil used in the present study was a fresh palm oil after the process at 205±5°C for four six-hour periods. L-Carnitine was obtained from Sigma-Aldrich (St. Louis, MO). The chemical was given using the forced-feeding method directly to the stomach of the rat. All experiments were conducted in accordance with guidelines on the welfare of experimental animals and with the approval of the Faculty of Veterinary Medicine Committee for Animal Experimentation, Udayana University.
Sample collection
At the end of the study, animals were anesthetized with ketamine (75 mg/kg) and xylazine (10 mg/kg). Peripheral blood was the collected using a sterile hematocrit tube from the retro-orbital plexus, allowed to clot on the ice, centrifuged at 3000 rpm for 10 min, and the serum was collected and stored at -20°C for further AST, ALT, and MDA analysis. The necropsy was performed afterward using the previously reported method with minor adaptation19. The liver tissue was collected and fixed with a 10% neutral buffered formalin (NBF) for histopathology preparation or with RNAlater Stabilization Solution for the examination of SOD, CAT, and GPx mRNA expression.
Histopathology preparation method
The liver was immersed in a fixative solution for 24 hours, sliced and put into a box of a cassette to be further processed in a tissue processor machine. In this box, the tissues were immersed in a solution of 70%, 80%, 90%, and 96% alcohol, and two toluene solutions for every 2 hours. The tissue was then inserted into liquid paraffin in 56°C for 2 hours followed by blocking. Tissue slicing was done by using a microtome machine with a thickness of around 4-5 microns in paraffin blocks. The excised tissue developed on the water in a water bath at a temperature of 37°C which was then captured with an object glass and was dried at room temperature. Paraffin sections were then stained with hematoxylin-eosin. Liver histology was then assessed using a binocular light microscope (Olympus BX50, Hamburg, Germany).
Biochemical analysis
Hepatic triglyceride contents were measured from 50 mg of liver tissue lysed in 1 ml of lysis buffer. The supernatants of the lysate were analyzed using assay kit F001-2 (Nanjing Jiancheng Biological Engineering Institute, Nanjing, China). Plasma AST and ALT level were measured by enzymatic kinetic UV assay and a kinetic colorimetric assay (Roche Diagnostics, Mannheim) following the manufacturer’s instructions. MDA was measured using the thiobarbituric acid reactive substances (TBRAS) method. For hepatic MDA lever, the liver tissue was prepared as follow: one gram of liver tissue was washed with 0.9% NaCl, homogenized in 1.15% KCl, and centrifuged for 10 min at 1000 g. The supernatant was used to measure liver MDA level. The working suspension was used to measure the absorbance at 525 nm for excitation and 547 nm for emission. Hepatic MDA levels were normalized to the protein content measured using the Lowry’s method.
RNA extraction and real time quantitative PCR
Total RNA was isolated from liver tissue using Trizol (Roche, Mannheim, Germany) in accordance with manufacturer’s instructions and was converted to cDNA using a previously described method5. The PCR primer sequences used are as follows: Rat GAPDH, 5′-CAACTACATGGTTTACATGTTC-3′ (forward, Fw) and 5′-GCCAGTGGACTCCACGAC-3′ (reverse, Rv); rat SOD1, 5′-AATGTGTCCATTGAAGATCGTGTGA-3′ (Fw) and 5′-GCTTCCAGCATTTCCAGTCTTTGTA-3′ (Rv); rat GPx1, 5′-GAAGCCACGTGATCTCAGCC-3′ (Fw) and 5′-CTTGGGGTCGGTCATGAGCGC-3′ (Rv); rat Cat, 5′-CCCAAGCAACATGCCCCCTGGCAT-3′ (Fw) and 5′-AAGAGCCTGGACTCGGGCCCCG–3′ (Rv).
Statistical analysis
Data are presented as mean ± SD. Statistical significance were calculated by one-way ANOVA followed by Tukey post-hoc test.
Results
The histopathological examination of the hepatic tissues stained with H&E revealed that the three-week deep-frying oil treatment increased the number of steatosis and that the supplementation of L-Carnitine prevented the formation of this steatosis (Fig. 1). Quantification of the number of steatosis showed that the average hepatic steatosis in the C group was 6.26 ± 1.66 lipid droplets/field of view, the NC group was 48.13 ± 9.41 lipid droplets/field of view, while in the T group was 12.67 ± 2.21 lipid droplets/field of view (p <0.001). Next, we examined the hepatic TG content due to its role in the pathogenesis of NAFLD. Expectedly, the hepatic TG content was elevated by deep-frying oil treatment as compared to the untreated rats, and the supplementation of L-Carnitine in the T group ameliorate increasing hepatic TG content (Fig. 2).
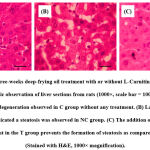 |
Figure 1: Effect of three-weeks deep-frying oil treatment with or without L-Carnitine supplementation on steatosis. |
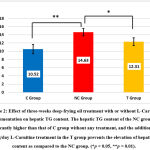 |
Figure 2: Effect of three-weeks deep-frying oil treatment with or without L-Carnitine supplementation on hepatic TG content. |
Next, oxidative stress and the impairment of the antioxidant system are known to play a crucial role in the pathogenesis and severity of NAFLD/NASH. This led us to examine the expression of endogenous antioxidant. Consistent with the widely approved theory, the deep-frying oil treatment caused a depletion on the expression of SOD, CAT and GPx; and that the supplementation protects the impairment of these antioxidant systems (Fig. 3). The improvement of antioxidant status was followed by the prevention of oxidative damage to the hepatocyte. As listed in Table 1., serum AST and ALT which are the marker of liver function were decreased in the NC group as compared to the C group, and lower in the T group as compared to the NC group. Similar trends were also observed for both serum and hepatic MDA, which is the marker of oxidative stress-induced lipid peroxidation. Together, these data suggesting that L-Carnitine prevent the deep-frying oil treatment-induced oxidative stress and damage.
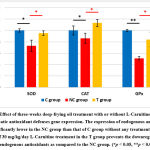 |
Figure 3: Effect of three-weeks deep-frying oil treatment with or without L-Carnitine treatment on the hepatic antioxidant defenses gene expression. |
Table 1: Effects of three-weeks deep-frying oil treatment with or without L-Carnitine supplementation on hepatic function and oxidative damage.
| C Group | NC Group | T Group | |
| Serum AST level (U/L) | 93.47 ± 8.93 | 187.83 ± 19.98** | 112.07 ± 29.44†† |
| Serum ALT level (U/L) | 60.43 ± 11.87 | 98.17 ±13.94** | 72.29 ± 12.66†† |
| Serum MDA (nmol/ml) | 6.54 ± 0.61 | 12.14 ± 1.87** | 15.74 ± 2.34†† |
| Hepatic MDA (nmol/gr.prot) | 487.32 ± 35.86 | 552.68 ± 21.76** | 503.77 ± 12.43†† |
Values are mean ± SD, *p<0.05 and **p<0.01 of C vs NC group, †p<0.05 and ††p<0.01 of NC vs T group.
Discussion
Studies showed that deep-frying oil causes lipid peroxidation, moderate to severe cytoplasmic vacuolization, hepatocyte hypertrophy, and necroinflammation that indicate the initiation of NAFLD and progression of the disease 20,21. Here, similarly, we also showed that the three-weeks deep-frying oil treatment increased the number of steatosis, induced oxidative stress marked by the depletion of endogenous antioxidant expression, and causing oxidative damage as shown by the elevation of liver function marker (AST and ALT) as well as lipid peroxidation marker (hepatic and systemic MDA). The treatment of L-Carnitine in the present study can prevent the process of fatty liver, as demonstrated by our data showing that the group of rats with L-Carnitine exhibited a significantly lower number of hepatic steatosis than that of negative control group. The L-Carnitine treatment also prevented oxidative stress by improving the expression of antioxidant and decrease the damage to the liver. This effect is might be mediated by various mechanisms, including reducing lipid levels, preventing oxidative stress, and modulating the inflammatory response in the liver 22,23.
Several studies have shown that L-Carnitine improves lipid profiles. For instance, Malaguarnera et al. 24 observed that Ox-LDL, TBARS, and conjugated diene were reduced after 12 weeks of treatment compared to baseline. Similarly, another study found that L-Carnitine treatment for 30 days in the rabbit model of cholesterol-induced atherosclerosis can reduce LDL and increase HDL levels 25. Until now, the mechanisms by which L-Carnitine regulates lipid profiles are not known but may involve the improvement of fatty acids transport into the mitochondria for metabolism and energy. Mobilization, oxidation, and excretion of fatty acids also increased with L-Carnitine supplementation 26,27.
Apart from increasing levels of LDL and VLDL and decreasing systemic HDL, decreased fatty acid oxidation in the liver is also an important process that causes fat accumulation due to disturbances in mitochondrial β-oxidation in the hepatocyte. The oxidation system in the mitochondria and peroxisomes as well as the ω-oxidation in the microsomes are controlled by peroxisome proliferator-activated receptor alpha (PPAR-α) 28,29. The epidemiologic study revealed that the expression of hepatic PPARα is significantly lower in the patients with NAFLD 30. Study showed that L-Carnitine can increase the protein level of PPAR-α in the liver, therefore enhances fatty oxidation 31. Furthermore, the results of this study are also supported by various studies showing that L-carnitine supplementation led to the restoration of liver lipid metabolism in dyslipidemic animals 32.
We also found that L-Carnitine can prevent oxidative stress by ameliorating the effect of deep-frying oil on the depletion of endogenous antioxidants mRNA. Our study supports previous finding by Montesano et al. 15 who reported that beneficial effects of L-Carnitine on the prevention of hepatic steatosis is mediated by inhibition of oxidative stress. Mechanistically, Gülçin et al. 33 and Lee et al. 34 separately reported that L-Carnitine is a good antioxidant that works on free radicals including 2,2-diphenyl-1-picrylhydrazyl (DPPH), •O2– (superoxide anion) radical, H2O2 (hydrogen peroxide). In addition to its direct antioxidant activity, L-Carnitine also has been proved to increase the expression and activity of endogenous antioxidants through the activation of PPAR-α 35, nuclear factor erythroid 2 (NFE2)-related factor 2 (Nrf2) 36, and Forkhead box O3 (FOXO3) 37, both in vitro and in vivo.
Next, we showed here that oral L-carnitine can reduce levels of ALT and AST as well as decrease the MDA levels which are biomarkers of liver function deterioration and oxidative damage respectively; hence, preventing the progression of NAFL to NASH caused by deep-frying oil. Interestingly, we found that both hepatic and serum MDA levels were decreased by the treatment of L-carnitine, suggesting that the antioxidant activity of L-carnitine is systemic. A cross-sectional study revealed that serum MDA is associated with NAFLD and NASH in men but not women, presumably due to the protection of estrogen 38. L-Carnitine has been previously reported to have a protective effect on mitochondria and reduces oxidative stress. It was found that L-Carnitine inhibited hydroperoxide radical production and induction of enzymatic antioxidant system in mitochondria. L-Carnitine also reduces oxidative damage to the mitochondrial DNA 39–41. Inhibition of oxidative stress by L-carnitine, in turn, prevents the peroxidation of hepatic membrane polyunsaturated fatty acids which eventually results in the depletion of hepatic and serum MDA.
Conclusion
In summary, L-Carnitine possesses both an anti-dyslipidemia and antioxidant activity that can protect the liver from the aging phenotype caused by unhealthy food, including deep-frying oil. We demonstrated that L-carnitine prevents the development of steatosis, improves antioxidant defense and inhibits oxidative liver damage. The current research constraints are: 1) the composition of deep-frying oil such as content of unsaturated trans fatty acids and the peroxidized acids that lead to NASH is unclear; 2) the intracellular direct target of L-Carnitine remain unknown, therefore the mechanistic explanation of L-carnitine beneficial effects remain partial; 3) various factors involved in lipid metabolism (i.e., PPAR-α, CPT1, and acyl-CoA oxidase) and oxidative stress response (i.e., Nrf2 and FOXO3) were not measured; 4) the findings in this study are correlative in nature and the causative relationships were not directly examined. To this end, further study is required to: 1) profile the composition of deep-frying oil, 2) elucidate the intracellular direct target of L-Carnitine, 3) examine the effects of L-carnitine on the factors involved in lipid metabolism and oxidative stress response, and 4) study the causative relationships between these factors and L-carnitine by using genetically-knockout rats. Additionally, further study to examine the beneficial effects of L-carnitine consumption epidemiologically in the context of prevention and healing of diseases is necessary.
Conflict of interests
The authors declare that there are no conflicts of interest.
Funding Sources
There is no funding Sources.
Author Contributions
I.N.S., I.G.A.A.S., and F.M.S. designed the study; R.C.P. and H.S. performed experiments and prepared the histopathology analysis; F.M.S. performed biochemical analysis, quantitative PCR, data analyses and prepared the manuscript; I.N.S., I.G.A.A.S., and F.M.S. reviewed the manuscript; all authors approved the final version of the manuscript.
References
- Widhiantara, I. G., Permatasari, P., Rosiana, I. W., Sutirtayasa, I. W. P. & Siswanto, F. M. Role of HIF-1, Siah-1 and SKN-1 in Inducing Adiposity for Caenorhabditis elegans under Hypoxic Conditions. Indones. Biomed. J. 12, 51–6 (2020).
- Bhat, S., Maganja, D., Huang, L., Wu, J. H. Y. & Marklund, M. Influence of Heating during Cooking on Trans Fatty Acid Content of Edible Oils: A Systematic Review and Meta-Analysis. Nutrients 14, 1489 (2022).
- Oteng, A.-B. & Kersten, S. Mechanisms of Action of trans Fatty Acids. Adv. Nutr. 11, 697–708 (2020).
- Liu, Y., Li, J., Cheng, Y. & Liu, Y. Effect of frying oils’ fatty acid profile on quality, free radical and volatiles over deep-frying process: A comparative study using chemometrics. LWT 101, 331–341 (2019).
- Kartiko, B. H. & Siswanto, F. M. Overtraining elevates serum protease level, increases renal p16INK4α gene expression and induces apoptosis in rat kidney. Sport Sci. Health 14, 1–7 (2018).
- Pouwels, S. et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr. Disord. 22, 63 (2022).
- Alqahtani, S. A. & Schattenberg, J. M. NAFLD in the Elderly. Clin. Interv. Aging 16, 1633–1649 (2021).
- Nassir, F., Rector, R. S., Hammoud, G. M. & Ibdah, J. A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. (N. Y). 11, 167–75 (2015).
- Widhiantara, I. G., Arunngam, P. & Siswanto, F. M. Ethanolic Extract of Caesalpinia bonducella f. Seed Ameliorates Diabetes Phenotype of Streptozotocin- Nicotinamide-Induced Type 2 Diabetes Rat. Biomed. Pharmacol. J. 11, 1127–1133 (2018).
- Samarghandian, S., Afshari, R. & Farkhondeh, T. Effect of long-term treatment of morphine on enzymes, oxidative stress indices and antioxidant status in male rat liver. Int. J. Clin. Exp. Med. 7, 1449–53 (2014).
- Nyandra, M., Kartiko, B. H., Arunngam, P., Pangkahila, A. & Siswanto, F. M. Overtraining Induces Oxidative Stress Mediated Renal Damage in Male Wistar Rats. Transylvanian Rev. 26, 7659–7666 (2018).
- Guo, Q. et al. Taurine Attenuates Oxidized Fish Oil-Induced Oxidative Stress and Lipid Metabolism Disorder in Mice. Antioxidants 11, 1391 (2022).
- Li, Y. et al. Effects of polar compounds in fried palm oil on liver lipid metabolism in C57 mice. J. Food Sci. 85, 1915–1923 (2020).
- Ribas, G. S., Vargas, C. R. & Wajner, M. L-carnitine supplementation as a potential antioxidant therapy for inherited neurometabolic disorders. Gene 533, 469–476 (2014).
- Montesano, A. et al. L-Carnitine counteracts in vitro fructose-induced hepatic steatosis through targeting oxidative stress markers. J. Endocrinol. Invest. 43, 493–503 (2020).
- Sawicka, A. K., Renzi, G. & Olek, R. A. The bright and the dark sides of L-carnitine supplementation: a systematic review. J. Int. Soc. Sports Nutr. 17, (2020).
- Salic, K. et al. Combined Treatment with L-Carnitine and Nicotinamide Riboside Improves Hepatic Metabolism and Attenuates Obesity and Liver Steatosis. Int. J. Mol. Sci. 20, 4359 (2019).
- Serban, M.-C. et al. Impact of L-carnitine on plasma lipoprotein(a) concentrations: A systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 6, 19188 (2016).
- Flecknell, P. et al. Preanesthesia, Anesthesia, Analgesia, and Euthanasia. in Laboratory Animal Medicine 1135–1200 (Elsevier, 2015). doi:10.1016/B978-0-12-409527-4.00024-9
- Estévez-Vázquez, O. et al. Fat: Quality, or Quantity? What Matters Most for the Progression of Metabolic Associated Fatty Liver Disease (MAFLD). Biomedicines 9, 1289 (2021).
- Uno, K. et al. Influence of trans-fatty acids on non-alcoholic steatohepatitis with hepatic fibrosis induced by a choline-deficient, methionine-lowered, L-amino acid-defined diet in male Harlan Sprague Dawley rats. Fundam. Toxicol. Sci. 8, 221–228 (2021).
- Fathizadeh, H. et al. The effects of L-carnitine supplementation on indicators of inflammation and oxidative stress: a systematic review and meta-analysis of randomized controlled trials. J. Diabetes Metab. Disord. 19, 1879–1894 (2020).
- Emran, T. et al. L-carnitine protects cardiac damage by reducing oxidative stress and inflammatory response via inhibition of tumor necrosis factor-alpha and interleukin-1beta against isoproterenol-induced myocardial infarction. Biomed. Pharmacother. 143, 112139 (2021).
- Malaguarnera, M. et al. L-Carnitine Supplementation to Diet: A New Tool in Treatment of Nonalcoholic Steatohepatitis—A Randomized and Controlled Clinical Trial. Am. J. Gastroenterol. 105, 1338–1345 (2010).
- Bahrami-Bukani, M. et al. Evaluation of L-carnitine in an animal model of cholesterol induced atherosclerosis. Trop. J. Pharm. Res. 18, 1661–1666 (2021).
- Longo, N., Frigeni, M. & Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta – Mol. Cell Res. 1863, 2422–2435 (2016).
- Odo, S., Tanabe, K., Yohda, M. & Yamauchi, M. Liver-Oriented Acute Metabolic Effects of A Low Dose of L-Carnitine under Fat-Mobilizing Conditions: Pilot Human Clinical Trial. J. Nutr. Sci. Vitaminol. (Tokyo). 66, 136–149 (2020).
- Han, L., Shen, W.-J., Bittner, S., Kraemer, F. B. & Azhar, S. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part I: PPAR-α. Future Cardiol. 13, 259–278 (2017).
- Todisco, S. et al. PPAR Alpha as a Metabolic Modulator of the Liver: Role in the Pathogenesis of Nonalcoholic Steatohepatitis (NASH). Biology (Basel). 11, 792 (2022).
- Montagner, A. et al. Liver PPARα is crucial for whole-body fatty acid homeostasis and is protective against NAFLD. Gut 65, 1202–1214 (2016).
- Jiang, F. et al. L-carnitine ameliorates the liver inflammatory response by regulating carnitine palmitoyltransferase I-dependent PPARγ signaling. Mol. Med. Rep. 13, 1320–1328 (2016).
- Savic, D., Hodson, L., Neubauer, S. & Pavlides, M. The Importance of the Fatty Acid Transporter L-Carnitine in Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients 12, 2178 (2020).
- Gülçin, İ. Antioxidant and antiradical activities of l-carnitine. Life Sci. 78, 803–811 (2006).
- Lee, B.-J., Lin, J.-S., Lin, Y.-C. & Lin, P.-T. Effects of L-carnitine supplementation on oxidative stress and antioxidant enzymes activities in patients with coronary artery disease: a randomized, placebo-controlled trial. Nutr. J. 13, 79 (2014).
- Li, J.-L., Wang, Q.-Y., Luan, H.-Y., Kang, Z.-C. & Wang, C.-B. Effects of L-carnitine against oxidative stress in human hepatocytes: involvement of peroxisome proliferator-activated receptor alpha. J. Biomed. Sci. 19, 32 (2012).
- Li, J. et al. L-carnitine protects human hepatocytes from oxidative stress-induced toxicity through Akt-mediated activation of Nrf2 signaling pathway. Can. J. Physiol. Pharmacol. 94, 517–525 (2016).
- Wu, C. et al. L-carnitine ameliorates the muscle wasting of cancer cachexia through the AKT/FOXO3a/MaFbx axis. Nutr. Metab. (Lond). 18, 98 (2021).
- Zelber-Sagi, S. et al. Serum Malondialdehyde is Associated with Non-Alcoholic Fatty Liver and Related Liver Damage Differentially in Men and Women. Antioxidants 9, 578 (2020).
- Terruzzi, I. et al. L-Carnitine Reduces Oxidative Stress and Promotes Cells Differentiation and Bone Matrix Proteins Expression in Human Osteoblast-Like Cells. Biomed Res. Int. 2019, 1–13 (2019).
- Lee, Y.-C. G. et al. L-Carnitine reduces reactive oxygen species/endoplasmic reticulum stress and maintains mitochondrial function during autophagy-mediated cell apoptosis in perfluorooctanesulfonate-treated renal tubular cells. Sci. Rep. 12, 4673 (2022).
- Virmani, M. A. & Cirulli, M. The Role of l-Carnitine in Mitochondria, Prevention of Metabolic Inflexibility and Disease Initiation. Int. J. Mol. Sci. 23, 2717 (2022).







