Manuscript accepted on :27-06-2022
Published online on: 25-08-2022
Plagiarism Check: Yes
Reviewed by: Dr. Tukaram Dudhamal, Dr. Randa Salah Gomaa Mahmoud
Second Review by: Dr. Foram Joshi
Final Approval by: Dr. Patorn Piromchai
I Gusti Agung Ayu Dharmawati1,2 , Nur Habibah1
, Nur Habibah1 , I Gusti Agung Ayu Putu Swastini1
, I Gusti Agung Ayu Putu Swastini1 and Heri Setiyo Bekti1,2*
and Heri Setiyo Bekti1,2*
1Medical Laboratory Technologist, Polytechnic of Health Denpasar, Bali, Indonesia.
2Center of Excelence in Science and Technology, Polytechnic of Health Denpasar, Bali, Indonesia
Corresponding Author E-mail:herisetiyob7@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2502
Abstract
Wild mango (Spondias pinnata (L.f) kurz) or Cemcem or Kecemcem is one of the famous plants in Bali. It is widely used by Balinese as both food and traditional medicine. Several study has shown that S. pinnata leaf extract has antibacterial activity against several Gram-positive and Gram-negative bacteria. S. mutans is a Gram-positive bacteria that causes dental caries. In Indonesia, the prevalence of dental caries is 88.8% and most suffered by toddlers. The purpose of this study was to determine the effect of ethanol extract of S. pinnata leaves in inhibiting the growth of S. mutans. Inhibition zone test was carried out using the diffuse disc method with two extract concentrations of 60% and 80%, respectively. From the results, it was found that the inhibition zone of 60% concentration was 12.95 mm and 80% concentration was 15.77 mm. Both fall into the category of strong inhibition zones. Based on this, the ethanol extract of S. pinnata leaves can be used as a natural antibacterial agent.
Keywords
Antibacterial Activity; Ethanol Extract; Spondias pinnata; Streptococcus mutans
Download this article as:| Copy the following to cite this article: Dharmawati I. G. A. A, Habibah N, Swastini I. G. A. A. P, Bekti H. S. Antibacterial Potential of Spondias pinnata (L.f) kurz Leaf Ethanol Extract against Streptococcus mutans Bacterial Growth. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Dharmawati I. G. A. A, Habibah N, Swastini I. G. A. A. P, Bekti H. S. Antibacterial Potential of Spondias pinnata (L.f) kurz Leaf Ethanol Extract against Streptococcus mutans Bacterial Growth. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3R9sWUd |
Introduction
Wild mango (Spondias pinnata (L.f) kurz) or Cemcem or Kecemcem is one of the famous plants in Bali. This plant has many benefit and it is widely used by Balinese as both food and traditional medicine for generations. “Loloh cemcem” is a tradiotional drink from Bali. It is made from cemcem (S. pinnata) leaves1. Roots, bark, fruit, and leaves can be used in traditional medicine2. S. pinnata is widely used by Balinese people as a medicine for fever and toothache. The pharmacological effects of S. pinnata are also used as food flavoring, antimicrobial, and anti-tuberculosis3,4.
Research conducted by Wulansari and Armayanti (2008) on the use of S. pinnata leaves extract concentrations of 20%, 40%, and 60% to inhibit the growth of S.aureus, E. coli, and S. typhi showed significant differences in the average inhibition power (p<0.05) in all treatment, as well as the positive and negative controls5. Research conducted by Sudirga (2020) states that the S. pinnata plant is one of the traditional medicinal plants that has been used for generations by the people of the Trunyan Village. This plant has efficacy as a medicine for fever and toothache by using the leaves and sap from the S. pinnata which contains alkaloids, citric acid, and Ca-oxalate6.
Dental and oral health is often neglected by Indonesian people. The low awareness of maintaining dental and oral health is one of the causes of dental and oral disease in Indonesian society. The results of the 2018 Basic Health Study (Riskesdas) showed that 57.6% of the Indonesian population experienced dental and oral problems and only about 10.2% received medical services. The largest proportion of dental problems in Indonesia is dental caries at 88.8% with the most suffers being children under five7.
Dental caries is an infection of the teeth caused by Streptococcus mutans bacteria which causes demineralization of the tissue, causing localized damage to the tissue. The main habitat of S. mutans is the mouth, pharynx, and intestines. Dental caries has several factors such as adhesion to the enamel surface, production of acidic metabolites, ability to build glycogen, and to synthesize extracellular polysaccharides8.
Seeing the phenomenon of dental caries caused by the influence of the proliferation of S. mutans contained in dental plaque, so the researcher wanted to examine the quality and antibacterial inhibition of the ethanol extract of S. pinnata leaves on the growth of bacteria that cause plaque formation. As well all known that dental plaque is the initial source of dental and oral disease.
Material and methods
Preparation of S. pinnata Leaves
S. pinnata leaves are dried at 500C for 15 hours, then ground with a blender machine to fine powder. The obtained S. pinnata leaf powder is then used in the extraction process.
S. pinnata Leaves Extraction Process
Extraction is performed by weighing 300 g of dried leaves, which are then dissolved in 96% ethanol up to 4500 ml. In addition, agitation and extraction were performed for 15 minutes using a microwave with a power of 450 watts. The obtained extract was filtered with Whatman Paper number 1. The obtained filtrate was concentrated in the rotary evaporator vacuum at 300C.
In this study, the concentrations of the ethanol extract of S. pinnata leaves used were 60% and 80%, respectively. There are 4 treatments in this study. A negative control which is given treatment by giving ethanol solvent. A positive control that was treated with 2% chlorhexidine. Six times replication were conducted for high accuracy.P1 was treated with ethanol extract of S. pinnata leaves with a concentration of 60%. P2 is a treatment with ethanol extract of S. pinnata leaves with a concentration of 80%.
Culture of S. mutans
Bacterial strains used were S. mutans ATCC. S. mutans were cultured into BHI-A with vitamin K. The agar media was made by 10 µl vitamin K, 50 µl hemin solution, BHI-A 37 g in 100 ml sterile distilled water and 500 µl yeast extract. One bacteria used from the ATCC bacterial stock and was inoculated, then incubated at 370C for 24 h.
Preparing the S. mutans bacteria suspension
S. mutans suspension was made by incorporating one colony of S. mutans from BHI-A into liquid media with total volume of 10 ml containing 0.37 g BHI-B, 5 µl hemin, 1 µl vitamin K, and 50 µl yeast extract. Then the suspension was incubated for 24 h, and the concentration was measured to obtain turbidity equivalent to 1.5 x 106 CFU/ml.
Inhibition test of S. mutans
For antibacterial activity, the disc diffusion method was used. The S. mutans suspension was swabbed on the entire MH agar surface. The paper discs containing different concentrations of S. pinnata ethanol extract 60% and 80% respectively were placed on the agar surface. Then incubated at 370C for 24 h.
The area without visible bacterial growth or clear zone around each disc was observed. The diameter of the clear zone was measured using a calliper.
Statistical analysis
The data obtained were analyzed for diversity with the One Way Anova test.
Results and Discussion
The activity of S. pinnata leaves ethanol extract in inhibiting the growth of S. mutans, the One Way Anova test was used. The significance analysis are presented in Table 1.
Table 1: The Differences in Bacterial Inhibition Zone between Groups after Treatment
| Variable | Groups | n | Average±SD | p |
| S. mutans | Positive control | 6 | 20.38±0.12 | <0.001 |
| P1 | 6 | 12.95±0.17 | ||
| P2 | 6 | 15.77±0.13 |
Table 2 shows that the average inhibition of the control positive against S. mutans was 20.38±0.12. The inhibitory power of P2 was 15.77±0.13 and the P1 was 12.95±0.17. Based on the results of the analysis using the One Way Anova test, it was shown that there was a significant difference between the three groups (p<0.05).
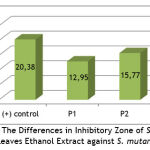 |
Graph 1: The Differences in Inhibitory Zone of S. pinnata Leaves Ethanol Extract against S. mutans. |
Graph 1 shows that there is a significant difference in the average inhibition zone between the three treatment groups.
The antibacterial test results of the ethanol extract of S. pinnata leaves against S. mutans showed a significantly different mean of inhibition zone ( p < 0.05) between the ethanol extract of S. pinnata leaves 60%, 80%, and positive control (using chlorhexidine 2%). The diameter of inhibitory zone showed the increase in the average according to the increase in concentration. The average diameter of the inhibitory zone at concentration of 60% was 12.95 mm and a concentration of 80% was 15.77 mm. Pan et al (2009) claimed that the category of inhibition with an inhibitory diameter of 0 to 3 mm was classified as weak, while an inhibitory diameter of 3 to 6 mm was classified in the medium category, and an inhibition diameter greater than 6 mm was classified in the string category9.
The results of this study are in line with research conducted by Wulansari et al., (2018) on the effectiveness of S. pinnata leaves extract to inhibit the growth of S. aureus, E. coli, and S. typhi bacteria. From the results of this study it was proven that there was a significant inhibition of bacterial growth5. Asnani et al. (2017) suggested that the ethanol extract of S. pinnata leaves contains steroids, flavonoids, tannins, and saponins is able to inhibit the growth of S. aureus, K. pneumonia, M. morganii, and P. aeruginosa10.
The content of phenolic compounds, flavonoids, saponins, and tannins in S. pinnata leaves extract will form a complex on the bacterial cell wall which causes inhibition and death of bacterial cells5,11–13. Flavonoids are responsible for the observed antimicrobial activity. Flavonoids are a group of promising bioactive substances with low systemic toxicity. The leaves and stems are rich in flavonoids. The study by Adamczak et al. (2020) demonstrated moderate antibacterial properties of flavonoids against clinical strains of E. coli and P. aeruginosa14. Some studies identified that the antibacterial mechanism of flavonoids are inhibiting nucleic acid synthesis, inhibiting cytoplasmic membrane function by affecting biofilm formation, porins, permeability, and interaction with some key enzymes14–17.
S. mutans is a Gram-positive spherical bacterium that typically pairs or forms chains during its growth and is a normal flora of the oral cavity. S. mutans is able to synthesize large polysaccharides such as dextran from sucrose which is a sticky polysaccharide, and plays an important role in caries formation. The prevention and control of dental caries have been a major challenge for decades18,19.
To date, prevention and treatment of dental caries is not limited to traditional methods used such as regular dental visits, brushing teeth with fluoride toothpaste, and low-sugar diets. Sogandi and Nilasari (2019) reported on the use of some natural ingredients as problem-controlling agents in the oral cavity and they found that noni fruits extract could inhibit the growth of S. mutans bacteria that cause dental caries20. Suhendar et al. (2019) showed that the methanol extract of Kasturi mango contains alkaloids, flavonoids, phenolics, terpenoids, and saponins has an inhibitory activity against S. mutans21.
From the results of this study, it was found that the diameter of the inhibition zone of the ethanol extract of S. pinnata leaves was quite strong and could be used as an alternative treatment for dental caries.
Conclusion
This study demonstrated that the ethanol extract of S. pinnata leaves (cemcem) has the potential to prevent the formation of dental caries caused by S. mutans bacteria. S. pinnata leaves extract with 80% concentration provides an inhibition zone greater than 60% concentration. It can be used as a natural antibacterial agent.
Acknowledgement
None
Conflict of Interest
The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript. We certify that the submission is original work and is not under review at any other publication
Funding Source
This study was supported by, Polytechnic of Health Denpasar, Board for Development and Empowerment Human Resources of Health – The Ministry of Health Republic Indonesia
References
- Laksemi DAAS. Biological activity of Spondias pinnata: A review. Indones J Biomed Sci. 2019;13(2):88–93.
CrossRef - Badoni A, Bisht C. Importance and Problems in Natural Regeneration of Spondias pinnata. Rep Opin. 2009;1(5):12–3.
- Hazra B, Biswas S, Mandal N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med. 2008;8:1–10.
CrossRef - Savitri, Ariantari, Dwija. Potensi Antituberkulosis Ekstrak. J Farm Udayana [Internet]. 2013;2(3). Available from: http://ojs.unud.ac.id/index.php/jfu/article/view/7385/5639
- Wulansari, N.T. & Armayanti LY. Efektivitas Ekstrak Daun Cem-cem (Spondias pinnata (L.f) Kurz) dalam Menghambat Pertumbuhan Staphylococcus aureus, Escherichia coli dan Salmonella typhi. J Media Sains. 2018;2(2):59–63.
- Sudirga S. Pemanfaatan Tumbuhan sebagai Obat Tradisional di Desa Trunyan Kecamatan Kintamani kabupaten Bangli. E J Bumi Lestari [Internet]. 2012;4(2):7–18. Available from: http://ojs.unud.ac.id/index.php/blje/article/view/2379
- Kementerian Kesehatan RI. Profil Kesehatan Indonesia 2018. Jakarta; 2018. 111–112 p.
- Forssten SD, Björklund M, Ouwehand AC. Streptococcus mutans, caries and simulation models. Nutrients. 2010;2(3):290–8.
CrossRef - Pan X, Chen F, Wu T, Tang H, Zhao Z. The acid, bile tolerance and antimicrobial property of Lactobacillus acidophilus NIT. Food Control [Internet]. 2009;20(6):598–602. Available from: http://dx.doi.org/10.1016/j.foodcont.2008.08.019
CrossRef - Asnani A, Rahayu WP, Jenie BSL, Yuliana ND. Aktivitas Antibakteri Dan Sitotoksisitas Ekstrak Daun Kedondong Hutan. Vol. 28, Jurnal Teknologi dan Industri Pangan. 2017. p. 169–79.
CrossRef - Gupta VK, Roy A, Nigam VK, Mukherjee K. Antimicrobial activity of spondias pinnata resin. J Med Plants Res. 2010;4(16):1656–61.
- Jain P. Antioxidant and Antibacterial Activities of Spondias pinnata Kurz. Leaves\. European J Med Plants. 2014;4(2):183–95.
CrossRef - Das J, Mannan A, Rahman MM, Dinar MAM, Uddin ME, Khan IN, et al. Chloroform and ethanol extract of Spondias pinnata and its different pharmacological activity like- antioxidant, cytotoxic, antibacterial potential and phytochemical screening through in-vitro method. Int J Res Pharm Biomed Sci [Internet]. 2011;2(4):1805–12. Available from: http://www.ijrpbsonline.com/files/RC38.pdf%0Ahttps://www.cabdirect.org/cabdirect/abstract/20123074681
- Adamczak A, Ożarowski M, Karpiński TM. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J Clin Med. 2020;9(1).
CrossRef - Górniak I, Bartoszewski R, Króliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Vol. 18, Phytochemistry Reviews. 2019. 241–272 p.
CrossRef - Barbieri R, Coppo E, Marchese A, Daglia M, Sobarzo-Sanchez E, Nabavi SF, et al. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol Res [Internet]. 2016; Available from: http://dx.doi.org/10.1016/j.micres.2016.12.003
CrossRef - Xie Y, Yang W, Tang F, Chen X, Ren L. Antibacterial Activities of Flavonoids: Structure-Activity Relationship and Mechanism. Curr Med Chem. 2014;22(1):132–49.
CrossRef - Dianawati N, Setyarini W, Widjiastuti I, Ridwan RD, Kuntaman K. The distribution of Streptococcus mutans and Streptococcus sobrinus in children with dental caries severity level. Dent J (Majalah Kedokt Gigi). 2020;53(1):36.
CrossRef - Friedman JY. The Role of Streptococcus Mutans in the Formation of Dental Caries: An Ecological Perspective. Sci J Lander Coll Arts Sci. 2011;5(1):40–6.
- Sogandi S, Nilasari P. Identifikasi Senyawa Aktif Ekstrak Buah Mengkudu (Morinda citrifolia L.) dan Potensinya sebagai Inhibitor Karies Gigi. J Kefarmasian Indones. 2019;9(2):73–81.
CrossRef - Suhendar U, Fathurrahman M, Sogandi S. Antibacterial Activity and Mechanism of Action of Methanol Extract from Kasturi Mango Fruit (Mangifera casturi) on Caries-Causing Bacterium Streptococcus mutans. J Kim Sains dan Apl. 2019;22(6):235–41.
CrossRef







