Manuscript accepted on :08-07-2022
Published online on: 25-07-2022
Plagiarism Check: Yes
Reviewed by: Dr. Jagdish Joshi
Second Review by: Dr. Hanefi ÖZBEK
Final Approval by: Dr. Ayush Dogra
Dhanya Sasidharan Palappalil1* , Jitha Sushama2
, Jitha Sushama2 and Kala Parvathy Kesavan2
and Kala Parvathy Kesavan2
1Department of Pharmacology, Government Medical College, Kottayam, Kerala, India 686008,
2Department of Pharmacology, Government Medical College, Thiruvananthapuram, Kerala, India.
Corresponding Author E-mail: drspdhanya@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2501
Abstract
Objectives: Drug-drug interactions (DDIs) are an important issue in clinical practice as management of co-morbidities necessitates polypharmacy and some of these interactions can transmute into or accentuate adverse drug reactions (ADRs). The objective was to estimate the proportion of ADRs due to DDIs and to describe the pattern of drug-drug interactions that resulted in ADRs. Materials and Methods: Cross-sectional study was done in the Department of Pharmacology of a Government Medical College in Kerala for a period of 1 year after getting clearance from the Institutional Ethics Committee. ADR reports submitted to the ADR Monitoring Centre from June 2015 to May 2017 formed the study material and details were entered in a structured proforma. Each suspected drug and concomitant drugs were entered in MICROMEDEX®, MEDSCAPE, and LEXICOMP drug interaction softwares to identify all potential DDIs (pDDIs). The interactions which matched with ADR description were considered to be the probable cause of that ADR. SPSS software version 16 was used for data analysis. Descriptive data were expressed as frequencies and percentages. Results: Of the 345 ADR patients reported during the study period, 249 had concomitant drugs (mean 2.84±1.85 drugs/patient) from whom we identified 295 pDDIs (mean 1.18 ± 1.59 pDDIs/patient). Of the 295 pDDI, 30 matched the description of ADR, thus the proportion of ADRs due to DDIs was 12.05% (30 out of the 249 ADRs). Aspirin with Clopidogrel (n=5) and Heparin with Clopidogrel (n=5) topped the list of interactions contributing to ADR. Amongst the 30 suspected drug interactions causing ADR, 23 (76.67%) were pharmacodynamic, 21(70%) were of major severity and in 27(90%) the time of onset were not specified. Conclusions: Drug-drug interactions attributed to 12.05% of the ADRs in which data on concomitant drugs were available. Pharmacodynamic interactions (76.7%) contributed to sADRs more than pharmacokinetic interactions.
Keywords
Adverse Drug Reactions; Drug Interaction; Pharmacokinetic interaction; Pharmacodynamaic interaction
Download this article as:| Copy the following to cite this article: Palappalil D. S, Sushama J, Kesavan P. K. Drug Interactions as a cause of Adverse Drug Reactions in a Tertiary Care Hospital. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Palappalil D. S, Sushama J, Kesavan P. K. Drug Interactions as a cause of Adverse Drug Reactions in a Tertiary Care Hospital. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3oozmCJ |
Introduction
More than one drug is prescribed to a patient either to achieve a synergistic effect or because more than one drug is required to treat multiple conditions.1 This can lead to drug-drug interactions (DDIs). Potential drug-drug interactions (pDDIs) are common and they can be apparent or become clinically significant. When there are marked alterations in the effects of some drugs it may be therapeutically beneficial or cause adverse drug reactions(ADRs), toxicity, or therapeutic failure which complicate drug co-administration.1,2 DDIs pose a crucial concern in clinical practice and drug utilization because we cannot stop the drug from causing the interaction as the specific drug might be essential for the patient and the benefits of its use outweigh the risk posed by the drug interaction (DI).3 Recognition of every potential DDI when prescribing or dispensing is a challenge. While the incidence of pDDI is near 40% when a patient is on five concomitant drugs, it doubles to 80% with the use of seven or more drugs. 4 In patients with multiple co-morbidities, it is yet another challenge to identify adverse effects due to DDI and classify them as pharmacokinetic, pharmacodynamic, or combinations.1 However, with the use of computerized screening softwares, DDIs are now predictable and we can identify potential drug therapy problems and prevent ADRs due to DDIs. 5
ADRs are unintended, noxious reactions that occur in the therapeutic dose of the drug and add to the pharmacoeconomic burden of society by causing increased hospitalization.[6] Depending on various study designs, populations, and periods, the proportion of patients developing ADR due to drug interactions has varied from 0.63 to 56%.7,8 Of the ADRs that necessitate hospitalization, 26% are due to DDIS.2 Due to controlled settings in clinical trials the ADRs due to DDI which we encounter in real-life settings are seldom discovered or systematically investigated during the development of drugs.9 ADRs due to co-administration of drugs can be an enhancement of the already known ADR of a drug or it may be a new unanticipated effect that cannot be associated with either of the drugs when used alone. Based on the similarity of drug molecular structure, drug targets, drug-side effects, and drug target protein sequence several computational methods that predict DDIs have been published. There are also published models that predict the therapeutic potential of drugs and adverse drug reactions based on genome-wide drug-protein interactions.10 More than 60 free and paid software such as Medscape Drug Interaction checker, Lexi-Interact by Lexicomp online, Micromedex drug interaction checker, Epocrates, Harmavista, Stockley’s Drug interactions, British National Formulary are available which can detect potential DDIs.11 The data regarding the proportion of ADRs caused by drug interactions are sparse in the Indian population. This study aimed to estimate the proportion of ADRs that occurred due to drug-drug interactions during the study period and to describe the pattern of drug-drug interactions that resulted in ADRs.
Materials and methods
This cross-sectional study was done in the Department of Pharmacology, Government TD Medical College, Alappuzha for a period of one year from July 2016 to June 2017 after getting Institutional Research Committee and Ethics Committee clearance number EC 04/2016 dated 26.05.2016. All suspected ADR (sADR) reports submitted to the ADR Monitoring Centre, Department of Pharmacology from June 2015 to May 2017 formed the study material. Any reports of poisonings due to insecticides/pesticides/acids/ alkalis/kerosene/plant products as well as sADR reports without concomitant drugs were excluded from the study. A sample size of 284 was calculated considering a prevalence of 26% of hospitalized ADRs due to drug interactions.2 Data from the sADR reports collected in the common standardized format IPC-ADR form were entered in a structured proforma regarding the gender, description, and type of ADR, the suspected drug and its class, the number and names of concomitant drugs, the causality using Naranjo Scale, severity using Hartwig and Siegel Scale and preventability assessment using Schumock and Thornton Scale.12-14 ADRs with no concomitant drugs were excluded from further study. Each suspected drug and concomitant drugs were entered in the MICROMEDEX Drug Interaction Checker Software, MEDSCAPE drug interactions checker, and LEXICOMP Online software Lexi-Interact.15-17 Drug interaction identified in any of the software was considered as a potential drug-drug interaction. The description of the interaction was matched with that of the ADR and reported by the investigators independently. Criteria for selection of ‘ADR due to drug interaction’ were an involvement of the suspected drug in the drug interaction and a match in the description of the ADR reported and drug interaction detected through software. The suspected drugs were entered along with the concomitant drugs in the software and any drug interactions which matched with the adverse drug reactions were selected manually. If in a patient more than one interaction with the suspected drug could result in an adverse drug reaction, all the interactions were included for analysis. The type of interaction, the severity of interaction, and the onset of interaction were entered in the proforma. SPSS software version 16 (SPSS Inc., Chicago, USA) was used for data analysis. Descriptive data were expressed as frequencies and percentages.
Results
A total of 345 patients were reported to develop ADR from June 2015 to May 2017 of which 93 were eliminated from the study because of a lack of details or absence of concomitant drugs. Of the remaining 249 patients with ADRs, 90 were reported from June to December 2015, 127 were reported in the year 2016, and 32 from January to May 2017. The mean age was 44.84±19.60 years with females being 135(54.2%) and males 114(45.8%). Cefotaxime (n=18), followed by Amoxicillin (n=17) and Azithromycin as well as Ciprofloxacin (each n=13) topped the list of drugs that resulted in sADRs. Antibiotics (n=106) followed by NSAIDs (n=27) topped the list of drug classes contributing to sADRs. Pruritus (n=78) followed by rash (n=60) topped the list of sADRs. Of the 249 patients, 38.5% (n=96) received the drug intravenously, 30.52% (n=76) received it through oral route, 32(12.9%) intradermally, 8(3.2%) intramuscularly, 4(1.6%) subcutaneously and 1(0.4%) each as inhalation and suppository. Type B-Bizarre ADRs occurred in 79.11% (n=197) and the rest were Type A-Augmented (n=52). The ADRs were not serious in 185 patients and were serious in 64 patients, 34 resulting in prolonged hospitalization, and 30 were life-threatening. All sADRs were managed symptomatically and at the time of the report of sADR, 227 patients had recovered from it and 22 were recovering. The severity of ADRs were mild in 130 patients (Level 2), moderate in 88 [Level 3 (n=50), Level 4(n=38)], and severe in 31(Level 5). The ADR was definitely preventable in 242 patients, probably preventable in 2, and not preventable in 5.
In the 249 ADR patients, we identified 295 potential drug interactions as shown in table 1, the maximum number in a patient being 8 and the least 1. The average number of potential interactions per patient was 1.18±1.59. The number of concomitant drugs ranged from 1(n=62, 24.9%) to 9(n=1, 0.4%), with a mean of 2.84±1.85. There was a positive correlation between the number of concomitant drugs and the number of pDDIs (correlation coefficient r=0.313) as shown in Fig 1. Of the 295 pDDI, 117 pDDI were due to the suspected drug with the concomitant drugs and 178 pDDI were between the concomitant drugs. The potential interactions of the suspected drug were pharmacodynamic (n=77), pharmacokinetic (n= 32), pharmaceutical (n=2) and unknown (n=6). There were 59 major, 49 moderate, and 9 minor interactions. The interactions were delayed (n=16), rapid (n=11) and non-specified (n=90).
Table 1: Number of potential Drug-Drug Interactions.
| Number of DDIs/
ADR patient |
Number of ADR patients (%) | Frequency of DDIs (%) |
| 0 | 119 (79.8%) | 0 |
| 1 | 60 (24.09%) | 60 (20.3%) |
| 2 | 22 (8.83%) | 44 (14.91%) |
| 3 | 20(8.03%) | 60 (20.3%) |
| 4 | 18 (7.22%) | 72 (24.40%) |
| 5 | 4 (1.60%) | 20 (6.77%) |
| 6 | 4(1.60%) | 24 (8.13%) |
| 7 | 1(0.40%) | 7 (2.37%) |
| 8 | 1(0.40%) | 8 (2.71%) |
| Total | 249 | 295 |
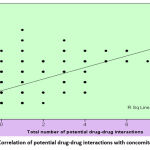 |
Figure 1: Correlation of potential drug-drug interactions with concomitant drugs. |
Of the 117 potential interactions of the suspected drug, we identified 30 drug interactions whose description matched with that of the ADR. Thus the proportion of ADRs due to DDIs was 12.05% (30 out of the 249 ADRs). The mean age of patients who developed ADR following DDI was 54.73±17.36 years and 18(60%) were females and the rest males. Table 2 shows the distribution of drug interactions concerning age group and gender. As shown in table 2 majority of drug interactions that resulted in ADR occurred in the 19-60 years age group 14(46.7%) of which 9 occurred in females and 8 in males. Type A-Augmented reactions occurred in 24(80%) and Type B-Bizarre in 6 (20%). Skin and appendages (5, 16.7%) followed by Central Nervous System (4, 13.3%) topped the list of organ systems affected. In 16(53.3%) patients ADR due to DDI resulted in prolonged hospitalization, in 5(16.7%) it was life-threatening, and in 9(30%) it was not serious. Of the 30 ADRs due to DDI, 6(20%) were severe (Level 5), 21(70%) had moderate [Level 3=8(26.7%), Level 4=13(43.3%)] severity, and 3(10%) were mild (Level 2) in severity. Twenty-seven (90%) were definitely preventable and 3(10%) were probably preventable ADRs. Causality assessment revealed that 28(93.4%) were probable, 1(3.3%) was possible and 1(3.3%) was certain. At the time of reporting, in 24 patients the suspected drug was stopped, in 3 the dose was reduced and 29 (96.7%) had recovered and 1(3.3%) was recovering from it.
Table 2: Demographic profile of patients with drug interactions that resulted in adverse drug Reactions.
| Age Group | Gender | Number of Interactions
n=30 |
|
| Female n=18 | Male n=12 | ||
| 0-18 years | 3 (16.7%) | 0 | 3 (10 %) |
| 19-60 years | 9 (50 %) | 8 (66.7%) | 17(56.7%) |
| >60 years | 6 (33.3%) | 4(33.3%) | 10(33.3%) |
As summarized in Table 3, Aspirin with Clopidogrel (n=5) and Heparin with Clopidogrel (n=5), topped the list of drug interactions contributing to ADR. Twenty-two (73.3%) patients who developed ADR as a result of DDI received more than 1 concomitant drug. Amongst the 30 DDIs, 23(76.67%) were pharmacodynamic, 2(6.66%) were pharmacokinetic, 4(13.33%) were both pharmacodynamic and pharmacokinetic and 1(3.33%) had unknown interaction type. The severity was major in 21(70%) interactions and moderate in 9(30%). The onset of interaction was delayed in 2(6.7%), immediate in 1(3.3%), and not specified in 27(90%). All the interactions occurred at the normal therapeutic dose of the suspected and concomitant drugs. In 5 out of the 30 interactions the suspected drug was Fixed-Dose Combination of Aspirin+ Clopidogrel interacting among themselves as shown in Table 3.
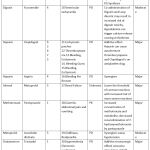 |
Table 3: Profile of drug interactions that caused ADR. |
Discussion
“An adverse drug reaction is any response to a drug which is noxious and unintended, and which occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of disease, or for the modification of physiological function”.18 While adverse drug reactions can occur in appropriately prescribed, dispensed, or administered drugs they can also occur when the pharmacological effect of one drug is modified by another. A total of 345 patients developed ADR during the study period, the commonest ADR was pruritus, the commonest suspected drug class was antibiotic and the drug was a Beta-lactam, Cefotaxime. In a study done earlier from the same institution the commonest ADR was maculopapular rash, the drug class was antibiotics and the drug was a Beta-lactam, Amoxicillin.19
Drug-drug interactions are seldom recognized in clinical practice. With the advent of electronic decision support tools, the physician can be alerted about potential DDIs and careful selection and monitoring can help in preventing adverse outcomes.20 The role of DDI is an important predisposing factor for ADR. The theoretical probability of occurrence of potential interactions is higher than clinically relevant adverse reactions.21 In a study by Palappallil et al., 12.73% of ADRs were due to DI, of which the majority were significant pharmacodynamic interactions and were delayed in onset and DDIs had more probability of causing severe ADRs with an Odds ratio of 1.75.19A study by Fotker et al, found that of the potential DDIs identified in 51% of patients on admission, only in 1.2 % it was the reason for the admission.22 Faspie et al, identified 1851 pDDIs among 118 patients receiving drugs for Chronic kidney disease.11 Pichala et al, identified that drug interactions resulted in the majority of the problems identified by drug therapy assessment, however only 20% were clinically significant, and most required monitoring of the patients.3 Lucca et al., found that the incidence of pDDI in psychiatric patients was 55.2% and 12% of patients with DDI developed ADRs.23 In this study, we identified 295 pDDIs of which 117 pDDI were due to the suspected drug of which 30(10.17% ) resulted in ADR cases. Of the 30 adverse drug reactions due to drug interactions 18 occurred in females and 12 in males and the majority occurred in the 19-60 years age group. Lucca et al., found that amongst the 97 patients who developed ADRs in which DDI was suspected majority were males (57).23 This is in contrast to our study. Lucca et al., found that 87(89.6%) developed ADR due to drug interaction in the adult age group as compared to the pediatric and geriatric group.23 This is in line with this study in which 17(56.7%) developed ADR in the adult age group, 10% developed in the pediatric age group, and 33.3% developed in the geriatric age group.
Co-administration of drugs in the same infusion can lead to pharmaceutical interaction among drugs or with the intravenous fluid.24 Of the 117 potential interactions of the suspected drugs, two were pharmaceutical. We did not encounter any pharmaceutical interaction which resulted in ADR in this study.
When the action of one drug is modified by another without change in the serum levels it results in pharmacodynamic interactions. These interactions are difficult to classify as some drugs instead of directly affecting the object drug may interfere with the physiological mechanisms. [24] Amongst the potential DDIs with the suspected drug, 65.81% were pharmacodynamic and of the 30 DDIs which resulted in ADR, 76.7% were pharmacodynamic. Aspirin and Clopidogrel are two antiplatelet drugs and the combined use of these drugs helps in preventing ischaemic episodes due to synergism. The Clopidogrel in Unstable angina to prevent Recurrent ischemic Events (CURE) trial has shown a 20% reduction in myocardial infarction (MI), stroke, and cardiovascular deaths.[25] This dual antiplatelet therapy also helps in checking the restenosis of stented coronaries. However, the Management of ATherothrombosis with Clopidogrel in High-risk patients with a recent transient ischaemic attack or ischaemic stroke (MATCH) trial has shown that serious bleeding in high-risk stroke patients doubles with dual antiplatelet therapy.[26] In this study, five patients developed bleeding causing hemoptysis, hematuria, hematemesis, and black tarry stools because of the additive effect. Two patients received concomitant aspirin and tirofiban and one developed gum bleeding and the other thrombocytopenia. Another patient received aspirin as a concomitant drug in a patient who developed bleeding per rectum after receiving heparin. Five patients who received heparin and clopidogrel developed bleeding, ecchymotic patches, and thrombocytopenia. Bleeding is the most serious complication of heparin therapy. Heparin-induced thrombocytopenia due to platelet aggregation is mild and of brief duration. Clopidogrel also causes thrombocytopenia and has an additive effect with heparin.24 Warfarin an oral anticoagulant had synergistic interactions with aspirin causing hypoprothrombinemia (pharmacodynamic interaction) as well as displaced warfarin from the plasma protein binding site (pharmacokinetic) resulting in hematuria and bleeding gums in a patient and ecchymosis in another. [24]Administration of streptokinase with aspirin and heparin resulted in bleeding in two patients. Clinical use of Streptokinase has reduced to a large extent as it exhausts the circulating fibrinogen thereby causing bleeding.24 Diclofenac is a non-steroidal anti-inflammatory drug that decreases prostaglandin synthesis and this can affect the diuretic effect of Furosemide as renal vasodilatation mediated through prostaglandin is blunted and this can lead to edema as seen in one of the ADRs. The increase in systemic venous capacitance is mediated through prostaglandin and accounts for the rapid respite it offers in left ventricular failure and pulmonary edema.24 There is also competition between furosemide and diclofenac for tubular secretion which delays the action of furosemide.27 Ondansetron and tramadol caused hyperesthesia in a patient because of the additive serotonergic effect (excessive stimulation of 5HT1A and 5HT2A). Risperidone when administered with lithium, an antimanic drug resulted in dyskinesia as extrapyramidal symptoms are aggravated in the presence of lithium. Some moderate severity pharmacodynamic interactions were also noted as shown in Table 3.
Pharmacokinetic interactions result in variation of serum levels of the drug due to interference at the level of absorption, distribution, metabolism, or excretion. This can alter the effectiveness of the drug which may be identified by scientific knowledge or detected by therapeutic drug monitoring.11 There were six ADRs due to pharmacokinetic interactions of which three had pharmacodynamic interactions also. Levetiracetam enhanced the toxic effect of Carbamazepine and resulted in Stevens Johnson’s syndrome. Sisodiya et al., described a series where four patients who developed adverse reactions due to carbamazepine with concurrent use of Levetiracetam, and this was presumed to be due to additive central nervous system depressant effects as there was no change in the carbamazepine levels.28 Increased concentration of methotrexate, as well as decreased renal elimination of 7-hydroxymethotrexate by pantoprazole, resulted in gastritis viz abdominal pain and vomiting. Several case reports have been published establishing that the abnormality in methotrexate concentration and elimination were not evident with prior or subsequent administration of methotrexate without concurrent proton pump inhibitor and concurrent use was significantly associated with delayed methotrexate elimination.17, 29 The possible mechanism proposed is the inhibition of renal elimination of hydrogen ions by proton pump inhibitors, which interferes with the secretion of methotrexate which is actively secreted in the distal renal tubules with hydrogen ions produced via the hydrogen-potassium ATPase pump.29 Clopidogrel can inhibit the metabolism of Phenytoin and this resulted in hypersensitivity reactions due to Phenytoin. Clopidogrel and Phenytoin utilize Cytochrome Pigment (CYP) 2C19 enzyme and Clopidogrel is also an inhibitor of CYP2C19 causing raised Phenytoin levels. [30]Two patients developed bleeding in the form of hematuria and ecchymosis with concomitant use of Warfarin and Heparin. Apart from having synergistic pharmacodynamic interaction Heparin can displace Warfarin from the plasma protein binding site and result in an increased concentration of free warfarin resulting in bleeding. [24] Similarly, Cyclosporine and Dexamethasone in addition to the synergistic interaction increase serum Dexamethasone concentration by affecting CYP3A4 metabolism and P-glycoprotein efflux transporter resulting in drug-induced hyperglycemia. [24] This study was conducted based on the ADRs reported to the Department of Pharmacology and may not be representative of all the ADRs that occurred in the institution during the study period. Of the 345 ADRs reported to the department 93 were eliminated from the study because of lack of details or absence of concomitant drugs.
Conclusions
A total of 345 ADRs were reported during the study period, of which 249 were included in the study. Pruritus was the most frequent sADR, antibiotics topped the list of drug classes causing sADR and the most frequent suspected drug was Cefotaxime. The majority of the ADRs were bizarre, not serious, and mild in severity. In the 249 ADR reports, 295 potential Drug-Drug Interactions were found out of which 117 were due to the suspected drug which caused ADR. In 30(10.17%) ADRs drug-drug interaction was suspected as the cause. The majority of the ADRs due to DDI were augmented, affected Skin and appendages, were serious, severe, definitely preventable, and probable in causality. The most common drug interaction resulting in ADR was that of Aspirin with Clopidogrel and Heparin with Clopidogrel. The majority were pharmacodynamic and of major severity. Intense monitoring for pDDI and constant vigilance may improve the therapeutic outcome and reduce the burden of extremely harmful and preventable ADRs.
Acknowledgment
We thank the State Board of Medical Research, Government of Kerala for funding this study. We acknowledge Mrs. Adwaida Raveendran who helped in retrieving the data from the Micromedex Drug Interaction checker. We thank all the staff of Govt Medical College TDMCA who actively reported the ADRs. We thank the Pharmacovigilance Programme of India for supporting our institution in being an ADR monitoring centre.
Conflict of Interest
There are no conflict of Interest.
Funding Sources
There is no funding source.
References
- Brunton LL, Hilal-Dandan R, Knollmann BC. Goodman & Gilman’s The Pharmacological Basis of Therapeutics.13th Ed 2018;New York:Mc Graw Hill Medical.
- Busca C, Farcas A, Cazacu I, Leucuta D, Achimas-Cadariu A, Mogosan C, et al. How many potential drug-drug interactions cause adverse drug reactions in hospitalized patients? Eur J Intern Med 2013;24(1):27-33.
CrossRef - Pichala PT, Kumar BM, Zachariah S, Thomas D, Sanchez L, Gerado A. An interventional study on intensive care unit drug therapy assessment in a rural district hospital in India. J Basic Clin Pharm 2013;4:64-7.
CrossRef - Grattagliano I, Portincasa P, D’Ambrosio G, Palmieri VO, Palasciano G. Avoiding drug interactions:here’s help. J Family Pract 2010;59:322-9.
- Zarowitz, BJ, Stebelsky LA, Muma BK., Romain TM. and Peterson, E. L. (2005), Reduction of High-Risk Polypharmacy Drug Combinations in Patients in a Managed Care Setting. Pharmacotherapy, 25: 1636–1645. doi: 10.1592/phco.2005.25.11.1636
CrossRef - Juurlink DN,Mamdani M,Kopp A,Laupacis A, Redelmeier DA. Drug-Drug interactions among elderly among elderly patients hospitalized for drug toxicity.JAMA 2003;289:1652-8.
CrossRef - Doubova SV, Reyes-Morales H, del Pilar Torres-Arreola L, Suárez-Ortega M. Potential drug-drug and drug-disease interactions in prescriptions for ambulatory patients over 50 years of age in family medicine clinics in Mexico City. BMC Health Serv Res 2007;7:147.
CrossRef - Zhan C, Coreea de Araujo R, Bierman AS, Sangl J, Miller MR, Wickizer SW, et al. Suboptimal prescribing in elderly outpatients: potentially harmful drug-drug and drug-disease combinations. J Am Geriatr Soc 2005;53:262-7.
CrossRef - Percha B, Altmans RB. Informatics confronts drug-drug interactions. Trends Pharmacol Sci 2013;34(3):178-84.
CrossRef - Liu R, AbdulHameed MDM, Kumar K, Yu X, Wallqvist A, Reifman J. Data-driven prediction of adverse drug reactions induced by drug-drug interactions. BMC Pharmacol Toxicol 2017;18:44.
CrossRef - Fasipe OJ, Olayemi SO, Akinyede AA, Osho PO, Ibiyemi-Fasipe OB, Osho ES. How do we prevent the burden of extremely harmful and clinically nonbeneficial drug-drug interactions among chronic kidney disease patients? Toxicol Res Appl 2018;2:1-14.
CrossRef - Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239-45.
CrossRef - Hartwig SC, Siegel J, Schneider PJ. Preventability and severity assessment in reporting adverse drug reactions. Am J Hosp Pharm. 1992;49(9):2229-32.
CrossRef - Schumock GT, Thornton JP. Focusing on the preventability of adverse drug reactions. Hosp Pharm. 1992;27(6):538.
- Interaction Checking [database on internet].Greenwood Village(CO)[cited 2020 Feb 25]. Available from:www.micromedexsolutions.com
- WebMD LLC. Drug Interaction Checker. Copyright© 1994- 2016. Available from: http://www.reference.medscape.com/ drug-interactionchecker. [Last cited on 2019 April 25].
- Lexi-Interact. Lexicomp(2018).Wolters Kluwer Health , Inc.Riverwoods,IL. Available at:http://online.lexi.com. [Last Accessed 2020 February 25]
- World Health Organization for International Drug Monitoring. Glossary of Terms Used in Pharmacovigilance. Sweden: Uppsala Monitoring Centre; 2015. Available from: http:// who-umc.org/graphics/28401.pdf [Last cited on 25 February 2020]
- Palappallil DS, Ramnath SN, Gangadhar R. Adverse drug reactions: Two years’ experience from a tertiary teaching hospital in Kerala. Natl J Physiol Pharm Pharmacol 2017;7(4):403-11.
CrossRef - Schreiber R, Gregoire JA, Shaha JE, Shaha SH.Think time: A novel approach to analysis of clinician’s behaviour after reduction of drug-drug interaction alerts. Int J Med Inform 2017;97:59-67.
CrossRef - Pirmohamed M. Drug-drug interactions and adverse drug reactions: separating the wheat from the chaff. Wien Klin Wochenschr 2010;122:62-64.
CrossRef - Fokter N, Mozina M, Brvar M. Potential drug-drug interactions and admissions due to drug-drug interactions in patients treated in medical departments. Eien Klin Wochenschr 2020;122:81-88.
CrossRef - Lucca JM, Ramesh M, Ram D, Kishor M. Incidence and predictors of adverse drug reactions caused by drug-drug interactions in psychiatric patients: An empirical study. Trop J Med Res 2016;19:29-35.
CrossRef - Tripathi KD. Essentials of Medical Pharmacology.8th New Delhi. Jaypee Brothers Medical Publishers;2019.
CrossRef - Fox KA, Mehta SR, Peters R, Zhao F, Lakkis N, Gersh BJ, et al. Benefits and risks of the combinations of Clopidogrel and aspirin in patients undergoing surgical revascularization for non-ST-elevation acute coronary syndrome: the Clopidogrel in Unstable angina to prevent Recurrent ischemic Events(CURE) Trial. Circulation 2004;110(10):1202-8.
CrossRef - Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. Aspirin and Clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients(MATCH): randomized, double-blind, placebo-controlled trial. Lancet 2004;364(9431):331-7.
CrossRef - Katzung Basic and Clinical Pharmacology.14th Ed.Chennai. McGraw Hill Education(India) Private Limited;2018
- Sisodiya SM, Sander JW, Patsalos PN. Carbamazepine toxicity during combination therapy with Levetiracetam: a pharmacodynamic interaction. Epilepsy Res 2002;48(3):217-9.
CrossRef - Suzuki K, Doki K, Homma M, Tamaki H, Horis S, Ohtani H, et al. Co-administration of proton pump inhibitors delays elimination of plasma methotrexate in high-dose methotrexate therapy. Br J Clin Pharmacol 2009;67(1):44-9.
CrossRef - Abushammala I, Abususoud A,Shammaleh KFA. Pharmacokinetic interaction study between Clopidogrel and Phenytoin in healthy male rabbits. World J Pharm Pharmaceutical Sci 2017;6(5):222-30.
CrossRef







