Manuscript accepted on :31-08-2022
Published online on: 12-09-2022
Plagiarism Check: Yes
Reviewed by: Dr. Ankur Singh Bist, Dr. Hassan Mumtaz
Second Review by: Dr. Mazhar Ozkan
Final Approval by: Dr. Josphert Ngui Kimatu
Department of Biology, AL-Iraqia University, Baghdad, Iraq
Corresponding Author E-mail: dr.zubaidalattef@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2512
Abstract
In this research , cuticular with epidermal anatomical characters were described , as well as the anatomical features of stems and leaves for ten wild species belong to Amaranthaceae and Compositae in Iraq. The species belong to Amaranthaceae were: Amaranthus blitum L., Beta vulgaris L., Amaranthus viridis L. , Atriplex prostrata Boucher ex DC. and Chenopodiastrum murale (L.) S.Fuentes, Uotila & Borsch. While the species belonging to Compositae were: Lactuca virosa Habl., Crepis vesicaria L., Senecio peregrinus Griseb., Crepis capillaris (L.) Wallr and Silybum marianum L. Gaernt. Anatomical differences between the species were studied. Results showed that several of the internal characters of leaves, stems, and leaf epidermis have a taxonomic importance that support to identify species. These characters are stem form, layers number of palisade with spongy layers of leaf, in addition to the different in the foliar epidermal cell shapes and stomatal index
Keywords
Amaranthaceae; Anatomical characters; Compositae; Epidermal characters; Leaf anatomy; Stem anatomy
Download this article as:| Copy the following to cite this article: Ismaeel Z. A. L. Comparative Anatomical Study for Some Wild Species Belong to Amaranthaceae and Compositae in Iraq. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Ismaeel Z. A. L. Comparative Anatomical Study for Some Wild Species Belong to Amaranthaceae and Compositae in Iraq. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3d7UdIo |
Introduction
Plant anatomy was survey of the plant internal structures, it was a source of fascination with scientific inquiry field from the time of earliest microscopists , It plays an important role in the understanding of plant biology 1 . The internal characters of leaves , roots, stems , trichomes, petioles , plant surface, stomata complex, in addition to the shape and location of crystals with other characters were useful and important features in systematic botany like using of the modern techniques and chemical composition 2,3. Amaranthaceae contains annual in addition to perennial, dioecious, monoious, or polygamousherbs, hermaphroditic, shrubs or rarely trees. It is composed of approximately 800 species of 60 genera with two sub families (Amaranthoideae with Gomphrenoideae)4 .
Compositae ( Asteraceae) , a huge angiospermic family among the dicotyledonous, because the big number of the species approximately 1,620 genera and about 23,600 species which made the family cosmopolitan distribution 5 . From almost 10% of flowering plants worldwide, Compositae is composed of 12 subfamilies that includes edible, medical, noxious and invasive with endangered species. Large number of plant species of this family are herbaceous, shrubs with trees, in addition to creepers as well as climbers 6 . This research is dealt with comparative anatomical features of 10 wild dicots species belong to Amaranthaceae and Compositae growing in different parts in Iraq by using light microscopy. It was conducted that the internal features in view of its great role in establishing the taxonomic position of the studied plant species and it is an attempt to gathering as much datum as possible about these species as well as an aim of providing useful taxonomical information that would give further insight into proper taxonomy and identify species.
Materials and Methods:
Collection and identification of the studied plants
Plants were collected of different regions in Iraq between 2018 and 2020 during the flowering period. After that, the wanted parts were slit into small pieces and fixed in formalin acetic acid solution . After 24 hours, the plant parts were washed several times with ethyl alcohol solution with concentration of 70% then save in it even use 7. The taxonomic identification of the plants was conducted according to AL-Rawi (8) to precise identification and compared with previously identified specimens stored at the Research Center and Museum of Iraqi Natural History, National Herbarium of Iraq.
Anatomical study
The method of AL-Musawi 9 and AL-Zubaidy 10 were used with some modifications. Some sections stained by safranin and glycerine mixture 1:10.
Measurements by micrometers were taken using Altay binocular microscope made in Italy, and photos were taken by a Sony digital camera. Stomatal index and stomatal frequency calculated by the formula mentioned by Salisbury11 :
S indicates number of stomata in unit, while E represents epidermal cells number in the area of the unit.
Results and discussion
Epidermal cells and stomatal complexes
Anatomical research has used successfully to explain taxonomic status and support in identification the species 12,13. The results gained from the study clarify that: the anatomical characters can help to separate taxa.
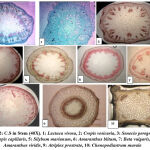
The data for the micromorphological features of foliar epidermis of the examined species were abbreviated in Table (1) and illustrated in Figure (1). Epidermal cells in the examined species varied, they have undulating walls in Crepis capillaris, Lactuca virosa and Amaranthus blitum and with straight walls in Crepis vesicaria , Senecio peregrinus , Silybum marianum , Beta vulgaris and Chenopodiastrum murale،. While it has semi-undulant walls in Amaranthus viridis and Atriplex prostrata. In all the examined species, leaves are amphistomatic with anomocytic complexes in Crepis capillaris , Lactuca virosa , Amaranthus blitum ,Amaranthus viridis and Chenopodiastrum murale. In addition to anisocytic in Crepis vesicaria , Senecio peregrinus , Silybum marianum and Beta vulgaris. Paracytic type was observed only in Atriplex prostrata. Stomatal index varied between adaxial and abaxial layers of the studied species and the largest stomatal index was recorded in Crepis capillaris, while the smallest was in Amaranthus viridis. The results taken from this study showed that, the anatomical features could separate species. Leaf epidermal characters have a good role in classification and determination number of plant species with genera. The variation in the foliar epidermal cells could be used to separate these species. In addition, the study found that epidermal cell walls on the lower surface were more undulating than the cell walls of the upper side. This observation was ensured by Esaue 14, this may be due to the sudden growth of cells during leaf differentiation 15,16. The undulation patterns have an important taxonomic value 17,18. Stace 19 improved that undulating of cell walls is a mesomorphic feature and that environmental state like wetness that play an important function to determining the pattern of anticlinal cell walls. Straight and curved patterns were diagnosis as features of species which develop in arid conditions, however undulate walls were showed mostly in plant growing in area of more humidity. The reasons of decrease or absence of stomatal complexes in some studied species may be due to genetic reason 20 or environmental factors such as drought, as well as, increasing in day length which means increasing exposure to sun light. This opinion was ensured by 16,17 .
Table 1: Quanitative data of foliar epidermis of the studied species
| Species | Upper epidermis | Lower epidermis | ||||
| length of stomata (µm) | Width of stomata (µm) | Index of stomata (µm) | Length of stomata (µm) | Width of stomata (µm) | Index of stomata | |
| Crepis capillaris | 28.5-29.8 (29.5) | 22.2-25.6 (23.4) | 20.1 | 27.5-30.6 (29.1) | 23.4-26.3 (25.2) | 23 |
| Crepis vesicaria | 38.6-39.5 (38.9) | 27.4-29.1 (28.5) | 18.5 | 38.5-39.6 (39.5) | 34.5-36.7 (36.1) | 22 |
| Lactuca virosa | 22.3-25.4 (23.1) | 17.5-20.7 (19.5) | 16.5 | 30.1-33.2 (32.7) | 26.5-29.7 (28.4) | 21.3 |
| Senecio peregrinu | 26.9-28.4 (27.5) | 21.3-24.3 (23.7) | 16 | 29.1-34.5 (33.6) | 22.3-25.5 (24.9) | 20 |
| Silybummarianum | 33.5-35.7 (35.1) | 21.3-23.1 (23.0) | 19 | 35.6-38.9 (37.9) | 25.2-28.1 (27.3) | 22 |
| Amaranthus blitum | 27.1-29.6 (28.5) | 17.5-19.4 (18.4) | 16.4 | 30.2-34.9 (33.7) | 22.1-26.7 (26.1) | 20 |
| Amaranthus viridis | 22.3-27.5 (26.9) | 15.5-16.7 (16.2) | 13.5 | 25.6-27.4 (26.3) | 16.4-18.7 (18.1) | 15.5 |
| Atriplex prostrata | 29.6-30.1 (29.4) | 18.4-20.1 (19.4) | 16.1 | 29.5-32.2 (31.2) | 20.1-23.4 (22.1) | 20 |
| Beta vulgaris | 40.1-42.3 (41.5) | 33.2-35.5 (35.1) | 12.5 | 40.0-43.2 (42.5) | 35.6-36.4 (35.9) | 19.5 |
| Chenopodiastrum murale
|
27.8-29.5 (28.1) | 16.1-17.4 (17.1) | 18.1 | 30.1-33.2 (33.1) | 17.4-19.8 (17.5) | 20.3 |
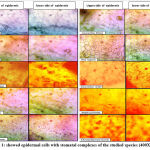 |
Figure 1: showed epidermal cells with stomatal complexes of the studied species (400X). |
Stem anatomy
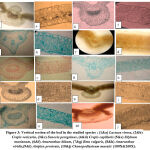
The anatomical characters of the stem of studies species are shown in Table (2) and illustrated in Figure (2). Cross section shape of the stem was genetically controlled, so it was an important taxonomic characters. Tannin filled cells were observed only in the cortex and pith regions of: Lactuca virosa. This result shows a parallelism with the record of AL-Mayah and Hammadi (22) and AL-Helfy (23) . Differences appeared in the thickness of phloem between the studied species; this difference may occur due to the need of plant to the food because, as known, phloem was the food conducting tissue of the vascular plants. While, increase or decrease in phloem fibers was related to support needed, according to the plant species. Tracheary elements of wood resembled by vessels and trachieds. Wood arms projected clearly towards the pith.
Table 2: Qualitative and quantitative data of stem anatomy of the studied species.
| Species | Stem cross section shape | Epidermis thickness (µm) | thickness of Cortex (µm) | thickness of Vascular bundle (µm) | Diameter of xylem vessel (µm) |
| Lactuca virosa | Ovate to circular | 13.3-17.5
(15.2) |
216.7-225.4 (223.1) | 498.2-520.4
(510.5) |
19.2-22.5 (20.1) |
| Crepis vesicaria | Sub-circular to ovate | 25.2-35.00
(33.7) |
183.6-189.1 (186.1) | 606.7-612.3
(610.5) |
16.7-19.4
(17.8) |
| Senecio peregrinus | Quadrangular | 18.3-24.5 (21.4) | 108.2-115.7 (111.9) | 130.1-136.3 (132.2) | 9.5-14.4 (11.9) |
| Crepis capillaris | Circular to sub-circular with slightly wavy outline | 19.8-28.2
(24.3) |
99.5-108.1
(102.4) |
188.0-192.1
(190.3) |
12.2-17.7
(15.6) |
| Silybum marianum | Circular with opposite concavity | 12.5-18.4
(17.5) |
75.4-84.2
(80.1) |
223.4-235.1
(233.5) |
17.2-22.1
(19.6) |
| Amaranthus blitum | Subcircular with slightly observed angles | 26.3-30.1 (28.5) | 230.1-236.4 (233.2) | 512.5-522.3 (520.5) | 19.4-23.4 (22.5) |
| Beta vulgaris | Sub-circular to ovate | 20.3-25.5 (22.5) | 110.2-115.7 (112.5) | 140.1-146.3 (142.4) | 8.5-13.4 (10.1) |
| Amaranthus viridis | circular | 16.6-23.4
(22.5) |
43.2-50.1
(49.1) |
66.2-74.6 (72.5)
|
28.2-31.2
(30.3) |
| Atriplex prostrata | Sub-circular | 17.2-22.6 (20.1) | 36.1-49.4 (46.4) | 68.6-75.1 (71.8) | 10.2-13.5 (11.4) |
| Chenopodiastrum murale
|
Quadrangular | 13.6- 19.4 (14.5) | 67.9-76.8 (72.1) | 407.0-413.2 (410.2) | 11.2-15.4 (14.1) |
Leaf anatomy
The anatomical features of the leaf of the studies species are summarized in Table (3) and illustrated in Figure (3). This study revealed differences in the leaf anatomical characters of the studied species, these characters could be used to separate these species. Leaf blade comprised of an upper epidermis, lower epidermis and mesophyll between them. The upper and lower epidermis was monolayered cells of different shapes and sizes, depending on species. Epidermal external walls of cells were slightly cutinized and protected by cuticle (24). The upper epidermal cells were larger than that of the lower epidermis except in Lactuca virosa. Mesophyll was heterogenous comprised of palisade and spongy layers (Dorsiventral leaves) in all the studied species. In dorsiventral leaves, the results revealed that there were differences between palisade and spongy layer thickness and cell rows number. Palisade cells were highly compacted; the compactness of the palisade parenchyma depending directly upon light intensity. This layer increased in row numbers in some of the studied species, due to obtain the energy suitable for the photosynthesis.
Table 3: Quanitative data of leaf anatomy of the studied species.
| Species | upper epidermis Thickness (µm) | lower epidermis Thickness (µm) | palisade layers No. | Thickness of palisade layer (µm) | Thickness of spongy layers (µm) | No. of midrib vascular bundles | Thickness of midrib vascular bundle (µm) |
| Lactuca virosa | 13.0-18.6
(15.5) |
17.0-21.3
(19.0) |
2-3 | 29.1-38.8
(35.4) |
61.5-65.1
(63.1) |
1 | 140.2-146.1 (143.5) |
| Crepis vesicaria | 16.3-19.0
(17.5) |
13-17.3
(15.0) |
2-3 | 66.7-79.6
(72.5) |
101.7-115.4
(108.0) |
3 | 303.4-313.2
(308.5) |
| Senecio peregrinus | 9.2-11.7
(10.6) |
8.5-13.4
(10.5) |
2-3 | 38.1-56.4
(47.1) |
33.2-39.4
(36.8) |
1 | 97.1-102.4
(99.5) |
| Crepis capillaris | 10.5-18.7
(16.3) |
9.5-14.2
(13.4) |
2-3 | 26-34.8
(31.5) |
26.2-36.4
(35.5) |
1 | 291.5-297.3
(292.8) |
| Silybum marianum | 7.8-13.4
(9.2) |
6.5-9.5
(7.5) |
2-3 | 29.1-33.8
(30.4) |
35.7-43.1
(40.3) |
1 | 127.7-135.4
(133.2) |
| Amaranthus blitum | 15.5-26.3
(25.5) |
14.0-17.3
(16.1) |
2-3 | 65.0-70.1
(67.3) |
37.5-40.9
(63.1) |
1 | 86.5-92.3
(90.7) |
| Beta vulgaris | 15.0-18.1
(16.2) |
10.3-16.3
(12.5) |
2-3 | 59.1-66.7 (64.3)
44.5-53.1 (50.5) |
35.2-39.8
(37.1) |
1 | 119.2-125.7
(123.5) |
| Amaranthus viridis | 13.3-18.4
(17.1) |
9.9-12.6 (11.2) | 2-3 | 33.7-39.4
(35.2) |
20.0-28.7
(25.5) |
1 | 155.0-159.6
(158.5) |
| Atriplex prostrata | 17.3-18.9
(18.1) |
14.7-17.6
(17.1) |
3-4 | 31.8-39.5
(35.4) |
38.8-41.5
(40.5) |
1 | 212.2-218.1
(216.5) |
| Chenopodiastrum murale
|
16.5-19.4
(17.4) |
13.8-15.5
(15.1) |
2-3 | 104.2-113.5
(110.2) |
135.5-146.3
(144.5) |
1 | 312.6-318.1
(314.5) |
Conclusion
The present study proved very helpful and resulted in exploration of valuable distinct variations: Presence of distinct leaf patterns of epidermal cells as well as distinct stomatal complexes in some species, such as: paracytic on the epidermis of Atriplex prostrata. Tannin filled cells observed only in the stem cortex and pith of Lactuca virosa , as well as the Leaf upper epidermal cells of the same species were smaller than the lower cells.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding Source.
References
- Dengler, N. G. An integral part of Botany (Book review). Amer. J. of Bot. 2002.89(2):369-374.
CrossRef - Tripathi, S. and Mondal, A. K. Comparative (quantitative and qualitative) studies of the stomata of selected six medicinally viable species of Cassia Int. J. of Sci. Bt. & Pharm. Res. 2012. 1(3):104-113.
- Yetisen, K; Ozdemir, C.; Kucuoduk, M. and Akyol, Y. A morphological and anatomical study of Hyacinthella glabrescens (Liliaceae). Phytol. Balcan. 2012. 18(3): 319-322.
- Iamonico, D. A nomenclatural survey of the genus Amaranthus (Amaranthaceae) 7: names published by Willdenow. Botanic Garden and Botanical Museum Berlin. 2020. 50(1) : 147-155.
CrossRef - Faust, W.Z. and Jones, S.B.J.. The systematic value of trichome complements in the North American group of Vernonia (Compositae). Rhodora. 1973.75:517-528.
- Basu, S., Zandi, P., Cetzal-Ix, W., & Sengupta, R. 2014.Asteraceae: The sunflower family. Retrieved from http://www.eoearth.org/view/article/53cd57450cf2d022a359c79b
- Esmaeel,Z.A.L. Comparative anatomy of some wild Dicots Spp. grown in Baghdad Province. Ph.D. Thesis. University Of Baghdad. Iraq. 2014.
- Al-Rawi A. Wild plants of Iraq. Ministry of Agriculture & Irrigation . Abu Ghiraib- Iraq .1964.
- AL-Musawi, A.H. A systematic study of the genus Hyoscyamus (Solanaceae) .Ph.D. thesis.Uni .of Reading . U.K. 1979 .P.96.
- AL-Zubaidy , A.M.A. Systematic Study of the genera (Ajuga,Marrubium L., Lallemanita Fisch . and C.A. Mey And Laminm L. ) of Labiatae in Iraq . Ph.D. thesis .Univ. of Baghdad .Iraq . 1998 .
- Salisbury , E.J. On the cause and ecological significance of stomatal frequency with special reference to the woodland flora. Philosophical Transactions of the Royal Society of London. Biol. Sci. 1972. 216:1-65.
CrossRef - Scatena, V.L.; Giulietti, A.M.; Borba, R.L. and Vander, B.C. Anatomy of Brasilian Eriocaulaceae: correlation with taxonomy and habitat using multivariate analysis. Plant syst. Evol. 2005.253:1-22.
CrossRef - Munir, M. Khan; M.A. Ahmad, M.; Abbasi, A.M; Zafar, M.; Khan, K.Y; Taria,K.; Tabassum,S.; Ahmed,S.N.; Habiba,U. and Bano, A. Taxonomic Potential of Foliar epidermal anatomy among the wild culinary vegetables of Pakistan. J.Med. Plant Res. 2011. 5(13):2857-2862.
- Esaue,K. Plant anatomy.1stChapman Hall. 1953.767 PP.
- Avery,G.S.Jr. Comparative anatomy and morphology of embryos and seedlings of Maize, Oats.And wheat.Bat.Gaz. 1933.89:1-39.
CrossRef - Esaue,K.Plant anatomy.2ndToppanCompany,Ltd. 1965. 767 PP.
- Metcalf,C. andChalk,L. Anatomy of Dicotyledons leaves, Stems and wood in Relation to taxonomy with notes on Economic uses. Clarendon Press. Oxford. 1950. 1500 PP.
- Culter, D.; Botha; T. and Stevenson,D. Plant Anatomy and Applied Approach. Black well Publishing. 2007. P:52-60
- Stace, C.A. Cuticular studies as an aid to plant taxonomy. Bull. Br. Museum (Natural History).Bot. 1965.4:1-78.
- Aliwy, S. A. A. Comparative anatomical study of selected Compositae species grown in Jadiriyah Campus, Baghdad University. MSc. Thesis. Baghdad Univ Iraq. 2009. (In Arabic).
- Fahn, A. Plant anatomy. 2nd Pergamon Press. Oxford. 1974. PP.611.
- Hammadi, K.J.; Al-Mayah, A.A. and Al-Rubai. Comparative anatomical study of some genera of Polygonaceae in Iraq. Basrah Res. J. 2002. 28:90-109.
- Al-Helfy, M. A. A. Systematic study of the genus Erodium in Iraq. Ph.D. Thesis. Univ. of Basrah. Iraq. 2000. (In Arabic).
- Batanouny, K.H. Anatomy of plants. Univversity Press of Cairo, Cairo. 1992. pp.104.