Manuscript accepted on :17-09-2022
Published online on: 26-09-2022
Plagiarism Check: Yes
Reviewed by: Dr. Anish Khan
Second Review by: Dr. Nicolas Padilla
Final Approval by: Dr. H Fai Poon
Abdeljaouad Ez-zahir1 , Ali Lahna1
, Ali Lahna1 , Farida Marnissi2, Mounia Oudghiri1*
, Farida Marnissi2, Mounia Oudghiri1* , Abdallah Naya1
, Abdallah Naya1
1Laboratory of Immunology and Biodiversity, Department of Biology, Faculty of Sciences Ain Chock, Hassan II University of Casablanca B.P. 5366, Maarif, Casablanca, Morocco 2Pathological anatomy laboratory, Ibn Rochd Hospital, Lahcen El Arjoun Street, Casablanca, Morocco
Corresponding Author E-mail: mouniaoudghiri@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2500
Abstract
Objectives: Psoriasis is a chronic skin inflammation disease, which is requires prolonged treatment, thereby interfering with the side effects of synthetic drugs. Traditional herbal medicine, and recent plant-based drugs, have been proven beneficial in reducing these side effects of synthetic drugs. Ammi visnaga L. species contains 2 furanochromones (khelline and visnagine) with a similar structure than psoralens that possess antipsoriatic activity. The objective of the present study was to verify the influence of extraction solvent on these two furanochromone content of Ammi visnaga and to study its immunomodulatory and antipsoriatic effect in vivo. Materials and Methods: In the present study, khellin and visnagin contents were compared between aqueous, hydroethanolic and ethyl acetate extracts of Ammi visnaga L. by highperformance liquid chromatography (HPLC), and their immunomodulating and antipsoriatic effects were studied for the hydroethanolic extract, in vivo, by hemagglutination test after immunization using human erythrocytes and by Psoriasis-like dermatitis induced using ultraviolet-C (UV-C) irradiations of two hours, during 3 successive days or by application of 2 a formaldehyde and Complete Freund adjuvant (CFA) mixture (1:10 ratio) during 3 successive days. Results: Ethanol at 60% showed the best results in the extraction of furanochromones (khellin and visnagin), followed by ethyl acetate and then by water. This hydroethanolic extract at 100 mg/kg showed an immunostimulating effect of the humoral response, by increasing the value of the hemagglutination antibody (HA) titer. The same extract, at a dose of 300 - 600 mg/kg orally or 2-4% topically, has a considerable antipsoriatic effect, reducing the psoriatic severity score (erythema and squaling), the epidermal thickness and the leukocytic infiltration. Conclusion: the 60% hydroethanol extract is better in the extraction of furanochromones from Ammi visnaga. This extract has considerable immunostimulant and antipsoriatic effects.
Keywords
Ammi visnaga L.; Antipsoriatic; Furanochromones; HPLC; Immunomodulatory
Download this article as:| Copy the following to cite this article: Ez-zahir A, Lahna A, Marnissi F, Oudghiri M, Naya A. Immuno-Modulatory, Anti-Psoriatic Effects and Furanochromone (Khellin and Visnagin) Contents of Ammi Visnaga (L.) Hydeoethanolic Extract. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Ez-zahir A, Lahna A, Marnissi F, Oudghiri M, Naya A. Immuno-Modulatory, Anti-Psoriatic Effects and Furanochromone (Khellin and Visnagin) Contents of Ammi Visnaga (L.) Hydeoethanolic Extract. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3DUKAYs |
Introduction
Psoriasis is a common autoimmune disease, which manifests as skin lesions, resulting from hyperproliferation of keratinocytes, in response to a massive infiltration of cytokine-secreting immune cells.1 This chronic disease requires lifelong treatment, which could lead to more serious side effects, in the skin, and even elsewhere, especially for the synthetic drugs. This emphasizes the importance of the search of therapeutic alternatives, which could be from plant origin; as long as these are recognized to be tolerable by the body.2-4 Psoralens are among the many natural substances with a proven antipsoriatic effect in several previous studies. 5 These active principles have a significant structural similarity with the two most abundant furanochromones of Ammi visnaga L., namely khellin and visnagin.6,7 Both furanochromones differentially modulate aryl hydroc arbon rece ptor (AHR) signaling and Cytochrome P450 1A (CYP1A) activity, which play a crucial role in therapy of psoriasis and of other skin diseases.8,9 This may suggest an antipsoriatic effect in this plant, or in one of its active compounds, already shown to be active in vitiligo and psoriasis treatment.10-12
Against this background, and knowing that the qualitative and quantitative chemical composition might vary between extracts;13,14 the present study was conducted to evaluate the two concerned furanochromones (khelline and visnagine) content in different type of Ammi visnaga L. extracts in first and then the antipsoriatic and imunomodulating effects of theses furanochromones in vivo by induction of two type of a psoriasiform-like skin phenotype animal models.
Materials and methods
Animals
The acute dermal toxicity, immunomodulatory and anti-psoriatic studies in vivo, were carried out on young and adult Swiss albino mice (20 – 30g) or Wistar albino rats (150 – 250g). The animals were housed in polypropylene cages under standard conditions (Temperature: 22°C, Relative humidity: 60-70%, Light/dark period: 12 hours, frequent air change by extractor), with free access to drinking water and food. They were obtained from the Faculty of Sciences Ain Chock, Hassan II University; Casablanca. The experiments were conducted in accordance with internationally acceptable guidelines for assessing efficacy of plant-derived drugs, and in vivo tests have been approved by the ethics committee of the Mohammed V University of Rabat/Morocco: clearance N° PR062022JE.
Ammi visnaga L. seed Extracts preparation
Ammi visnaga L. seeds were milled to powder, and then 5 g of powder were soaked in 50 ml of extraction solvent (distilled water, 60% ethanol, or ethyl acetate) and left under magnetic stirring in a water bath at 40 °C for 1 h. After cooling, each crude extract was filtered through a cloth square and then through 0.45 µm Wattman filter paper. The crude filtrate was directly used for the comparative study of khellin and visnagin content. However, for the in vivo studies, this filtrate was concentrated to dryness, using a rotary evaporator, and then the residue was regained in warm water.
Comparative study of khellin and visnagin content by HPLC
The contents of the two main furanochromones, khellin and visnagin, in the aqueous, hydroethanolic and ethyl acetate extracts, prepared from the mature seeds of Ammi visnaga L., were investigated using HPLC, adopting the protocol described by Badr et al, with some adapptation.[15] The crude filtrate of each extract was filtered through a single-use syringe filter of approximately 0.22 µm pore size and then diluted 1:10 in methanol. The HPLC method used was isocratic reverse phase, on a C18 column of 4.6 µm, 250×4 mm. The mobile phase was a mixture of water, methanol and tetrahydrofuran (50: 45: 5, v/v/v), with a debit of 1 ml/min. The injection volume was 20 µl. Quantification was performed at room temperature, with UV detection at 245 nm based on the peak area.
Evaluation of the effect of the Ammi visnaga L. seed hydroalcoholic extract on humoral immune response
Pretreatment and experimental design
The study was carried out on a total of 10 rats, which were divided into two groups of five animals left together for one week for acclimatization. The control group was treated with distilled water orally, while the other group was treated with Ammi visnaga seed hydroalcoholic extract at 100 mg/kg by the same route. This treatment lasted 3 successive days before the sensitization by the antigen (Ag) and then 7 days after.
Sensitisation and treatment
After receiving the Ammi visnaga L. hydroethanolic extract or vehicle for three consecutive days, rats were immunised by injection of a sample of human erythrocytes (HRBCs), prepared as follows: a blood sample was extracted into an EDTA tube and centrifuged at 2500 RPM/10min to remove the plasma, then the pellet was washed three times with 0.9% NaCl solution, and then a 30% (v/v) suspension of red blood cells was prepared in Phosphate Buffered Saline at pH=7.4. Each rat in the tow groups was injected intraperitoneally with 0.1 ml of the red cell suspension.
Evaluation of the production of anti–HRBC antibodies by Hemagglutination assay
According to the protocol described by Pravansha et al,16 seven days after immunisation, blood was collected from all rats in dry tubes by cardiac puncture and left at 37°C for one hour before centrifugation at 2500 RPM to extract the serum. The serum was incubated at 56°C for 30 minutes to inactivate the complement. Simultaneously, a human red blood cell suspension was prepared, in the same manner as that used during the sensitisation phase, but at 1% concentration. The hemagglutination assay was performed in 96-well microplates, so that 50 μl of serum from each rat was placed separately in the first well, while 25 μl of PBS solution were placed in the rest of the wells, then 25 μl of the contents of each well were transferred to the next well, after mixing them well, at a frequency of 36 wells for each serum, starting with the first well containing the serum only. In this way we established a series of 1/2 dilutions well to well. After adding 25 μl of HRBCs suspension at a concentration of 1% to each well, the mixture was incubated at room temperature for 2 hours before reading and determining the last dilution of serum that caused the agglutination of human erythrocytes, so that the inverse of this dilution can be value that was adopted to estimate the effect of the substances to be tested (Hemagglutination antibody (HA) titer) on the production of anti-HRBC antobodies.
Evaluation of the acute dermal toxicity of Ammi visnaga L. seeds hydroalcoholic extract
The acute dermal toxicity of the hydroalcoholic extract of Ammi visnaga L. seeds in mice was tested according to OECD guideline 402.17 Approximately 10% of the body area was carefully shaved at the dorsal portion for nine female mice, which were divided into three groups. 48 hours after hair shaving, mice in two groups were treated once topically with the plant extract at a dose of 1000 mg/kg or 2000 mg/kg (limit dose), while mice in the control group were treated with the vehicle. The treated mice were observed for any signs of toxicity during 14 days.
Induction of a UV-psoriasiform-like skin phenotype animal models and evaluation of the anti-psoriatic activity of AV hydroethanolic extract
To induce an animal model which presents the similar characteristics as psoriasis, on the skin, we have used the protocol described by Kulkarni et al., with some addaptations.18 The last area of the back (about 2.5 x 2. 5 cm) was shaved and then exposed to ultraviolet light (UV-C/253,7nm) for 1 hour on three successive days using a 15-watt lamp after covering the rest of the body with foil paper and attaching their paws around their abdomens to restrain their movement, in such a way that the lamp was positioned at a height of 20 cm from the rats’ backs.
After the appearance of the required symptoms (erythema and scaling), one day after the 3rd irradiation, the animals were randomly divided into four groups of five rats each, in addition to a normal group of the same number, which was not exposed to irradiation. Then the study of the antipsoriatic effect of Ammi visnaga L. (AV) hydroethanol extract was carried out by daily oral gavage, during a period of 14 days, as follows:
Group I: Normal + vehicle (Normal control);
Group II: Psoriasis + vehicle (Psoriasis control);
Group III: Psoriasis + retinoic acid (RA) at 0,5mg/kg (Standard);
Group V: Psoriasis +AV extract at 300 mg/kg;
Group VI: Psoriasis + AV extract at 600 mg/kg.
Clinical examination
Redness / erythema and scaling of psoriatic lesions of all rats were visually assessed by a psoriatic severity index graded from 0 to 4: 0, none; 1, mild; 2, moderate; 3, severe; 4, very severe, and then the results obtained were used to calculate the cumulative psoriatic severity index (CPSI).
Histopathological examination
The rats were euthanized at the end of the treatment period and skin fragments from the treated area were removed and preserved in 10% formalin for histological sections (5 µm) with hematoxylin-eosin staining. The sections were evaluated microscopically for any inflammatory signs characteristic of psoriasis, and the epidermal thickness of all mice was measured at 5 different sites in 5 sections for each animal. The percentage of epidermal thickness of all groups compared to the positive control group was calculated.
Induction of a formaldehyde/CFA psoriasis-form-like skin phenotype animal models and evaluation of the anti-psoriatic activity of AV hydroethanolic extract
To induce psoriasis-like dermatitis in mice, an area of 3 cm x 2 cm was shaved on the posterior dorsal part in 20 animals, and then 120 microliters of 1:10 CFA in 10% formaldehyde was applied once a day for three successive days to the shaved part of skin. 19
To evaluate the antipsoriatic activity of the hydroalcoholic extract of Ammi visnaga L., a group of 5 mice was used as a normal control, while the mice previously treated with the CFA/formaldehyde mixture were randomly distributed into four groups of 5 animals. The mice in all groups were treated topically, once a day, for 15 successive days, using a cream (3g of beeswax in 10ml of paraffin oil) as a vehicle for the substances to be tested:
Group I: Normal + vehicle (Normal);
Group II: Psoriasis + vehicle (Psoriasis control);
Group III: Psoriasis + Retinoic acid (RA) at 0,5% (Standard);
Group V: Psoriasis +AV extract at 2%;
Group VI: Psoriasis + AV extract at 4%.
Clinical examination
The antipsoriatic activity of the tested substances (Ammi visnaga L. extract and retinoic acid) was evaluated every 5 days, based on a psoriatic severity index PSI: 0- none (clear); 1- mild (redness); 2- moderate (redness and erythema); 3- severe (redness, erythema and scaling).
Histopathological examination
idem to the same section above.
Evaluation of the T cell lymphocyte infiltration
The anti-CD3; anti-CD4 and anti-CD8 antibodies were used in immunohistochemical staining with an automate (Dako Autostainer Link 48) on the different tissus sections to evaluate the number of T cell infiltration after the different treatment.
Statistical analysis
Analysis of variance (ANOVA) followed by Dennett’s post hoc test was adopted to compare the results between the different groups. This analysis, as well as the graphics, was performed by Graphpad Prism 7 software and the differences were considered statistically significant at p <0.05. All data are expressed as mean ± standard deviation (S.D.).
Results
Khellin and visnagin levels in the different types of AV extracts
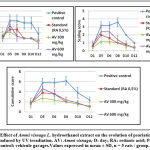
A comparative study of the khellin and visnagin content between the three extracts, aqueous, hydroethanolic and ethyl acetate was carried out by HPLC. The Figure 1 show the chromatograms obtained and table 1 gives a recapitulation of the main results obtained. It can be observed that the two substances of interest existed in the three extracts and that the protocol adopted gave a clear resolution between the two substances to be tested (khelline = peak 4 and visnagine = peak 5), and that no interference was detected between them, nor with other compounds.
 |
Figure 1: Chromatograms of HPLC analysis of furanochromone content (khellin and visnagin). |
Based on the peak air, it can be seen that the 60% hydroethanol extract gave the highest khellin and visnagin efficiency. Compared to this extract, the efficiency of the aqueous extract was 67.26% for khellin and 60.14% for visnagin, while the efficiency of the ethyl acetate extract was 92.08% for khellin and 83.56% for visnagin.
Table 1: HPLC analysis of furanochromone content (khellin and visnagin).
| Substance | Extraction solvent | Solvent temperature | Extract color | Retention time | Peak air | % compared to the most cost-effective extract |
| Khellin | Distilled water | ≃40 °C | brown | 6,760 | 21574172 | 67,26% |
| Ethanol 60% | ≃40 °C | brown | 6,801 | 32077550 | 100% | |
| Ethyl acetate | ≃40 °C | greenish | 6,741 | 29538734 | 92,08% | |
| Visnagin | Distilled water | ≃40 °C | marron | 8,611 | 13756094 | 60,14% |
| Ethanol 60% | ≃40 °C | marron | 8,675 | 22871453 | 100% | |
| Ethyl acetate | ≃40 °C | greenish | 8,599 | 19113400 | 83,56% |
Immunomodulatory effect of AV hydroethanolic extract
The study of the influence of Ammi visnaga L. seed hydroethanolic extract on the humoral immune response was carried out by the hemagglutination test using rats, after immunisation with a suspension of human red blood cells. The Figure 2 illustrates the results of this study, where the inverse of the highest dilution that induces hemagglutination represents the value of the hemagglutinating antibody (HA) titer. We can observe that a 60% hydroethanolic extract of Ammi visnaga L. induces an increase in the HA, compared to the control group. This index reached 853,33±264,39 for the rats treated with the plant extract at 100 mg/kg, statistically different in comparison to the control group (26,66±8,26).
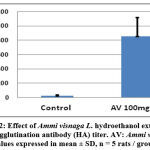 |
Figure 2: Effect of Ammi visnaga L. hydroethanol extract on Haemagglutination antibody (HA) titer. AV: Ammi visnaga. Values expressed in mean ± SD, n = 5 rats / group. |
Acute dermal toxicity of AV hydroethanol extract
For the acute dermal toxicity test, the only topical treatment with the plant extract at a limit dose of 2000 mg/kg did not induce any mortality during the 14 days of observation and the mice showed no apparent signs of toxicity.
Effect of AV hydroethanol extract treatment on the induced UV-psoriasis-like model
Clinical examination
It showed that one hour of UV irradiation at low wattage for three successive days was effective in inducing dermatitis with the same clinical characteristics as described for psoriasis. As shown in Figure 3 the psoriatic severity index increased rapidly in the control group (which had received the vehicle) with both erythema and desquamation. After 8-10 days of the first irradiation, there was a decrease in these scores. We observed that oral gavage with Ammi visnaga L. seed extract at doses of 300 and 600 mg/kg, significantly prevented the increase of psoriatic severity scores for erythema and desquamation compared to control rats. In addition, the decrease in these scores and in the cumulative score was faster than without treatment. The antipsoriatic effect observed in Ammi visnaga L. seed extract was found to be similar to that of a 0.5 mg/kg retinoic acid suspension (standard).
Histopathological examination
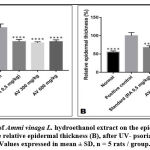
Histological sections (Figure 4 and figure 5) showed a large increase in epidermal thickness in the vehicle-treated control group (188, 82± 16, 18 µm) at the inflammation site compared to normal skin (105, 25±5, 08 µm) (p<0, 0001). These sites were also characterized by leukocyte infiltration and a high level of parakeratosis and dilation of capillary loops. Oral gavage with hydroethanolic extract of Ammi visnaga L. seeds showed a significant reduction in epidermal thickness (121,69±4,41 µm) for 300 mg/kg dose and 127,02±7,63 for 600 mg/kg, which represent, respectively, 64,45% and 67,27% compared to the control group, significantly different compared to the positive control group (p<0,0001). A comparable effect was observed in the standard group, after treatment with a 0.5 mg/kg retinoic acid suspension, epidermal thickness was 127.24±9.58 µm, which is 67.39% compared to the positive control group.
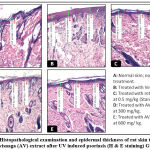 |
Figure 4: Histopathological examination and epidermal thickness of rat skin treated by Ammi visnaga (AV) extract after UV induced psoriasis (H & E staining) G X 100. |
Effect of AV hydroethanol extracts treatment on the induced Formaldehyd/CFA psoriasis-like model
Clinical examination
Repeated topical application of a CFA/formaldehyde mixture to the shaved skin of mice was proven effective in inducing phenotypic symptoms of psoriasis, immediately after the first application redness/erythema appeared, than the scalps after two or three application (Figure 6).
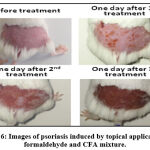 |
Figure 6: Images of psoriasis induced by topical application of formaldehyde and CFA mixture. |
Macroscopic examination of psoriasis severity was performed every three days during the entire treatment period (Table 2). A progressive decrease in psoriasis severity index was observed in groups of mice treated with retinoic acid or Ammi visnaga L. extract. Concerning redness and erythema, the decrease in severity index was statistically significant compared with the control only after 3 days for the standard group (retinoic acid 0.5%; p≤0.001) and after 12 days for the plant extract (AV 2% and AV 4%; p≤0.001). For scaling, the decrease in severity score was statistically significant between retinoic acid or Ammi visnaga L. extract treated groups and the control group after 12 days of treatment only. The cumulative severity score, considering redness/erythema and scaling (Figure 7), was progressively decreased during treatment with Ammi visnaga L. extract or retinoic acid. At the end of the study, this cumulative score was 2.4 for the standard group (AR 0.5%), 2.2 for the group of mice treated with Ammi visnaga L. cream 2% and 2 for those treated with a dose of 4%. These results was highly significant compared to the control group (p≤0.001)
Table 2: Effect of Ammi visnaga L. hydroethanol extract on erythema and scaling severity scores for Formaldehyde/CFA psoriasis induction animals.
|
|
Days of treatment | Control | Standard
(RA 0,5%) |
Ammi visnaga hydroethanol extract | |
| AV 2% | AV 4% | ||||
| Redness /Erythem | 0 | 4±0 | 4±0 | 4±0 | 3,8±0,45 |
| 4 | 4±0 | 3,2±0,45*** | 4±0 | 4±0 | |
| 7 | 4±0 | 2,8±0,84** | 3,4±0,55 | 4±0 | |
| 10 | 4±0 | 2,4±1,34* | 2,8±0,84 | 3,4±0,55 | |
| 13 | 3,8±0,45 | 1,8±0,84**** | 1±0**** | 2±0**** | |
| 16 | 3,2±0,45 | 1±1,22*** | 1±0*** | 1±0,45*** | |
| Scaling | 0 | 4±0 | 4±0 | 4±0 | 3,8±0,45 |
| 4 | 4±0 | 3,6±0,55 | 4±0 | 4±0 | |
| 7 | 4±0 | 3,2±1,09 | 4±0 | 4±0 | |
| 10 | 4±0 | 2,6±1,52 | 3,6±0,9 | 4±0 | |
| 13 | 4±0 | 2±1*** | 2±0,7*** | 2,6±0,55* | |
| 16 | 3,6±0,55 | 1,4±1,52** | 1±0*** | 1,2±0,45*** | |
| RA: retinoic acid, AV: Ammi visnaga. Values expressed in mean ± SD, n = 5 rats / group. | |||||
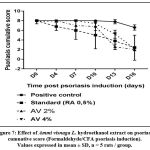 |
Figure 7: Effect of Ammi visnaga L. hydroethanol extract on psoriasis cumuative score (Formaldehyde/CFA psoriasis induction). Values expressed in mean ± SD, n = 5 rats / group. |
Histopathological examination
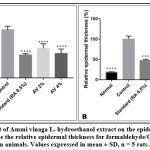
The characteristics of dermatitis induced by formaldehyde/CFA treatment (Figure 8) were similar to those described for psoriasis in the control group; especially parakeratosis, increase of epidermal thickness, elongation of ridges and leukocyte infiltration in the inflammation site. The thickness of the epidermis was increased from 57.73±2.57 in the no treated group, to 303.69±23.06 in the Formaldehyde/CFA treated group (Figure 9). Compared to the Ammi visnaga extract-treated mice, there was less inflammation (less leukocyte infiltration) in the skin, and a highly significant (<0.0001) dose-dependent decrease in epidermal thickness; the latter reached 186.75±29.03 and 153.98±28.45 in the groups treated by the 2% and 4% plant extract, respectively. The same results were observed in the group treated with 0.5%retinoic acid, in which the epidermal thickness was 144.68±8.94.
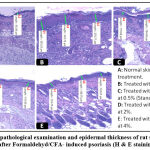 |
Figure 8: Histopathological examination and epidermal thickness of rat skin treated by AV extract after Formaldehyd/CFA- induced psoriasis (H & E staining) G X 100. |
Evaluation of T cells infiltration
The effect of Ammi visnaga L. seeds extract on leukocyte infiltration was studied by immunohistochemical staining (Figure 10). This study showed that psoriasis induced by a combination of formaldehyde and CFA is characterized by a severe infiltration of cells that express membrane markers such as CD3 (T cells), CD4 (Th) and CD8 (Tc), compared to the skin of normal mice, and that the application of a cream based on the extract of Ammi visnaga L. strongly attenuated this infiltration.
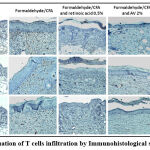 |
Figure 10: Evaluation of T cells infiltration by Immunohistological staining (GX 100). |
Discusion
Khellin and visnagin are two natural furanochromones of high therapeutic importance.[8] Both of these active principles are the most abundant secondary metabolites in Ammi visnaga L..20 But their levels in these plant extracts depend on various factors: the used part of the plant, the development stage and location, and the extraction method and solvent.[21,22] There are different methods for separation and quantification of these two compounds in extracts, such as capillary electrophoresis (CE), high performance liquid chromatography (HPLC), high performance thin layer chromatography (HPTLC).15,,23,24 In terms of efficiency, based on the results of HPLC analysis, the present study showed that the 60% hydroethanol extract gave the best yield in extraction of the two furanochromones concerned, from the seeds of Ammi visnaga L., followed by ethyl acetate extract, then by aqueous extract, and that visnagin is more sensitive to such changes in extraction solvent than khellin, but in terms of availability, safety and cost, water remains the solvent of the first choice.25 Regarding these results, the 60% hydroethanol extract was chosen for the rest of studies.
The immune system is influenced by multiple substances known as immunodulators; these are either immunostimulators that accentuate the immune response or immunosuppressors, which inhibit this response. Such agents can be very beneficial for homeostasis maintenance.26 The determination of an antibody titer after antigenic stimulation is a suitable procedure to test the effect of a substance or a mixture of substances on humor-mediated immune response.27 This test realized on rats, using HRBCs as antigen, showed that Ammi visnaga L. hydroethanolic extract at 60% was attributed a significant immunostmulatory activity, considering its capacity to increase the value of the antibody titer. This increase is mainly related to a stimulation of antibodies production by B plasma cells.28 Such activity could be useful in the neutralization and elimination of some antigens, particularly in immunodeficiency cases.9
Psoriasis is a chronic skin inflammation, resulting from the interaction between several environmental, genetic and immunological factors. Erythema and scaling are the main clinical characteristics of this disease, while it is characterized histologically by an abnormal proliferation of skin cells, accompanied by an increase in the epidermal thickness, ridge elongation and capillary loop dilatation.30 Traditional medicine, based on natural herbal substances, has an important place in available treatments for psoriasis, considering the body’s tolerance to these substances, compared to synthetic drugs.31-33 This demonstrates the importance of ascertaining the antipsoriatic activity, in plants used for these popular practices. This became possible after discovering the possibility of generating a disease state with the same characteristics as psoriasis in laboratory animals.34 In the present study, two models were used to test the antipsoriatic effect of the Ammi visnaga L. seeds hydroethanol extract; the first one was based on the UV skin irradiation, while the other one was based on the skin topical treatment by a mixture of formaldehyde and complete freund adjuvant at 1:10 ratio. Both protocols showed their efficacy in inducing dermatitis with the same clinical and histological characteristics as psoriasis, since erythema and scaling rapidly appeared in the positive control group in both tests. In addition, the histological study performed at the end of these two tests showed the persistence of epidermal thickness increase during 15 days, and the higher leukocyte infiltration to the treated skin site in this group. These results are in accordance with previous studies based on the same models.[19,35] In this work, the treatment with Ammi visnaga L. seeds hydroethanol extract, orally at 300 and 600 mg/kg, or topically at 2% and 4%, for two weeks, showed efficacy in reducing psoriatic symptoms, especially the increase in epidermal thickness, scaling and lymphocytes infiltration. The antipsoriatic effect showed by Ammi visnaga extract, in both in vivo models, is probably due to the khellin’s ability to inhibit the epidermal growth factor (EGFR); this factor plays an important role in cell homeostasis and its abnormal activation is strongly linked to several cases of dermatological pathologies such as psoriasis. Moreover, treatments with inhibitors of this factor have already shown efficacy in ameliorating psoriatic lesions.36,37 Another explanation that may be the basis for this antipsoriatic effect is the ability of khellin and visnagin to activate aryl hydrocarbon receptor (AhR) signaling and inhibit cytochrome P450 1A (CYP1A) activity.8 Indeed, several previous studies have shown that activation of AhR improves psoriatic lesions, while inhibition of AhR or a deficiency in its expression exacerbates them.38
Conclusion
High performance liquid chromatography analyses showed that the 60% ethanolic extract of Ammi visnaga L. seeds is richer in furanochromones (khellin and visnagin) than its aqueous or ethyl acetate homologs. Therefore, it is recomended to use this solvent for any extraction planning to obtain the highest proportion of these two active principles. It has also been shown that this extract may be useful in assisting in the treatment of disease states that require stimulation of the humoral immune response, inhibition of T lymphocytes infiltration as well as in decrease in symptoms of psoriasis, when given daily by oral gavages at 300 and 600 mg/Kg, or topically at 2 and 4 %, In light of the characteristics demonstrated in this plant in this context by the present study.
Acknowledgement
The author would like to thank the administration of the Faculty of Sciences Ain Cock, Hassan II University, for the efforts brought during the realization of this work.
Conflict of Interest
There are no conflicts of interest to be declared by the authors.
Funding Source
Not applicable.
References
- Hawkes J. E, Chan T. C and Krueger J. G. Psoriasis pathogenesis and the development of novel targeted immune therapies. Journal of Allergy and Clinical Immunology 2017; 140:645-653.
- Lebwohl M, Ting P. T and Koo J. Y. M. Psoriasis treatment: traditional therapy. Annals of the Rheumatic Diseases, 2005; 64(suppl_2):ii83-ii86.
- Balak D. M. W, Gerdes S, Parodi A and Salgado-Boquete L. Long-term Safety of Oral Systemic Therapies for Psoriasis: A Comprehensive Review of the Literature. Dermatol Ther (Heidelb), 2020; 10:589-613.
- Dabholkar N, Rapalli V. K and Singhvi G. Potential herbal constituents for psoriasis treatment as protective and effective therapy. Phytotherapy Research, 2020; 35(5):1-16.
- Tzaneva S, Seeber A, Honigsmann H and Tanew A. A comparison of psoralen plus ultraviolet A (PUVA) monotherapy, tacalcitol plus PUVA and tazarotene plus PUVA in patients with chronic plaque-type psoriasis. British Journal of Dermatology, 2002; 147:748-753.
- Abu-Hashem A. A and El-Shazly M. Synthesis, Reactions and Biological Activities of Furochromones: A review. European Journal of Medicinal Chemistry, 2015; 90:633-665.
- Borges M. L, Latterini L, Elisei F, Silva P. F, Borges R, Becker R. S and Maçanita A. L. Photophysical Properties and Photobiological Activity of the Furanochromones Visnagin and Khellin. Photochemistry and Photobiology, 2008; 67:184-191.
- Vrzal R, Frauenstein K, Proksch P, Abel J, Dvorak Z and Haarmann-Stemmann T. Khellin and Visnagin Differentially Modulate AHR Signaling and Downstream CYP1A Activity in Human Liver Cells. PLoS ONE, 2013; 8:e74917.
- Haarmann-Stemmann T, Esser C and Krutmann J.The Janus-Faced Role of Aryl Hydrocarbon Receptor Signaling in the Skin: Consequences for Prevention and Treatment of Skin Disorders. Journal of Investigative Dermatology, 2015; 135:2572-2576.
- Abdel-Fattah A, Aboul-Enein N, Wassel G and El-Menshawi B. Preliminary Report on the Therapeutic Effect of Khellin in Psoriasis. Dermatology, 1983; 167:109-110.
- Fenniche S, Zaouak A, Tanfous AB, Jrad M and Hammami H. Successful Treatment of Refractory Vitiligo with a Combination of Khellin and 308-nm Excimer Lamp: An Open-Label, 1-Year Prospective Study. Dermatology and Therapy, 2017; 8:127-135.
- Narayanaswamy R and Ismail I. S. Role of Herbal Medicines in Vitiligo Treatment Current Status and Future Perspectives. Asian Journal of Pharmaceutical and Clinical Research, 2018; 11:19-23.
- Arifianti L, Sukardiman S, Indriyanti N and Widyowati R. Anticancer Property of
Orthosiphon stamineus Benth. Extracts in Different Solvent Systems against T47D Human Breast Cancer Cell Lines. FABAD J Pharm Sci, 2020; 45:187-194. - Nasr A, Zhou X, Liu T, Yang J and Zhu G. P. Acetone-water mixture is a competent solvent to extract phenolics and antioxidants from four organs of Eucalyptus camaldulensis. Turkish Journal of Biochemistry, 2019; 44:231-239.
- Badr J. M, Hadad G. M, Nahriry K and Hassanean HA. Validated HPLC method for simultaneous estimation of khellol glucoside, khellin and visnagin in Ammi visnaga L fruits and pharmaceutical preparations. Natural Product Research, 2014; 29:593-601.
- Pravansha S, Thippeswamy, B. S and Veerapur, V. P. Immunomodulatory and antioxidant effect ofLeptadenia reticulataleaf extract in rodents: possible modulation of cell and humoral immune response. Immunopharmacology and Immunotoxicology, 2012 ; 34:1010-1019.
- Test No. 402: Acute Dermal Toxicity, OECD Guidelines for the Testing of Chemicals,
Section 4, Éditions OCDE, Paris. 2017. - Kulkarni S, Autade A, Awari D and Chitlange S. Pharmacological Evaluation of AntiPsoriatic
Activity of Madhuca longifolia on Experimental Animals. International Journal of
Pharmaceutical Sciences and Research, 2017; 8:218-235. - Srivastava A. K, Nagar H. K, Chandel H. S and Ranawat M. S. Antipsoriatic activity of ethanolic extract of Woodfordia fruticosa (L.) Kurz flwers in a novel in vivo screening model. Indian J Pharmacol, 2016; 48:531-536.
- Al-Snafi A. E. Chemical Constituants and Pharmacological Activites of Ammi majus and Ammi visnaga. A Review. Int J Pharm & Ind Res, 2013; 03:257-265
- Franchi G. G, Bovalini L, Martelli P, Ferri S and Sbardellati E. High performance liquid chromatography analysis of the furanochromones khellin and visnagin in various organs of Ammi visnaga (L.) at different developmental stages. Journal of Ethnopharmacology, 1985; 14:203-212.
- Sellami H. K, Napolitano A, Masullo M, Smiti S, Piacente S and Pizza C. Influence of growing conditions on metabolite profile of Ammi visnaga umbels with special reference to bioactive furanochromones and pyranocoumarins. Phytochemistry, 2013; 95:197-206.
- Günaydın K and Erim F. B. Determination of khellin and visnagin in Ammi visnaga fruits by capillary electrophoresis. Journal of Chromatography A, 2002; 954:291–294.
- Kamal A, Khan W, Ahmad S, Ahmad F. J and Saleem K. Development and validation of high-performance liquid chromatography andhigh-performance thin-layer chromatography methods for the quantifiation of khellin in Ammi visnaga J Pharm Bioall Sci, 2015; 7:308-13.
- Khalil N, Bishr M, El-Degwy M, Abdelhady M, Amin M and Salama O. Assessment of Conventional Solvent Extraction vs. Supercritical Fluid Extraction of Khella (Ammi visnaga
) Furanochromones and Their Cytotoxicity. Molecules, 2021; 26:1290. - Bascones-Martinez A, Mattila R, Gomez-Font R and Meurman J. Immunomodulatory drugs: Oral and systemic adverse effects. Med Oral Patol Oral Cir Bucal, 2014; 19:e24-e31.
- Shukla S and Mehta A. Comparative phytochemical analysis andin vivo immunomodulatory activity of various extracts of Stevia rebaudianaleaves in experimental animal model. Frontiers in Life Science, 2014; 8:55-63.
- Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. Journal of Immunology, 1978; 120:1809-1812.
- Nfambi J, Bbosa G. S, Sembajwe L. F, Gakunga J and Kasolo J. N. Immunomodulatory activity of methanolic leaf extract of Moringa oleifera in Wistar albino rats. Journal of Basic and Clinical Physiology and Pharmacology, 2015; 26:603-611.
- Raychaudhuri S. K, Maverakis E and Raychaudhuri S. P. Diagnosis and classification of psoriasis. Autoimmunity Reviews, 2014; 13:490-495.
- Bonesi M, Loizzo M. R, Provenzano E, Menichini F and Tundis Anti-Psoriasis Agents from Natural Plant Sources. Current Medicinal Chemistry, 2016; 23:1250-1267.
- Herman A and Herman A. Topically used herbal Products for the treatment of psoriasis-mechanism of Action, Drug Delivery, Clinical Studies. Planta Medica, 2016; 82:1447-1455.
- Sakthi Priyadarsini S, Vani P. B and Kumar P. R. A Comparative Review on Conventional and Traditional medicine in the Treatment of Psoriasis. Research J Pharm and Tech, 2020; 13:5642-5646.
- Schön M. P. Animal models of psoriasis: a critical appraisal. Experimental Dermatology, 2008; 17:703-712.
- Nagar H. K, Srivastava A. K, Srivastava R and Ranawat M. S. Evaluation of potent phytomedicine for treatment ofpsoriasis using UV radiation induced psoriasis in rats. Biomedicine & Pharmacotherapy, 2016; 84:1156-1162.
- Elgazwy A. S. S. H, Edrees M. M and Ismail N. S. M. Molecular modeling study bioactive natural product of khellin analogues as a novel potential pharmacophore of EGFR inhibitors. Journal of Enzyme Inhibition and Medicinal Chemistry, 2012; 28:1171-1181.
- Wang S, Zhang Z, Peng H and Zeng K. Recent advances on the roles of epidermal growth factor receptor in psoriasis. American journal of translational research, 2019; 11:520-528.
- Di Meglio P, Duarte J. H, Ahlfors H, Owens N. D. L, Li Y, Villanova F et al. Activation of the Aryl Hydrocarbon Receptor Dampens the Severity of Inflammatory Skin Conditions. Immunity, 2014; 40:989-1001.