Manuscript accepted on :08-07-2022
Published online on: 15-07-2022
Plagiarism Check: Yes
Reviewed by: Dr. Shabana Khatoon
Second Review by: Dr. Dini Damayanti
Final Approval by: Dr. Ian James Martin
Sk. Aminabee* , K. Indraja
, K. Indraja , K. Matha Manogna, K. Naga Devika
, K. Matha Manogna, K. Naga Devika , K. Ramya Sri
, K. Ramya Sri , A. Lakshmana Rao
, A. Lakshmana Rao
Department of Pharmacology, V. V. Institute of Pharmaceutical Sciences, Gudlavalleru, Andhra Pradesh, India.
Corresponding Author E-mail: aminaammi786@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2467
Abstract
Background: Worldwide, the major death causing diseases are cardiovascular diseases and today the need for herb based therapeutics is needed. Present study was undertaken the whole plant of Indigofera Barberi (IB) to evaluate its cardioprotective activity against cardiotoxicity on rats induced by Doxorubicin (DXR). Methods: Soxlet extraction was used to prepare extracts. Preliminary phytochemical tests and in-vitro antioxidant activity are the methods used for standardization of all the extracts. Chloroform extract of Indigofera barberi (CEIB) and aqueous extract of Indigofera barberi (AQIB) are two extracts obtained from above activity were selected against induced cardiotoxicity of DXR to determine in-vivo cardioprotective activity. Total flavonoid and phenol content was determined. Endogenous antioxidants (MDA, GSH), ECG and histophological studies are the parameters of serum (CK, CK-MB, LDH) and non serum to evaluate the cardioprotective activity. Results: Serum elevated levels of biomarker, decreased antioxidant activity, changes in electrocardiogram (ECG) and histopathological studies are shown by DXR alone treated rats. The toxicity produced by DXR has reversed on the rats pre-treated with CEIB and AQIB. CEIB has shown more activity when compared to AQIB. Compared to standard vitamin E the activity of CEIB was found to be significant. Conclusion: The protective effect of IB plant on DXR induced cardiotoxicity was revealed. To understand the mechanism of action and to reveal phytochemical responsible for the said activity the further research to be undertaken.
Keywords
Antioxidant; Cardioprotective; Cardiotoxicity; doxorubicin; heart failure; Indigofera barberi
Download this article as:| Copy the following to cite this article: Aminabee S. K, Indraja K, Manogna K. M, Devika K. N, Sri K. R, Rao A. L. Antioxidant and Cardioprotective Activity of Indigofera barberi on Doxorubicin Induced Toxicity on Rats. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Aminabee S. K, Indraja K, Manogna K. M, Devika K. N, Sri K. R, Rao A. L. Antioxidant and Cardioprotective Activity of Indigofera barberi on Doxorubicin Induced Toxicity on Rats. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3IDRGRB |
Introduction
Heart attacks and strokes are usually acute events and are mainly caused by a blockage that prevents blood from flowing to the heart or brain. The most common reason for this is a build-up of fatty deposits on the inner walls of the blood vessels that supply the heart or brain. Strokes can be caused by bleeding from a blood vessel in the brain or from blood clots. The most important behavioral risk factors of heart disease and stroke are unhealthy diet, physical inactivity, tobacco use and harmful use of alcohol. The effects of behavioral risk factors may show up in individuals as raised blood pressure, raised blood glucose, raised blood lipids, and overweight and obesity. These “intermediate risks factors” can be measured in primary care facilities and indicate an increased risk of heart attack, stroke, heart failure and other complications.
The antioxidant properties of medicinal plants which could be ascribed to antioxidants phytochemicals related to therapeutics actions. Numerous medicinal plants and plant products having cardioprotective effect are documented. In the management of cardiovascular related disorders, currently available synthetic drugs serves as viable alternatives obtained from sustainable agents from natural sources6. Understanding the toxic side effects & high cost of most synthetic drugs many patients in developing countries are not reading accessible.
Doxorubicin (DOX)1 which belongs to the class of anthracyclines is one among the effective antitumor antibiotics. Due to dilation and high incidence of myocardial damage its use is limited.
After cumulative DOX administration congestive heart failure, cardiomyopathy and electro cardiographic changes were demonstrated2. Oxidative myocardial damage produced by anthracyclines cause excess production of free radicals and oxidative stress at a very high level. Antioxidant effect of therapeutic interventions may offer considerable cardio protection with this regard3. The study analysis of oxidative stress in cardiac tissues and drugs therapeutic efficacyin laboratory animals which are cancerfree serves as appropriate models by DOX induced cardio toxicity. To decrease the risk of DOX induced cardio toxicity while maintaining its efficacy several approaches may be taken4.
The altered schedules of drug administration, modifications of anthracycline molecules, adjunctive treatment with β-adrenergic blockers, ACE inhibitors, dexrazoxane and probucol were included. None of these have been entirely successful. To prevent r treat DOX induced cardio toxicity a new drug is needed5.
Endemic herb of high value in Tirumala hills is Indigofera barberi of Fabaceae family. Vernacularly name is Adavineelimanadumokka. It is an under shrub grows upto 1m tall. It has faintly angled branchlets. 3 foliated leaves, oblong ovate leaflets, pubescent, obtuse, mucronate7. Arrangement of pink color floweres is axillary congested racemes. Pods appressed, deflexed, sub-terete, sharply pointed and white tomentose. 2-4 number seeds. September or December is flowering and fruiting season. For controlling diabetes, leaf powder (5g) is taken orally8.
Along with buttermilk, pills of peanut size are prepared by leaves (50g), garlic (1g), pepper(1g) which are made into paste9. As practice followed by Nakkala and many tribal physicians, for 5 days once a day 5 pills are takento cure jaundice. It is also used as dye and colouring agent10. For the treatment of many skin diseases, peptic ulcers and to expel intestinal worms whole plant powder (5g) is taken for 10 days with rice washed water once a day. To cure wounds, cuts, burns and boils leaf juice is used as an antiseptic11.
Using biochemical makers and histopathological analysis, the study was undertaken to investigate the possible effects of Indigofera barberi extract against doxorubicin induces cardiotoxicity in rats was undertaken in the present study keeping these facts in view.
Materials and Methods
Plant material
The plant was identified and collected in Tirumala hills (deciduous forest) in Andhra Pradesh, India. Dr. K. Madhava chetty, a Plant Taxonomist, Department of Botany, Sri Venkateswara University, Tirupathi, India authenticated the samples. Sorting, cleaning and at room temperature it was airdriedfor about 1 week were done to the whole plant of Indigofera by using the laboratory hammer mill. Until required, storage is done in water and air proof containers protected from heat and sun light.12
Preparation of extracts
In soxhlet apparatus using petroleum ether, chloroform, ethanol and distilled water the Indigofera barberi powdered materials were extracted successively for 18 hrs. To get free from the solvents, the extracts were concentrated to dryness in rota evaporator13.
Phytochemical analysis
For the presence of saponins,alkaloids,glycosides, tannins, terpenoids, flavonoids,carbohydrates, proteins, aminoacids, sterols and fixed oils phytochemical analysis of extracts were carried out by different methods14.
Total flavonoids estimation
Aluminium chloride calorimetric assay15 was used to measure the total flavonoid content. To a 10 ml volumetric flask containing 4 ml of distilled water an aliquot (1ml) of extracts and standard solution of cetechin (100mg/ml) were added. 10 % Alcl3 about 0.3ml was added after 5 min. Then after 1 min, the volume was made up to 10ml with distilled water by addition of 2 ml of 1M NaoH. The content of the solution were mixed well and an absorbance was measured against prepared blank reagent at 510 nm. Fresh weight expressed as mg catechin equivalents (CE)/100G for total flavonoid contents of roots. Analysis of samples are analysed in triplicates.
Estimation of total phenols
Based on Folin-Clocalteu reagent assay16 total phenolics are determined. To 25 ml of volumetric flask, containing 9 ml distilled water an aliquot (1ml) extracts and standard solution of Gallic acid (100mg/ml) were added. The distilled water was used as blank. To the mixture 1 ml of Folin-Ciocalteu reagent was added and shaken. 10ml of 7% Na2co3 solution was added to the mixture after 5 min. The solution was diluted with water and mixed to a volume of 25 ml. An absorbance of 750nm with UV-vis spectrophotometer was determined against prepared blank reagent after incubation for 90 min at room temperature. Fresh weight of root extracts expressed as mg gallic acid equivalents (GAE)/100G gives the total phenolic content. Samples are all analyzed in triplicates.
Acute toxicity studies
According to OECD guidelines17, for chloroform extract acute toxicity studies were conducted on albino mice (20-25g). Healthy adult mice, were divided into groups (n=5), at the doses of 5, 50, 300 and 200 mg/kg body weight, chloroform extract was administered orally. For mortality and physical/behavioural changes animals were observed for over 14 days.
In-vitro antioxidant studies
DPPH radical scavenging activity assay
The free radial scavenging activity of the Indigofera barberi (IB) extracts, in-vitro, was measured by using 2,2-diphencyl 1-1-picrylhydrazyl (DPPH). 24 mg of DPPH was dissolved in 100 ml methanol and stored at 20oc which is used as stock solution. To succeed an absorbance approximately 0.95 ±0.02 by using the spectrophotometer at an absorbance of 517 nm, DPPH solution was impoverished which serves as working solution. 100 μl of extracts at various concentrations ( 10-100 μg/ml) and aliquant of the solution (3-4ml) were mixed thoroughly18. At room temperature in a dark room for about 15 min the reaction mixture which is shaken well is then incubated. The absorbance was taken at 517 nm. As the procedure mentioned above, the control was prepared without any extract. Based on the following equation, the percentage scavenging activity of DPPH was estimated.
Scavenging effect (%) = Abs. of control – Abs. of sample X 100
Abs. of control
Hydrogen peroxide (H2O2) scavenging activity
In a 50 mM phosphate buffer freshly prepared H2O2 (2mM) was added19. Different extracts of aliquants (0.01ml) were transferred into test tubes by adding 50mM phosphate buffer (pH 7.4), and made volumes up to 0.4ml. Tubes are vortexed after addition of 0.6ml H2O2 solution and an absorbance of H2O2 was determined at 230 nm which is performed against blank after 10 min. The abilities to scavenge H2O2 was calculated by using the specified equation:
H2O2 scavenging action = Abs. of control – Abs. of sample X 100
Abs. of control
Nitric oxide scavenging activity
By taking 2ml of 10mM sodium nitroprusside and a saline phosphate buffer (pH 7.4) of 0.5ml which is mixed with various concentrations of 0.5 ml of extract prepared in ethanol and mixture was incubated for about 30 min at 25oC. To each test tube, 1.5 ml of Griess reagent (contains phosphoric acid 3%, sulphanilamide 1% and napthylethylenediame dichloride 0.1%) is added correctly20. The absorbance was measures at 546 nm and with reference to ascorbic acid as standard; the percentage scavenging activity was compared. Nitric oxide radicals scavenging activity was calculated by comparing the absorbance values of control and absorbance values of test samples (extracts) using the following equation, the % inhibition of nitric oxide liberated was determined:
% inhibition of nitric oxide =Abs. of control – Abs. of sample X 100
Abs. of control
In-vivo cardioprotective studies
Animals and experimental protocol
In accordance with the guidelines of CPCSEA, male wiser rats of about 250-300 g of weight used in the study. In a temperature controlled room at 22 ± 3oC with 12h light-dark cycle, well ventilated room animals were housed. Water was provided ad libitum21 and food consisted of normal rat chow. To avoid stressful conditions care taken between 8 and 10 m experimental procedures were performed. Rats were divided randomly into four groups (12 rats each).
Group I: Received normal saline (1 ml/200 g body weight/day) orally for 10 consecutive
days
Group II: Received normal saline orally for 10 consecutive days and a single dose of DOX
(15 mg/kg, i.p.) on day 7
Group III: Received Vitamin E combined with DOX
Group IV: Received the CEIB (250 mg/kg) combined with DOX
Group V: Received the CEIB (500 mg/kg) combined with DOX
Group VI: Received the AQIB (250 mg/kg)combined with DOX
Group VII: Received the AQIB (500 mg/kg) combined with DOX
For 10 consecutive days the plant was extracted was administered and on day 7 DOX was administered. ECG was recorded using Niviqure Inco polygraph system and changes in heart rate and QT and ST changes were computed from the ECG after 48 hours of DXR challenge.
With thiopentone (35 mg/kg, i.p.) rats were anesthetized. In serum separating tubes by orbital puncture blood samples were collected. To separate the sera the blood was centrifuged at 3000g for 15 min and for the biochemical analysis they were kept at 70oC. The hearts were rapidly dissected out, by opening the abdomen of each rat, in ice-cold isotonic saline they were washed and between two filter papers they were blotted. In 10% formalin four hearts from each group were fixed for histopathological examination and for subsequent analysis the remaining hearts from each group were homogenized in ice-cold 0.1 M potassium phosphate buffer (pH 7.4) and stored at -70oC.
Cardiac biochemical assay
According to method of Adams22, cardiac GSH was determined. According to the method of Hissin and Hilf23 GSH level was assessed and values were expressed as nmol/mg protein. According to the method of Mihara and Uchiyama24 by measuring malondialdehyde (MDA) content in tissue homogenates liquid peroxidation products were determined. At 532 nm by spectrophotometrically the MDA content was measured. Based on a standard curve using 1,1,3,3-tetra ethoxypropane as a standard the MDA content was calculated. As nmol/g protein values are expressed.
Serum biochemical assay
Using diagnostic kits the activities of creatine kinase (CK), creatine kinase isoenzyme-MB (CK-MB) and lactate dehydrogenase25 (LDH) were determined according to standard methods. By measuring the rate of NADPH formation at 340 nm the CK-MB activity was assayed by measuring the rate of nicotinamide adenine dinucleotide reduced from (NADH) formation at 340 nm, the LDH activity was determined26.
Histopathological study
Under a light microscope, examination of hearts which were cut at 0.5 μm are mounted on slides, stained with hematoxylin and eosin were examined.
Statistical analysis
As mean ± SEM results are presented by use of one-way ANOVA followed by Dunnetts multiple comparison, compares data between treatment groups were done. At p<0.05, <0.01 and <0.001 values were considered statistically significant.
Results
Phytochemical analysis
The results were carried out and displayed for the phytochemical screening of various extracts i.e., petroleum ether extract of IB (PEIB), chloroform extract of IB (CEIB), ethanol extract of IB (EEIB), and aqueous extract of IB (AQIB) in Table 1.
Table 1: Phytochemical screening of successive solvent extraction of IB.
| Phytoconstituents | Method | Aqueous extract |
Ethanolic extract |
Chloroform extract |
Pet. Ether extract |
| Flavonoids | Shinoda Test | + | + | + | – |
| Zn. Hydrocholride Test | + | + | + | – | |
| Lead acetate Test | + | + | + | – | |
| Volatile Oil | Stain Test | – | + | – | – |
| Alkaloids | Wagner Test | – | – | – | – |
| Hager’s Test | – | – | – | – | |
| Tannins & Phenols | Fecl3 Test | + | + | + | – |
| Pot.Dichromate Test | + | + | + | – | |
| Saponins | Foaming Test | + | + | – | – |
| Steroids | Salkowski Test | + | + | – | + |
| Carbohydrates | Molish Test | – | – | – | – |
| Acid Compounds | Litmus Test | – | – | – | – |
| Glycoside | Keller-Killani Test | + | + | – | – |
| Amino Acids | Ninhydrin Test | – | – | – | – |
| Proteins | Biuret | – | – | – | – |
“+”: Present; “-”: Absent
Total flavonoids and phenolic content of IB extracts
Presence of total flavonoids and phenols in different extracts of IB are given in table 2. The maximum amount of flavonoids and phenolic compounds are present in CEIB followed by AQIB, PEIB, MEIB shows the result.
Table 2: Total flavonoids and phenols of different extracts of IB.
| Extract | Total Flavonoids (% w/w) Mean ± SEM |
Total Phenols (% w/w) Mean ± SEM |
| PEIB | 5.61 ± 0.12 | 3.38 ± 0.19 |
| CEIB | 7.12 ± 0.61 | 5.76 ± 0.88 |
| EEIB | 5.51 ± 0.45 | 3.12 ± 0.21 |
| AQIB | 6.23 ± 0.59 |
5.4 ± 0.52 |
Acute toxicity studies
For chloroform extract acute toxicity studies were conducted on albino mice (20-25g). the extract was found to be safe at 2000 mg/kg.
In-vitro antioxidant studies
By DPPH medical scavenging activity assay, hydrogen peroxide (H2O2) scavenging activity and nitric oxide scavenging activity determines the antioxidant activity of various extracts of IB in table 3,4,5 respectively. The % inhibition at various concentrations was more for (10-100 μg/ml) CEIB when compared to others was the result revealed.
Table 3: DPPH radical scavenging activity of Indigofera barberi extracts.
| Extract | Concentration (µg/ml) and % inhibition | |||||
| 10 | 20 | 40 | 60 | 80 | 100 | |
| Ascorbic acid | 58.51±0.11 | 65.59±0.51 | 72.36±0.14 | 78.21±0.28 | 81.36±0.14 | 91.68±0.19 |
| PEIB | 11.89±0.19 | 18.45±0.46 | 20.76±0.05 | 23.18±0.52 | 25.51±0.33 | 27.33±0.10+ |
| CEIB | 30.47±0.22 | 33.52±0.64 | 45.09±0.14 | 51.17±0.64 | 54.71±0.46* | 63.15±0.01* |
| EEIB | 14.15±0.31 | 17.19±0.59 | 22.09±0.28 | 24.15±0.33+ | 26.61±0.28 | 29.16±0.08 |
| AQIB | 16.28±0.45 | 23.28±0.87 | 27.98±0.36 | 33.65±0.41+ | 39.71±0.91* | 43.31±0.11 |
Each value represents the Mean± SEM. *P<0.01, +P<0.05 equated to postive control
Table 4: Hydrogen peroxide scavenging activity of Indigofera barberi extracts.
| Extract | Concentration (µg/ml) and % inhibition | |||||
| 10 | 20 | 40 | 60 | 80 | 100 | |
| Ascorbic acid | 54.95±0.11 | 62.48±0.95 | 73.56±0.28 | 79.79±0.11 | 83.57±0.23 | 92.58±0.51 |
| PEIB | 10.15±0.28 | 12.74±0.62 | 15.01±0.32 | 19.08±0.08 | 21.73±0.28 | 23.85±0.33 |
| CEIB | 31.76±0.59 | 34.73±0.23 | 46.71±0.46 | 53.75±0.17 | 59.95±0.29+ | 63.09±0.12* |
| EEIB | 10.75±0.76 | 14.86±0.58 | 18.31±0.89 | 22.17±0.42 | 26.55±0.25+ | 30.91±0.28 |
| AQIB | 17.34±0.97 | 23.85±0.31 | 29.51±0.91* | 37.84±0.25 | 39.76±0.16* | 43.78±0.21 |
Each value represents the Mean± SEM. *P<0.01, +P<0.05 equated to postive control
Table 5: Nitric oxide scavenging activity of Indigofera barberi extracts
| Extract | Concentration (µg/ml) and % inhibition | |||||
| 10 | 20 | 40 | 60 | 80 | 100 | |
| Ascorbic acid | 57.41±0.11 | 66.34±0.25 | 71.63±0.26 | 76.91±0.78 | 83.08±0.91 | 91.67±0.48 |
| PEIB | 11.14±0.23 | 15.38±0.41 | 19.11±0.61 | 21.76±0.72 | 23.51±0.08 | 27.65±0.55 |
| CEIB | 23.16±0.56 | 27.85±0.85 | 32.16±0.58 | 42.74±0.41* | 57.99±0.15 | 67.56±0.66 |
| EEIB | 14.56±0.78 | 20.45±0.96 | 27.61±0.27+ | 34.11±0.26 | 38.67±0.61* | 42.56±0.72 |
| AQIB | 12.14±0.09 | 20.43±0.23 | 26.91±0.71 | 38.51±0.29+ | 52.13±0.35 | 59.61±0.77+ |
Each value represents the Mean± SEM. *P<0.01, +P<0.05 equated to postive control
In-vivo cardioprotective studies
Non-serum parameters
When compared to normal group on DXR administration there is a significant increase in ST and QT intervals whereas the heart rate was decreased. The changes caused by DXR administration were reversed by pretreatment with chloroform extract of Indigofera barberi (CEIB) as well as vitamin E. When compared to normal as well as test treated animals, a significant decrease in body weight, heart weight have shown on rats treated with DXR.
Table 6: ECG changes of IB extracts on DXR intoxicated rats.
| Group | Treatment | ST interval (msec) | QT interval (msec) | Heart rate (msec) |
| I | Normal | 36.12 | 66.88 | 353.8 |
| II | Control (DXR) | 48.14 | 81.86 | 301.3* |
| III | Vitamin E + DOX | 37.43* | 65.89 | 402.5 |
| IV | CEIB (250 mg/kg) + DOX | 38.52 | 69.23 | 369.2 |
| V | CEIB (500 mg/kg) + DOX | 40.12 | 71.45** | 375.1 |
| VI | AQIB (250 mg/kg) + DOX | 43.21 | 70.39 | 381.2* |
| VII | AQIB (500 mg/kg) + DOX | 46.69* | 75.21 | 389.2 |
Each value represents the Mean ± SEM. *P<0.01, **P<0.05 equated to postive control
Serum parameters
In DXR treated rats, the serum CKMB levels of IB extracts shown protective effects which is shown in table 7. DXR treated rats had serum CKMB level of 235.9 ± 1.06 UI/L which shows induced cardio toxicity on rats on DXR treatment. Serum CKMB level decreased to 156.32 ± 1.4 UI/L on rats treated with vitamin E, which was significantly lower when compared to DXR control group. A serum CKMB level of 196.6 ± 0.36 and 174.11±0.23 respectively was observed on treatment with CEIB at 250 mg/kg and 500 mg/kg. A serum CKBM level of 201.32 ± 1.56 and 193.6 ± 1.18 respectively was observed on treatment with AQIB at 250mg/kg and 500 mg/kg when compared to DXR control group, both values are significantly lower.
Table 7: Effect of IB extracts on CK, LDH, CK-MB, MDA and GSH.
| Group | CK (UI/L) | LDH (UI/L) | CK-MB (UI/L) | MDA (nmol/g heart tissue) |
GSH (nmol/g heart tissue) |
|
| I | Normal | 155.4 ± 1.57 | 239.5 ± 0.91 | 134.71 ± 1.5 | 16.95 ± 0.96 | 2.98 ± 0.05 |
| II | Control (DXR) | 328.2 ± 0.9 | 418.7 ± 1.18 | 235.9 ± 1.06** | 48.91 ± 0.39 | 1.53 ± 0.03 |
| III | Vitamin E + DOX | 226.3 ± 1.12* | 321.5 ± 1.32 | 156.32 ± 1.4 | 24.94 ± 0.35 | 2.46 ± 0.11* |
| IV | CEIB (250 mg/kg) + DOX | 281.51 ± 0.89 | 385.3 ± 1.16 | 196.6 ± 0.36 | 39.56 ± 0.51 | 1.92 ± 0.09 |
| V | CEIB (500 mg/kg) + DOX | 232.45 ± 0.89 | 336.5 ± 0.98** | 174.11 ± 0.23 | 29.12 ± 0.62 | 2.12 ± 0.06 |
| VI | AQIB (250 mg/kg) + DOX | 294.34 ± 1.32 | 393.61 ± 1.11 | 201.32 ± 1.56 | 40.26 ± 1.3** | 1.78 ± 0.21 |
| VII | AQIB (500 mg/kg) + DOX | 275.3 ± 0.36 | 356.21 ± 1.33 | 193.6 ± 1.18* | 38.21 ± 1.25 | 2.09 ± 0.06 |
Each value represents the Mean ± SEM. *P<0.01, **P<0.05 equated to postive control
Histopathological study
On doxorubicin intoxicated rats the cardioprotective activity of CEIB was given in fig 1. I. On treatment with vehicle to normal heart tissue exhibited histology with normal myocardial cells with well defined myoplasm, prominent nuclei and nucleolus. II histology of heart section shows fatty changes, inflammation and damage of myocardial architecture with myocardial necrosis when treated with doxorubicin. III. Histology of heart tissue showed potential recovery of normal myocyte when compared to doxorubicin treated group upon treatment with standard vitamin E. IV. Histology of heart showed less necrosis when treated with CEIB (250 mg/kg). V. Histology of heart tissue returned to quite normal from injured heart when treated with CEIB (500 mg/kg) VI. Histology of heart tissue showed less vacuolization of the cytoplasm when treated with AQIB (250 mg/ kg). VII histology of heart tissue showed less necrosis when compared to doxorubicin treated group when treated with AQIB (500 mg/kg).
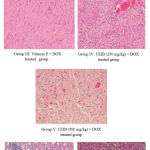 |
Figure 1: Cardioprotective activity of different extracts of IB. |
Discussion
Preparation of chloroform and ethanol extracts of IB was done and CBIE has highest yield. Many biological activities were exhibited by flavonoids and phenols through many studies. Thus, from CEIB and AQIB flavonoids and phenols are isolated27.
For the investigation of IB and DOX induced myocardial toxicity the present study was done28. The result suggests that DOX induced cardiomyopathy in rats is prevented by IB. For evidence the following lines are emphasized from the present study.
In the study, a significant elevation in the level of the diagnostic markers CK, CK-MB, LDH by DOX treated rats moreover, the severity of DOX induced myocardial damage is indicated by the elevated levels of these enzymes, which is in line with an earlier report29. DOX induced elevation of serum marker was significantly reduced which is shown by prior administration of CEIB 250mg/kg +DOX and 500mg/kg+ DOX. For maintaining architectural integrity and normal structural of cardiac myocytes can be done by restriction of leakage of these enzymes, CEIB is responsible which is confirmed by the reduction in the enzyme level through which membrane stabilizing property of CEIB can be accounted30.
For the definite diagnosis of myocardial injury, electrocardiograph abnormalities are the main criteria generally used. As compared to normal rats DOX induced rats shows significant alteration of ECG patterns. Decrease in the heart rate and prolongation of ST interval were the characteristic finding31. Prolongation of QT interval was also found in addition to this. The severity of DOX induced mayocardial damage is indicated by the ECG changes, which is in line with earlier report. By ST prolongation, oxidative stress caused due to consecutive loss of cellular damage might be characterized. A protective effect against DOX induced altered ECG patterns were shown of pretreatment of CEIB 250 mg/kg + DOX and 500mg/kg +DOX.
From the present study, the mechanism of cardiotoxicity induced by a DOX is not clearly known, although large body of evidence shows a decrease in endogeneous antioxidants, increased oxidative stress by increase in oxygen free radicals, which is followed by development of a variety of subcellular changes in the myocardium, typical of DOX induced cardiac injury. We found a significant increase in heart tissue in rat treated with DOX32.
Decrease in levels of GSH and increased lipid per oxidation are suggested by MDA levels. Depletion antioxidants and increased oxidative stress are responsible for the damage of the cardiac tissue as reported earlier. The oxidative stress and cardiac damage are confirmed by an increase in heart tissue MDA levels with an decrease in levels of GSH on DOX treated rats. The changes in MDA and enzyme levels by DOX induced changes are prevented by CEIB. In heart tissue of CEIB 250mg/kg + DOX, CEIB 500 mg/kg +DOX and vitamin E treated groups shown significant increase in the GSH activity and decrease in lipid peroxidation was found.
In the pathogenesis of DOX induced myocardial toxicity reactive oxygen species (ROS) are involved. In mitigating free radical-induced cell injury, the antioxidant enzyme GSH plays an important role. Because of its ability to use and remove organic and inorganic peroxide, in the heart, the GSH is extremely important. As observed in the present study, increased GSH consumption caused by enhanced lipid peroxidation by depletion of GSH in heart of rats is known to result33. The susceptibility to free radicals can be decreased by the prior administration of CEIB which protects the myocytes against DOX induced myocardial toxicity. The formation of hydroxyl radicals (OH), initiation, propagation of lipid peroxidation are promoted by declined GSH contents by DOX34.
The data of the present study clearly shows the beneficial action of CEIB as a cardioprotective agent is suggested by electrophysiological, biochemical and histological parameters which were maintained to near normal status in DOX treated rats, which are modulated by CEIB.
Conclusion
We this conclude that it is evident that CEIB whole plant possess promising antioxidant and cardioprotective activity in in-vivo model, through the research findings based on the observations and results obtained. As stated earlier, the presence of free-radical scavenging activity activity, which may be due to presence of flavonoids and phenolic compounds in the extracts may attribute to its cardioprotective activity. To elucidate detailed cardioprotective mechanism and to support the present assumption further studies are required.
Acknowledgement
Authors are sincerely thankful to Dr. K. Madhava Chetty, Plant Taxonomist, Department of Botany, Sri Venkateswara University, Tirupati, India for authentification of plant materials. Authors are thankful to V. V. Institute of Pharmaceutical Sciences, Gudlavalleru, India for providing necessary facilities of research work.
References
- Elbaky NAA, Ali AA, Ahmed RA. Cardioprotective effect of simvastatin on doxorubicin-induced oxidative cardiotoxicity in rats. Journal of Basic and Applied Sciences.(2010) 6:29-38.
- Ewer SM, Ewer MS. Cardiotoxicity profile of trastuzumab. Drug Saf. (2008) 31(6 ):459-67..
CrossRef - Deavall DG, Martin EA, Horner JM, Roberts R. Drug-induced oxidative stress and toxicity. Journal of Toxicology.(2012) 6:13.
CrossRef - Akinmoladun AC, Ibukun EO, Afor E, Akinrinlola, BL, Onibon TR, Akinboboye AO. and al. Chemical constituents and antioxidant activity of Alstoniaboonei. African Journal of Biotechnology.(2007) 6(10):1197-1201.
- Minotti G, Recalcati S, Menna P, et al. Doxorubicin cardiotoxicity and the control of iron metabolism: Quinone-dependent and independent mechanisms. Methods Enzymol. (2004) 378:340-61.
CrossRef - Kotamraju S, Chitambar CR, Kalivendi SV, et al. Transferrin receptor-dependent iron uptake is responsible for doxorubicin-mediated apoptosis in endothelial cells role of oxidant-induced iron signaling in apoptosis. J Biol Chem. (2002) 277(19 ):17179-87.
CrossRef - Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, EV, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin-treated children with high-risk acute lymphoblastic leukaemia: Long-term follow-up of a prospective, randomised, multicentre trial. Lancet Oncology.(2010) 11(10):950-961.
CrossRef - Cole MP, Chaiswing L, Oberley TD. The protective roles of nitric oxide and superoxide dismutase in adriamycin-induced cardiotoxicity. Cardiovasc Res. (2006) 69(1):186–97.
CrossRef - Daosukho C, Ittarat W, Lin SM, et al. Induction of manganese superoxide dismutase (MnSOD) mediates cardioprotective effect of tamoxifen (TAM). J Mol Cell Cardiol. (2005) 39(5 ):792-803.
CrossRef - Thippeswamy BS, Thakker SP, Tubachi S, Kalyani GA, Netra MK, Patil U. and al. Cardioprotective effect of Cucumistrigonusroxb on isoproterenol-induced myocardial infarction in rat. American Journal of Pharmacology and Toxicology.(2009) 4:29-37.
CrossRef - Shall S, Mohan MM, Kasture S, Sanna C, Maxia A. Protective effect of Ephedra n ebrodensis on dox orubicin-iiiduced cardiotoxicity in rats. Iranian Journal of Pharmacology and Therapeutics.(2009) 8:61-66.
- Madhava Chetty K, Sivaji K, Tulasi Rao K. Flowering plants of Chittoor District, Andhra Pradesh, India 4th edition, Student offset Printers, Tirupathi. (2013).
- SreeLatha Devi. A study on High valued Medicinal plants of Tirumala Hills, PhD Thesis, Rayalaseema University, Andhra Pradesh. (2011).
- Khandelwal KR. Practical Pharmacognosy Techniques and Experiments. 2000;149-156.
- Peterson DM, Emmons CL, Hibbs AH. Phenolic antioxidants and antioxidant activity in pearling fractions of oat groats. J Cereal Sci. (2001) 33:97-103.
CrossRef - Dos Santos MD, Almeida MC, Lopes NP. Evaluation of the antiinflammatory, analgesic and antipyretic activities of the natural polyphenol chlorogenic acid. Biol Pharm Bull. (2006) 29:2236–2240.
CrossRef - OECD Guidelines for testing of chemical, revised draft guidelines. Acute Oral Toxicity Up-and Down Procedure: (2008) 425:31.
- Kan S, Cheung WM, Zhou Y, et al. Enhancement of doxorubicin cytotoxicity by tanshinone IIA in HepG2 human hepatoma cells. Planta Med. (2014) 80:70–76.
CrossRef - Anu Kiruthika K, Sornaraj R. Assessment of antioxidant property of Quisqualis indica. Asian Journal of Pharmaceutical Science and Technology. 2013;4(2):35-37.
- Mandziuk S, Baj T, Sieniawska E, et al. Protective effect of Mutellina purpurea polyphenolic compounds in doxorubicin-induced toxicity in H9c2 cardiomyocytes. Drug Chem Toxicol. (2015) 38:1–8.
CrossRef - Fadillioglu E, Oztas E, Erdogan H, Yagmurca M, Ucar M, Sogut S, Irmak MK. Protective effects of caffeic acid phenethyl ester on doxorubicin-induced cardiotoxicity in rats. Journal of Applied Toxicology.(2004) 24:47-52.
CrossRef - Fraisse D, Felgines C, Texier O. Caffeoyl derivatives: major antioxidant compounds of some wild herbs of the Asteraceae family. Food Nutr Sci. (2011) 2:181–192.
CrossRef - Volkova M, Palmeri M, Russell KS, et al. Activation of the aryl hydrocarbon receptor by doxorubicin mediates cytoprotective effects in the heart. Cardiovascular Res. (2011) 90:305-314.
CrossRef - Neilan TG, Blake SL, Ichinose F. Disruption of Nitric Oxide Synthase 3 Protects Against the Cardiac Injury Dysfunction and Mortality Induced by Doxorubicin. (2007) 116(5 ):506–-514.
CrossRef - Tokarska-Schlattner M, Dolder M, Gerber I. Reduced creatine-stimulated respiration in doxorubicin challenged mitochondria particular sensitivity of the heart. Biochim Biophys Acta. (2007) 1767(11 ):1276-84.
CrossRef - Nagy N, Malik G, Tosaki A, et al. Overexpression of glutaredoxin-2 reduces myocardial cell death by preventing both apoptosis and necrosis. J Mol Cell Cardiol. (2008) 44(2):252–60.
CrossRef - Trachtenberg BH, Landy DC, Franco VI, et al. Anthracycline-associated cardiotoxicity in survivors of childhood cancer. Pediatr Cardiol. (2011) 32(3):342-53.
CrossRef - Cardinale D, Colombo A, Lamantia G. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. (2010) 55(3 ):213-20.
CrossRef - Koti BC, Vishwanathswamy AH, Wagawade J, Thippeswamy AH. Cardioprotective effect of lipistat against doxorubicin induced myocardial toxicity in albino rats. Indian Journal of Experimental Biology.(2009) 47:41‑46.
- You BR, Park WH. Gallic acid‑induced lung cancer cell death is related to glutathione depletion as well as reactive oxygen species increase. Toxicology In-Vitro. (2010) 24:1356‑1362.
CrossRef - Yoon GJ, Telli ML, Kao DP, et al. Left ventricular dysfunction in patients receiving cardiotoxic cancer therapies are clinicians responding optimally? J Am Coll Cardiol. (2010) 56(20 ):1644-50.
CrossRef - Franco YL, Vaidya TR, and Ait-Oudhia S. Anticancer and cardio-protective effects of liposomal doxorubicin in the treatment of breast cancer. Breast Cancer (Dove Med Press). (2018) 10:131-141.
CrossRef - Gianni L, Herman EH, Lipshultz SE, Minotti G, Sarvazyan N, and Sawyer DB. Anthracycline cardiotoxicity: from bench to bedside. J Clin Oncol. (2008) 26:3777–3784.
CrossRef - Singh G, Singh AT, Abraham A, Bhat B, Mukherjee A, Verma R, et al. Protective effects of Terminalia arjuna against doxorubicin‑inducedcardiotoxicity. Journal of Ethnopharmacology.(2008) 117:123‑129.
CrossRef







