Manuscript accepted on :11-03-2022
Published online on: 03-06-2022
Plagiarism Check: Yes
Reviewed by: Dr. Riham Abu-Zeid
Second Review by: Dr. Salman Ahmed
Final Approval by: Dr. Ian James martin
Aysam Fayed1 , Hala O Ramadan1
, Hala O Ramadan1 , Soha A. Hassan2
, Soha A. Hassan2 , Mohammed A. Hussein3*
, Mohammed A. Hussein3* and Tamer Roshdy1
and Tamer Roshdy1
1Department of Molecular Biology, Genetic Engineering and Biotechnology Research Institute, University of Sadat City, Menoufia, Egypt.
2Basic Science Department, Faculty of Dentistry, October 6 University, Sixth of October City, Egypt.
3Biotechnology Department, Faculty of Applied Medical Health Sciences Technology, October 6 University, Sixth of October City, Egypt.
Corresponding Author E-mail: prof.husseinma@o6u.edu.eg
DOI : https://dx.doi.org/10.13005/bpj/2438
Abstract
When used in excess, the analgesic paracetamol can cause hepatic centrilobular necrosis, which can be fatal. The goal of this study was to see if strawberry extract could protect rats' livers from paracetamol-induced hepatotoxicity. Strawberry (75 and 150 mg/kg bw) and vit C (1 g /kg bw) were given orally, daily for 15 days demonstrated a significant reduction in the effects of caused changes in plasma cholesterol, triacylglycerol, phospholipids and vit C, TBARS, GSH, TNF-α, IL-4 and NO, AST, ALT, ALP, LDH, SOD, GPx and GSH levels. Furthermore, strawberry extract significantly inhibits hepatocyte B-cell lymphoma 2 (Bcl2) but significantly induces p53, NF-KB and Trx1 gene expression compared to paracetamol- treated rats. Histological examination showed that significant normalization has been observed in strawberry extract treated rats. Conclusions Strawberry extract shows considerable hepatoprotective benefits in the case of paracetamol-induced liver damage, confirming it's essential use as a treatment for liver damage.
Keywords
Hepatoprotective; Inflammatory Mediators; Oxidative Stress Biomarkers; Paracetamol; Strawberry
Download this article as:| Copy the following to cite this article: Fayed A, Ramadan H. O, Hassan S. A, Hussein M. A, Roshdy T. Thioredoxin1 Gene Modulates Bcl2/p53/NF-KB Signaling Pathways in Strawberry Extract/Paracetamol-treated Rat Model of Acute Liver Injury. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Fayed A, Ramadan H. O, Hassan S. A, Hussein M. A, Roshdy T. Thioredoxin1 Gene Modulates Bcl2/p53/NF-KB Signaling Pathways in Strawberry Extract/Paracetamol-treated Rat Model of Acute Liver Injury. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3x9KJSL |
Introduction
Liver, as the core of metabolic processes, has role to perform in the metabolism of a wide range of xenobiotics and is more susceptible to their toxicity 1. Acetaminophen overdoses, which are both analgesics and antiparasitic, are the most common poisonings caused by pharmaceutical products in the U.S. now 2. Despite being regarded safe at moderate dosages, Overdosing on acetaminophen leads to centrilobular liver necrosis, which can be deadly 3. While early metabolism events of toxicity have been well described, Hepatocyte death’s precise mechanisms are unknown. Necrosis is the term for cell death, and apoptosis has been ruled out 4.
Acetaminophen is normally converted to an active intermediate, NAPQI, by cytochrome P450 enzymes, It was quickly detoxified by glutathione conjugation 5.
Excessive NAPQI binds to mitochondrial proteins in hepatocellular, destroying mitochondria and causing a flood of reactive oxygen species, peroxidation, eventually liver cell death. Natural antioxidants obtained from various alternative medicine systems have been shown in numerous studies to have a wide spectrum of biological actions 6-9. In animal models, a variety of antioxidant-rich alternatives have been utilised to reduce paracetamol-induced oxidative stress 8. Several plant extracts were shown to be beneficial in reducing organ toxicity 6-10. Strawberry is one of these plants. Strawberry is a crop grown all over the world, with different cultivars suited to different climates 11. It is the best source of minerals like mn, k, mg, cu, fe, and p 12, ascorbic acid 13, thiamine, riboflavin, niacin, vit B6, vit K, vit A, and vit E 14, folate 15, catechins, hydroxycinnamic acids, ellagitannins and ellagic acid have also been associated with the beneficial effect of strawberries on human health 16. Ascorbic acid, ellagitannins, and anthocyanins are the most important contributors to strawberries’ antioxidant capacity 17. Hepatoprotective 18, hypolipidemic 19, hypoglycemic, and antioxidant activities of extract berry fruits have been studied in vivo 20. Strawberry extract, on the other hand, has not been shown to have hepatoprotective or gastroprotective properties. We want to test therapeutic potential of strawberry extract on paracetamol-induced liver damage in rat model as a follow-up to our studies on biological value of neutral products19-24.
Materials and methods
Chemicals
El-Nile Pharmaceutical Company gave us paracetamol as a present (Cairo, Egypt). When used in vivo experiments, paracetamol was suspended in 0.5 % tween 80 and administered orally at a dose of 1 g/kg PB 25.
Prolabo and Farance collaborated on Tween 80.
Virgin Extracts (TM) in China provided the strawberry extract. Cranberry extract (75 and 150 mg/kg bw) was given to rats using an oral gastric gavage tube twice a day for two weeks.
Animals
Adult albino rats measuring roughly 20010 gms. They had been accustomed to the confines of an animal shelter. The animals were fed a regular feed and given free access to water. All through the trial, the rats were housed in the same habitat and were observed on a daily basis.
Experimental setup
The purpose of this study was to see if ethanolic and aqueous extracts of cranberry extract could prevent paracetamol hepatotoxicity in vivo when given periodically for two weeks.
The following treatments were given on a daily basis for 14 days. To intragastric intubation of rats, a 3 percent suspended solution was created.
Group I: Typical (Orally given a similar volume of tween 80, 1percent in saline)
Group II: Control (Orally administered a similar volume of tween 80, 1percent in saline)
Group III: Received a single daily dose of strawberry extract suspended in tween 80 [19].
Group IV: Strawberry extract suspended in saline (150 mg/kg bw) was given orally in a single daily dose [19].
Group V: vit C (1 g/kg bw) orally as a single daily dose suspended in tween 80 [8].
Through day 13, the day prior to final treatment. Animals belonging to categories II, III, IV, and V received on day 14, one hour after the last dosage of pharmaceutical therapy, paracetamol 26.
Treatment of blood samples
Samples of blood were collected in heparin-containing tubes from each animal’s retroorbital vein. Blood was heparinized and centrifuged at 1000 xg for 20 minutes cholesterol 27, triglycerides 28, (HDL) 29, phospholipids 30, ALT 31, AST 31, ALP 32, LDH 33 and vit C 34 levels were measured in separated plasma.
Preparation of liver samples
These animals were killed using cervical dislocation, livers were extracted swiftly. To make a homogenate with a 25 percent W/V ratio, Using glass homogenizer, a fraction of each liver was homogenised with saline and weighed. The homogenate was produced in three aliquots. The first was used 12 percent trichloroacetic acid, chilled on ice, and the resulting supernatant was employed for GSH measurement after centrifuged at 1000 xg35. The second was rotated at 1000 xg and resulting supernatant was used to estimate the levels of TBARS36, NO37, TNF-α 38and IL-4 39. The third was utilised create cytosolic fraction of liver using a cooling ultracentrifuge at 10500 xg for 15 minutes at 4 OC, and the clear supernatant was used to evaluate SOD 40 and GPx 41.
Real- time PCR
The manufacturer’s instructions were followed to obtain total hepatic RNA using the TRIzol technique (Life Technologies Corp., Grand Island, NY). 1 µg RNA was combined with 0.5 mmol/l each deoxyribonucleoside triphosphate, 10 nmol/l dithiothreitol, 25 pg oligo (dT) primer (dNTP), and 200 units of superscript II Rnase H reverse Transcriptase in reaction buffer. The reactions have been incubated for one cycle for 2 min at 42° C and again 50 min at 42° C, which they were heated for 15 min at 70°C and then chilled to 4° C.
(Table 1): Bcl2, P53, nuclear factor kappa, and Trx1. The PCR reaction mixtures were incubated for 3 minutes at 94° C, then for the appropriate number of cycles at 94° C for 45 seconds, then at their respective annealing temperatures for 30 seconds, and for 30 seconds at 72° C. After that, a 10 minute extension step at 72° C was performed 42.
Table 1: Primers used in real-time PCR.
| Gene | Primer sequence |
| Bcl2 | F: 5′-TGTGGATGACTGACTACCTGAACC3′
R: 5′CAGCCAGGAGAAATCAAACAGAGG3′ |
| p53 | F: 5′-CTACTAAGGTCGTGAGACGCTGCC-3
R: 5′-TCAGCATACAGGTTTCCTTCCACC-3 |
| NF-KB | F: 5′GCAAACCTGGGAATACTTCATGTGACTAAG-3′
R: 5′ATAGGCAAGGTCAGAATGCACCAGAAGTCC-3′ |
| Trx1 | F: 5-CCGCAACAGCCAAAATGGTGA-3
R: 5-AGCATGATTAGGCAAACTCCGTAA-3 |
| β-Actin (internal control for qRT-PCR) | F: 5′-GGCTGTATTCCCCTCCATCG-3’
R: 5′- CCAGTTGGTAACAATGCCATGT -3’. |
Histological assessment
Liver cut to little fragments then stored in formaldehyde solution containing 10% buffered formaldehyde 43. Under the microscope, the slices were evaluated for histological alterations.
Statistical analysis
To get mean, standard deviation, and error, the data analysed using statistical package for social science [44]. To establish statistical significance of differences between groups, data were analysed using one-way analysis of variance. Duncan’s test was examine intergrouping homogeneity by doing multiple comparisons among the groups.
Results
Table 2 When contrasted to normal group, oral administration of paracetamol at 1g/kg bw. caused increase in plasma cholesterol and triglycerides, In addition drop in HDL-C and phospholipids (p < 0.01). When contrast to the group receiving paracetamol, supplementation with strawberry extract (75 and 150 mg/kg bw) and Vit C (1 g/kg bw) as a result of substantial drop in plasma cholesterol and triglycerides,In addition rise in HDL-C and phospholipids (p < 0.05). Strawberry has dose-dependent impact (p< 0.05) .
Table 3 contrasted to normal group, oral administration of paracetamol at 1g/kg bw as a result of substantial increase in plasma ALT, AST, ALP, and LDH,In addition drop in vit C levels (p< 0.01). When contrast to group receiving paracetamol, supplementation with strawberry extract (75 and 150 mg/kg bw) and vit C (1 g/kg bw) as a result of substantial decrease in plasma ALT, AST, ALP, and LDH, In addition increase in vit C levels (p < 0.05).
Table 4 contrasted to control group (p < 0.01), orally administered paracetamol at dose of 1 gramme per kilogramme of body weight was shown as a result of substantial rise in lipid peroxides in the liver (TBARS). Strawberry extract (75 and 150 mg/kg bw) and vit C (1 g/kg bw) supplementation as a result of substantial reduction in liver TBARS when compared to paracetamol group (p <0.05). Furthermore, as compared with control group, orally administered paracetamol as a result of substantial decrease in reduced GSH, SOD, and GPx in liver (p< 0.01). When contrast to group that received paracetamol, supplement with strawberry and vit C substantial increase in GSH, SOD, and Gpx (p 0.05). Strawberry 150 mg / kg of strawberries has a stronger effect than vit C (p< 0.05).
Table 5 contrasted to control group, orally administered paracetamol at 1g/kg bw. a result of substantial rise TNF-α, NO, and IL-4 (p< 0.01). When compared to group that received paracetamol, orally administered strawberry (75 and 150 mg/kg bw) and Vit C (1 mg / kg bw) a result of substantial decrease TNF-α, NO, and IL-4 (p< 0.05). Strawberry 150 mg / kg had a stronger effect than vit C (p <0.05).
Table 2: shows effects of triglycerides, HDL cholesterol, and phospholipids in rats.
| No. | Groups | Plasma cholesterol (mg/dl) | Plasma triglycerides (mg/dl) | Plasma HDL-C (mg/dl) |
Plasma phospholipids (mg/dl) |
| (I) | Normal
1 % tween 80 |
172.67
± 9.22a |
105.79
± 9.97a |
37.14
± 5.16 a |
61.12
± 6.12 c |
| (II) | Control
Paracetamol (1 g/kg.b.w) |
202.48
± 7.48c |
177.80
± 10.98d |
28.13
± 4.56c |
36.70
± 3.45a |
| (III) | Paracetamol + Strawberry
extract (75 mg/kg.b.w) |
180.46
± 7.79b |
135.13
± 10.90c |
32.79
± 4.24b |
47.17
± 4.36 b |
| (IV) | Paracetamol + Strawberry
extract (150 mg/kg.b.w) |
170.56
± 9 .98a |
106.40
±9.98a |
38.26
± 2.62 a |
59.07
± 3.70 c |
| (V) | Paracetamol + Vitamin C
(1 g/kg.b.w) |
169.29
+± 8.93a |
118.59
± 7.20 b |
34.38
±3.21a |
62.95
±5.35c |
To 18h fasted animals, a single dosage of 1g/kg.b.w. of paracetamol was orally. Except for the regular group, it was provided to everyone else. For two weeks, strawberry extract and vitamin C strawberry and vitamin C on plasma cholesterol, were given orally, with the last dose of each given one hour before paracetamol. The numbers of the mean (n=6). numbers displayed are the mean and standard deviation of the number of observations in all treatment. At P ≤ 0.05, data following the same letter are not substantially different.
Table 3: shows the effects of strawberry extract and vitamin C in rats given on plasma ALT, AST, ALP, LDH, and vit C.
| No_ | Groups | Plasma ALT (U/L) | Plasma AST (U/L) |
Plasma ALP (U/L) | Plasma LDH (U/L) | Plasma Vti C (mg/dI) |
| (I) | Normal
1 % tween 80 |
27.10
± 3. .81a |
15.98
± 254 a |
154.15
± 950 a |
309-45
± 10.32 a |
83.49
± 7.02 d |
| (Il) | Control
Paracetamol (1 g/kg.b.w) |
60.48
± 5.05d |
4338
± 3.87 d |
28937
±14.98 e |
53734
± 14.3 1e |
30.79
+- 4.4 5 a |
| (111) | Paracetamol + Strawberry
extract (75 mg/kg.b.w) |
43.88
± 452 c |
32.24
± 6.08 c |
207 -49
±10.58d |
392-48
± 12.39 d |
67.42
± 4.48 b |
| (IV) | Paracetamol + Strawberry
extract (150 mg/kg.b.w) |
31.73
±3.38a |
21.98
±4.65b |
171.69
±12.18 b |
340.96
±17_ 73 b |
7526
±4.38 c |
| (V) | Paracetamol + Vitamin C
(1 g/kg.b.w) |
35.19
±3.08b |
3154
+3.80c |
189.30
±7.15c |
364.25
± 15.94c |
80.4 1
±4.44d |
To 18h fasted animals, a single dosage of 1g/kg.b.w. of paracetamol was given orally. Except for the regular group, it was provided to everyone else. For two weeks, strawberry extract and vitamin C were given orally, with the last dose of each given one hour before paracetamol. numbers of the mean (n=6). numbers displayed are the mean and standard deviation of number of observations in all treatment. At P ≤ 0.05, data following the same letter are not substantially different.
Table 4: shows the effects of strawberry extract and Vitamin C on TBARS, GPx, SOD, and GSH in rats.
| No. | Groups | TBARS
(mmol/mg wet tissue) |
GSH
(mg/ g tissue) |
SOD
(U/gm tissue) |
GPx
(U/mg tissue) |
| (I) | Normal
1 % tween 80 |
1.44
± 0.36a |
45.17
± 5.27 d |
26.60
± 3 .88 c |
153.72
± 5.40d |
| (II) | Control
Paracetamol (1 g/kg.b.w) |
5.39
± 0.51e |
23.76
± 5.3o a |
12.0 1
± 2.19 a |
55.19
± 3.09 a |
| (III) | Paracetamol + Strawberry
extract (75 mg/kg.b.w) |
3.79
± 0.52d |
3 1.62
± 4.232b |
18.87
± 2.30b |
132.14
± 7.0 1b |
| (IV) | Paracetamol + Strawberry
extract (150 mg/kg.b.w) |
2.66
±0. 3lb |
40.99
±4.38 c |
25.39
±3.4 1c |
148.23
± 9. 12 c |
| (V) | Paracetamol + Vitamin C
(1 g/kg.b.w) |
3.2 1
± 0.3 8c |
43 .63
± 4.42 c |
17.41
± 1.89b |
136.28
± 8.16b |
To 18h fasted animals, a single dosage of 1g/kg.b.w. of paracetamol was given orally. Except for the regular group, it was provided to everyone else. For two weeks, strawberry extract and vitamin C were given orally, with the last dose of each given one hour before paracetamol. numbers of the mean (n=6). mean and standard deviation of number of observations in all treatment are presented. At P ≤ 0.05, data following same letter are not substantially different.
Table 5: shows effects of strawberry extract and vitamin C on rat liver nitric oxide (iNOs), TNF-α, and IL-4 levels.
|
No. |
Groups | Hepatic iNOs
(pg/mg tissue) |
Hepatic TNF-a.
(pg/mg tis.sue) |
Hepatic IL-4 (U/gm tissue) |
| (I) | Normal
1 % tween 80 |
3.05
±0.42 a |
19.32
± 2.88 a |
3 1.97
±4.90 a |
| (II) | Control
Paracetamol (1 g/kg.b.w) |
8.18
± 0.45 b |
54.59
± 5.84 c |
78.06
± 5.70 c |
| (III) | Paracetamol + Strawberry
extract (75 mg/kg.b.w) |
5.38
± 0.87a |
34.07
± 3.28b |
44.25
± 5.48b |
| (IV) | Paracetamol + Strawberry
extract (150 mg/kg.b.w) |
3.12
±0 .46 a |
25. 36
±3.50 a |
33.63
±4.0 la |
| (\’) | Paracetamol + Vitamin C
(1 g/kg.b.w) |
4.20
±0 .85a |
35.07
±2.99b |
49.45
±4.77b |
To 18h fasted animals, a single dosage of 1g/kg.b.w. of paracetamol was given orally. Except for the regular group, it was provided to everyone else. For two weeks, strawberry extract and vitamin C were given orally, with the last dose of each given one hour before paracetamol. The numbers of the mean (n=6). mean and standard deviation of the number of observations in all treatment are presented. At P 0.05, data following the same letter are not substantially different.
Figures 1-4 show significant decrease in expression of hepatocyte Bcl2 in addition significant increase in expression of p53, NF-KB and Trx1 genes. the management of rats with strawberry extrct (75 and 150 mg / kg bw) and vit C (1g / kg bw) showed significant significant increase in Bcl2 in addition significant decrease in liver p53, NF-KB, and Trx1, compared to paracetamol-treated rats (p< 0.01).
The normal group’s liver slices were examined histopathologically (I) showed within normal arrangement and appearance of hepatocytes without fibrosis or inflammation x 200 H&E (Figure 5a).
However, in liver of paracetamol-treated control group (II), showed a greatly altered hepatic parenchyma due to diffuse hydropic degeneration and vacuolar degeneration in hepatocytes (yellow arrows), narrowed or occluded sinusoids with congested central veins (CV) and abnormal arrangement of the blood sinusoid (red arrows) (high magnification X: 400 bar 50 (figure 5b). Furthermore, the hepatocytes showed mildly disrupted hepatic parenchyma due to lesser degrees of hepatocyte degeneration (yellow arrows), some blood cells (#) between hepatocytes in some lobules and mild congested sinusoids (red arrows) in paracetamol-treated rats administrated with strawberry extract (75 mg / kg bw) as compared to the paracetamol-treated group (figure 5c) group (III) (high magnification X: 400 bar 50). Also, the histological examination of hepatocytes of rats treated with paracetamol + strawberries (150 mg / kg bw) groups (IV) showed mild lobular inflammation (yellow arrows) and lesser degrees degeneration of hepatocytes,, also nearly normal blood sinusoids(S) and normal portal area, (high magnification X: 400 bar 50) (Figure 5d). Also, Microscopic picture of H&E stained liver sections (I, J) showing improvement in histological morphology histological structure of the liver lobes, natural hepatocyte arrangements (*) with intact blood sinusoids (S) and normal portal area in rats administrated treated with paracetamol administered vitamin C (1g / kg) (Figure 5e) (high magnification X: 400 bar 50).
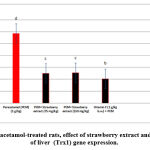 |
Figure 1: In paracetamol-treated rats, effect of strawberry extract and vit C on levels of liver (Trx1) gene expression. |
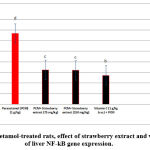 |
Figure 2: In paracetamol-treated rats, effect of strawberry extract and vit C on levels of liver NF-kB gene expression. |
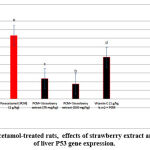 |
Figure 3: In paracetamol-treated rats, effects of strawberry extract and vit C on levels of liver P53 gene expression. |
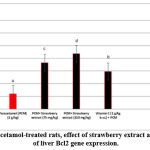 |
Figure 4: In paracetamol-treated rats, effect of strawberry extract and vit C on levels of liver Bcl2 gene expression. |
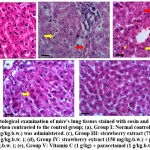 |
Figure 5: Histological examination of mice’s lung tissues stained with eosin and hematoxylin (H&E; 400 X) when contrasted to the control group; |
Discussion
Paracetamol (4′-Hydroxyacetanilide) is antipyretic and analgesic medication that is used orally 45. Sulfonation, glucuronidation, and oxidation are the three primary mechanisms by which it is metabolised by liver 46. The first two processes are more essential in terms of quantity than the third, but the oxidative pathway is the source of toxicity 47. Hepatic microsomes catalyse the oxidation of paracetamol, which is predominantly mediated by cytochrome P-450 48. The procedure yields NAPQI 49, a highly reactive arylating chemical. The P-4501A2 microsome was found to be the major catalysts of paracetamol activation in the human liver 50. When many NAPQI is created can be conjugated to GSH, unbound NAPQI becomes toxic by binding to macromolecules, including cellular proteins51.
Plant-based medicine Medications may contain phytoconstituents that occur naturally. which can decrease production of reactive oxygen species in variety of ways. pharmacological effects of phytoconstituents are diverse 51, 52.
The treatment of paracetamol to rats resulted increase in the levels of cholesterol, triglycerides, AST, ALT, ALP, LDH, iNOs, TNF-α , IL-4 and TBARs levels, however, decrease in vit C, HDL-C and phospholipid levels as well as GSH, SOD and GPx contrasted to normal rats. Orally administration extract and vit C to paracetamol treated rats showed normalization of plasma levels of cholesterol, triglycerides, AST, ALT, ALP, LDH, iNos, TNF-α , IL-4, TBARs vitamin C, HDL-C, GSH, SOD and GPx levels when contrasted to paracetamol treated rats 53. reported that paracetamol administration caused AST and ALT activities were elevated.
Because strawberry possesses scavenging free radical damage and antioxidants 41, goal investigation is to see if cranberry polyphenols may reduce diclofenac-induced liver damage in rats. Strawberry extract considerably reduces the peroxidative damage caused by diclofenac sodium in liver, as demonstrated by decreased levels of reactive chemicals thiobarbituric acid and lipid hydroperoxides.
could be related to polyphenols’ antioxidant properties 42. antioxidant is a chemical that can slow or stop other molecules from oxidising. Oxidation reaction in which electrons are transferred from material oxidizer. The lipid peroxidation mechanism causes cellular damage and functional problems in hepatocytes in response to paracetamol 7. Because the liver is involved in lipid homeostasis, increased iron may affect serum lipid concentrations, thereby reducing or increasing risk of atherosclerosis. Strawberry was found to be non-toxic in normal rats in preliminary investigations undertaken by this work. Hepatic necrosis after a large dose of paracetamol has been widely established 53. The current investigation found a significant increase in ALT, AST, ALP, and LDH after paracetamol administration (1g/kg bw) because of Ca2+ buildup, which activated phosphofructokinase and anaerobic glycolysis, culminating in lactate production 54.
The last stage of the paracetamol cell death process has been defined as the loss of Ca2+ homeostasis induced by oxidative damage and increase in intracellular Ca2+55. Strawberry treatment regulates plasma LDH activity.
Both light and TEM examinations demonstrated that indomethacin and strawberry extract cause inflammatory in hepatic and gastric mucosal cells. This finding backs up the fact that rats given strawberry extract with paracetamol had lower levels of TNF-, NO, IL-4, and thiobarbaturic acid (TBARS) in their livers.Our results indicated that the hepatoprotective effect of strawberry extract could be due to the presence of ascorbic acid 13, thiamine, riboflavin, niacin, vit B6, vit K, vit A and vitamin E 14, folate 15, flavonols, catechins, hydroxycinnamic acids, ellagitannins and ellagic acid 16.
The strawberry extract-treated groups had considerably lower liver TBARS levels than the paracetamol-treated groups in this study. Strawberry extract may have antioxidant properties and protect tissues from lipid peroxidation, according to this finding. protective effect of strawberry extract treatment strongly suggested that extracts are capable of stopping the release of marker enzymes to the bloodstream, conditioning Hepatocytes help to speed up the regeneration of parenchymal cells, and preserving integrity plasma membranes and thus restoring enzyme activities 56. In antioxidant defence, GSH plays a multifaceted role. It is a direct hunter of reactive oxygen species in addition co-substrate glutathione peroxidases’ detoxification of peroxide 57. According to Liu et al., 58, decrease in liver GSH levels could be attributable to decreased GSH production or increased GSH breakdown as a result of oxidative stress and tissue injury. considerable rise in aldehydic lipid peroxidation likely lowered GSH level, resulting in enhanced oxidative stress. GSH levels in liver were shown to be higher in strawberry extract-treated rats in this investigation. This suggests that strawberry extract may either boost GSH biosynthesis or decrease oxidative stress, resulting in less GSH breakdown, or it may have both effects.
SOD is thought to be essential because it catalyzed into H2O2 and molecular oxygen, lowering the harmful effects of their radicals 59. reduce in SOD activity found could be due to H2O2 inactivation 60. CAT, which is involved in hydrogen peroxide detoxification, is known to be inactivated by superoxide anion 61. As a result, increased SOD activity could be critical for catalase activity.
GPx serves in preventing oxidative stress. GPx, a selenium-containing enzyme, and GST collaborate with GSH to degrade H2O2 or other organic into non-toxic compounds 62.
SOD and GPx activity in the blood and liver were shown to be lower in rats given paracetamol. SOD and GPx activity were found to be lower in paracetamol-treated mice by several authors 63, 64. The findings suggest that strawberry extract can either boost SOD and GPx production or decrease inflammation, resulting with less SOD and GPx breakdown, or both. Hepatotoxicity from paracetamol manifests as centrilobular necrosis 65. Biochemical findings are backed up by histopathological research. Fatty alterations, necrosis, and vacuoles. To paracetamol-treated rats, orally administration strawberry (75 and 150 mg/kg bw) and vit C 66. In paracetamol-treated rats, researchers found vacuolated hepatocytes, necrosis, and congested sinusoids in the liver. The injection of – and -amyrin to the liver of paracetamol-treated rats exhibited normal histoarchitecture, according to Oliveira et al. 67. presence of flavonoids was discovered during a phytochemical screening of strawberry extract. Flavonoids (or bioflavonoids) are natural compounds impact the behaviour numerous cell systems through enzyme activity (SOD and GPx) and have antihepatotoxic, antiallergic, anti-inflammatory, antiosteoporotic, anticancer, and antioxidant properties 68, 69.
Oxidative stress plays role in hepatotoxicity paracetamol. Induction of oxidative stress may suppress Bcl2 and trigger p53 activation, as well as induction of the expression of the NF-KB and Trx1 genes. The Trx1 system is a sensor of energy and glucose metabolism and contributes to cellular redox equilibrium [70]. Our study suggested that the overexpression of Trx1 in rats treated with paracetamol to modulate the redox state and liver cells and provide Cys for the synthesis of protein and coenzyme A, this Cys source is used for the de novo synthesis of GSH, thus putting the reduced sulfur derived from Met in a molecule that can support reductions in cytosolic disulfides 71. However, the results obtained explain that the administration of strawberry extract containing polyphenols and flavonoids can negatively regulate Trx1 gene expression its free radical scavenging activity and the induction of GSH biosynthesis.
p53 plays role in a variety of diseases. Evidence does, however, support a pro-survival function in specific circumstances. Paracetamol poisoning has been demonstrated to activate p53 72.
Activation of NF-KB and p53 serves as signal to trigger cytotoxicity. However, increasing body of evidence supports function of NF-KB and p53 73-75. oxidative stress can induce activation of p53. In fact, p53 is activated in response to paracetamol exposition in animal models 76-77. present, the administration of strawberry containing polyphenols and flavonoids can down-regulate of NF-KB and p53 by reducing oxidative stress.
Strawberry extract suppresses regulation of hepatic Bcl-2 overexpression in animal model of paracetamol-induced acute liver damage, according to this study. Our findings, which coincide with Boulares et al. 78, reveal that paracetamol overdose inhibits antiapoptotic Bcl-2. Furthermore, our findings are consistent with at least two studies 79, found that hypoxia-ischemia inhibited Bcl-2 levels in rat model of neonatal brain injury and mouse model of paraquat-induced liver injury, respectively, and that resveratrol treatment substantially increased Bcl-2 levels 80.
Strawberry extract, according to histopathological studies, can protect the liver from paracetamol-induced liver damage. A diminished inflammatory response is proof of this. Strawberry’s protective effect against paracetamol-induced liver injury has not before been described, and could be the first study of its kind.
Finally, the findings of this investigation showed that strawberry extract has a significant hepatoprotective effect in rats when exposed to paracetamol. This could be because of its antioxidative properties, which include the capacity to reactive oxygen species scavenge and prevent peroxidation of lipids.
Authors’ contributions
Experimental design, antioxidant and hepatoprotective activities of strawberry water extract were carried out by Hala O Ramadan, Aysam Fayed and Tamer Roshdy. Histopathological examination was carried out by Soha A. Hassan. Wrote the protocol, wrote the first draft of the manuscript, managed the analyses of the study, managed the literature searches was carried out in collaboration between Hala O Ramadan, Aysam Fayed, Mohammed A. Hussein and Tamer Roshdy. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
Funding Sources
There is no funding Source.
References
- Liaqat, H., I. Javaria, R. Kanwal, T. Muhammad,I. Muhammad and S. Muhammad,2014.Hepatoprotective effects of Malva sylvestris L. against paracetamol-induced hepatotoxicity. Turk J Biol; 38: 396-402.
CrossRef - Litovitz,T.L.,W.Klein-Schwartz,G.C. Jr. Rodgers, D.J. Cobaugh, J. Youniss,J.C. Omslaer,M.E.May,A.D.Woolf and B.E .Benson,2002. annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System. Am J Emerg Med; 20:391–452.
CrossRef - Prescott,L.F.,1980. Hepatotoxicity of mild analgesics Br J Clin Pharmacol 10 (Suppl 2):373S–379S.
CrossRef - Gujral,J.S.,T.R.Knight, A.Farhood,M.L.Bajt and H.Jaeschke,2002. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci ; 67:322–328.
CrossRef - McGill,M.R.,M.R.Sharpe,C.D.Williams,M.Taha,S.C. Curry and H.Jaeschke,2012.The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest; 122: 1574–1583.
CrossRef - Hussein,M.A.,N.A. El-GizawyGobba and Y.O.Mosaad,2017.Synthesis of Cinnamyl and caffeoyl derivatives of cucurbitacin-e-glycoside isolated from Citrullus colocynthis fruits and their structures antioxidant and anti-inflammatory activities relationship. Current Pharmaceutical Biotechnology, 18, 677 – 6931.doi: 10.2174/1389201018666171004144615.
CrossRef - Elgizawy,H.A.,A.A. Ali and M.A.Hussein,2021.Resveratrol: Isolation, and Its Nanostructured Lipid Carriers, Inhibits Cell Proliferation, Induces Cell Apoptosis in Certain Human Cell Lines Carcinoma and Exerts Protective Effect Against Paraquat-Induced Hepatotoxicity. J Med Food, 24(1), 89-100. doi: 10.1089/jmf.2019.0286.
CrossRef - El Gizawy,H.A.,M.A.Hussein and E.Abdel-Sattar,2019.Biological activities, isolated compounds and HPLC profile of Verbascum nubicum. Pharmaceutical Biology, 57, 485-497. doi: 10.1080/13880209.2019.1643378.
CrossRef - Mosaad,Y.O.,N.A.El Khalik Gobba and M.A.Hussein,2016. Astaxanthin; a promising protector against gentamicin-induced nephrotoxicity in rats. Current Pharmaceutical Biotechnology, 17, 1189 – 11971. doi: 10.2174/1389201017666160922110740.
CrossRef - El Gizawy,H.A., H.M. Abo-Salem, A.A. Ali and M.A .Hussein,2021.Phenolic Profiling and Therapeutic Potential of Certain Isolated Compounds from Parkia roxburghii against AChE Activity as well as GABAAα5, GSK-3β, and p38α MAP-Kinase Genes. ACS Omega, 6(31), 20492–20511. doi: 10.1021/acsomega.1c02340.
CrossRef - Neri,D.,G.Baruzzi, F.Massetani and W. Faedi,2012.Strawberry production in forced and protected culture in Europe as a response to climate change. Canadian Journal of Plant Sciences; 92: 1021–1036.
CrossRef - Giampieri ,F.,S.Tulipani, J.M. Alvarez, J.L.Quiles,B. Mezzetti and M.Battino,2012.The strawberry: Composition, nutritional quality, and impact on human health. Nutrition;28: 9–19.
CrossRef - Singh,R.,R.K. Gupta,R.T. Patil,R.R.Sharma,R .Asrey, A. Kumar and K.K.Jangra,2010.Sequential foliar application of vermicompost leachates improves marketable fruit yield and quality of strawberry (Fragaria × ananassa Duch.). Scientia Horticulurae ; 124: 34–39.
CrossRef - Tulipani,S.,J.M. Alvarez, F.Busco,S .Bompadre, J.L .Quiles, B.Mezzetti and M.Battino,2011. Strawberry consumption improves plasma antioxidant status and erythrocyte resistance to oxidative haemolysis in humans. Food Chemistry; 128: 180–186.
CrossRef - Tõnutare ,T.,K. Keert, L. Szajdak and U. Moor,2014.Composition of commercially produced organic and conventional strawberries. Nutrition and Food Science ; 44: 562–575.
CrossRef - Tulipani,S.,S.Romandini,S.Bompadre,F.Capocasa and B .Mezzetti,2009. Variation in strawberry micronutrients, phytochemical and antioxidant profiles: the combined effect of genotype and storage. Acta Horticulturae ; 842: 867–871.
CrossRef - Aaby,K.,D. Ekeberg and G. Skrede,2007. Characterization of phenolic compounds in strawberry (Fragaria × ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. Journal of Agricultural and Food Chemistry ; 55: 4395–4406.
CrossRef - Liao,X.,L.Wang,C.Yang, J.He,X.Wang and R .Guo,2011. Cyclooxygenase mediates cardioprotection of angiotensin-(1–7) against ischemia/reperfusion-induced injury through the inhibition of oxidative stress. Mol Med Rep; 4:1145–50.
- Soliman SM, Mosallam S, Mamdouh MA, Hussein MA, Abd El-Halim SM. 2022. Design and optimization of cranberry extract loaded bile salt augmented liposomes for targeting of MCP-1/STAT3/VEGF signaling pathway in DMN-intoxicated liver in rats, Drug Delivery, 29:1, 427-439, DOI: 10.1080/10717544.2022.2032875
CrossRef - Fayed AM, Abdalla EA, Hassan SA, Hussein MA, Roshdy TM. 2022. Downregulation of TLR4-NF-κB-p38 MAPK Signalling in Cholestatic Rats Treated with Cranberry Extract. Pakistan Journal of Biological Sciences, 25: 112-122.
CrossRef - Hussein MA. 2012. Synthesis of some novel triazoloquinazolines and triazinoquinazolines and their evaluation for anti-inflammatory activity. Medicinal Chemistry Research; 21: 876 – 1886.
CrossRef - Abdel-Gawad SM, Ghorab MM, El-Sharief AM, El-Telbany FA, Abdel-Alla M. 2003. Design, synthesis, and antimicrobial activity of some new pyrazolo[3,4-d]pyrimidines Heteroatom Chemistry. 14; 6: 530 – 534. DOI: 10.1002/hc.1018723.
CrossRef - Ghorab,M.M.,Z.H.Ismail and M. Abdalla,2010.Synthesis and biological activities of some novel triazoloquinazolines and triazinoquinazolines containing benzenesulfonamide moieties. Arzneimittel-Forschung/Drug Research, 60, 87-95. doi: 10.1055/s-0031-1296254.
CrossRef - Hussein,M.A.,2013.Prophylactic Effect of Resveratrol Against Ethinylestradiol-Induced Liver Cholestasis. Journal of Medicinal Food, 21, 246-254. doi: 10.1089/jmf.2012.0183.
CrossRef - Buhl,S.N. and K.Y. Jackson,1978.Optimal conditions and comparison of lactate dehydrogenase catalysis of the lactate to pyruvate to lactate reactions in human plasma at 25, 30 and 37 0C. Clin. Chem.; 2415: 828.
CrossRef - Luo,Z.,T.Harada,S. London,C. Gajdusek and M. Mayberg,1995.Antioxidant and iron chelating agents in cerebral vasospasm. Neurosurgery; 37: 1054.
CrossRef - Fossati,P.and L.Prencipe,1982.Serum triacylglycerols determined calorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem , 1: 2077-2080.
CrossRef - Allain,C.C.,L.S. Poon, C.S. Chan,W.Richmond and P.C .Fu,1974.Enzymatic determination of total serum cholesterol. Clin Chem , 4: 470-475.
CrossRef - Burnstein,M., H.R. Selvenick and R. Morfin,1970.Rapid method for isolation of lipoprotein from human serum with polyanions. J Lipid Res , 11: 583- 395.
CrossRef - Searcy,R.L. and A. Bergquist,1960.A new colour reaction for the quantitation of serum cholesterol. Clin. Chem. Acta .; 5: 1 92-1 99.
CrossRef - Reitman,S. and S. Frankel,1957.A colorimetric method for the determination of plasma oxaloacetic acid and glutamic pyruvic transaminases. Am. j. Clin. Pathol.; 28: 56 – 63.
CrossRef - Kind,P.R.N. and E.J.King,1954.Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J. Clin. Pathol ; 7: 322 – 326.
CrossRef - Buhl,S.N. and K.Y.Jackson,1978.Optimal conditions and comparison of lactate dehydrogenase catalysis of the lactate to pyruvate to lactate reactions in human plasma at 25, 30 and 37 0C. Clin. Chem.; 2415: 828.
CrossRef - Urbach,C.,K. Hickman and P.L.Harris,1951.Effects of individual vitamins A, C, E and carotene administered at high levels and their concentration in the blood. Exp. Med. Surg.; 1 0: 7-20.
- Chanarin,I.,1989.Text book of Laboratory Haematology: An Account of Laboratory techniques, Churchill Livingstone, New York PP.; 107.
- Uchiyama,M. and M.Mihara,1978.Determination of malondialdehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem.; 86:271.
CrossRef - Miranda,K.M.,M.G.Espey and D.A.Wink,2001.A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 5: 62-71.
CrossRef - Beyaert,R.and W.Fiers,1998. Tumor Necrosis Factor and Lymphotoxin. In Cytokines, A.R.M.-S. a. R. Thorpe, eds. Academic Press, San Diego, p. 335-360.
CrossRef - Favre,N., G.Bordmann and W.Rudin,1997.Comparison of cytokine measurements using ELISA, ELISPOT and semiquantitative RT-PCR. J. Immunol. Methods; 204, 57–66.
CrossRef - Marklund ,S. and D.Marklund,1974.Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem.; 47:469.
CrossRef - Paglia,D. and W.Valentine,1967.Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med; 70: 158-169.
- Clotman,F.,V.J.Lannoy, M.Reber, S.Cereghini,D.Cassiman, P.Jacquemin,T. Roskams,G.G.Rousseau and F.P.Lemaigre,2002.The onecut transcription factor HNF6 is required for normal development of the biliary tract. Development; 129:1819–1828.
CrossRef - Alturkistani,H.A.,F.M .Tashkandi and Z.M. Mohammedsaleh,2015. Histological Stains: A Literature Review and Case Study. Glob J Health Sci.;8(3):72-79. doi:10.5539/gjhs.v8n3p72
CrossRef - SPSS,2012.(SPSS 15), Inc., Chicago, IL, USA.
- Mitchell,J.R.,S.S.Thorgeirsson,W.Z.Potter,D.J. Jollow and H.Keisser,1974. Acetaminophen induced hepatic injury. V. Protective role of glutathione in man and rationale for therapy. Clin. Pharmacol. Ther.; 16:676.
CrossRef - Jollow ,D.J.,J.R. Mitchell,W.Z.Potter,D.C.Davis,J.R.Gillette and B.B.Brodie,1973.Acetaminphen-induced hepatic necrosis. II.Role of covalent binding in vivo. J. Pharmacol Exp. Ther.; 187: 195.
- Potter,W.Z.,D.C. Davis,J.R. Mitchell,G.J.Jollow,J.R. Gillette and B.B.Brodie,1973.Aetaminphen-induced hepatic necrosis. III. Cytochrome p450-mediated covalent binding in vitro. J. Pharmacol. Exp. Ther.; 187: 203.
- Dahlin,D.C.,G.T.Miwa,A.Y.Lu and S.D. Nelson,1984. N-acetyl-p-benzoquinoneimine: a cytochrome p-450 mediated oxidation product of acetaminophen. Proc. Natl. Acad. Sci. USA.; 81:1327.
CrossRef - Raucy,J.L.,H.M.Lasker,C.S.Lieber and M.Black,1989.Acetaminophen activation by human liver cytochromes p-450 II EI and p-450 IA2. Arch. Biochem. Biophys.; 27: 270.
CrossRef - Vermeulen,N.P.E., J.G.M. Bassems and R.Van de Straat,1992. Molecular aspects of paracetamol-induced hepatotoxicity and its mechanism based prevention. Drug Metab. Rev.; 24: 367.
CrossRef - Zayova,E.,I.Stancheva,M. Geneva,M. Petrova and L. Dimitrova,2013.Antioxidant activity of in vitro propagated Stevia rebaudiana Bertoni plants of different origins. Turk J Biol, 37: 106–113.
- Zia-Ul-Haq,M.,S.Ahmad,M.Qayum and S.Ercişli,2013.Compositional studies and antioxidant potential of Albizia lebbeck (L.) Benth. pods and seeds. Turk J Biol, 37: 25–32.
- Mitra,S.K.,M.V.Venkataranganna,R .Sundaram and S.Gopumadhavan,1998.J Ethnopharmacol ; 63: 181-186.
CrossRef - Sies,H.,1997. Oxidative stress, oxidants and antioxidants, Exp. Physiol. 82: 291–295.
CrossRef - Veerappan,R.M.,S.Senthil,M.Rao and M.Ravikumar,2004.Redox status and lipid peroxidation in alcoholic hypertensive patients and alcoholic hypertensive patients with diabetes, Clin. Chem. Acta 340: 207–212.
CrossRef - Kumar,G.,G.Sharmila Banu,V.Kannan and M.Rajasekara Pandian,2005.Ind J Exp Biol; 43: 351-355.
- Waters,E.,J.H. Wang,H.P.Redmond,Q.D.Wu,E.Kay and D.Bouchier Hayes,2001.Role of taurine in preventing acetaminophen-induced hepatic injury in the rat. Am. J. Physiol. Gastrointest. Liver Phsiol.; 280: G1274.
CrossRef - Wohaieb,S. and D.V.Godin,1987. Alterations in free radical tissue – defense mechanisms in streptozotocin diabetes in rats: Effect of insulin treatment. Diabetes ; 36: 1014.
CrossRef - Liu,P.T., C.Ioannides,A.M.Symons and D.V.Parke,1993.Role of tissue glutathione in prevention of surgical trauma. Xenobiotica,; 23: 899.
CrossRef - Mc Crod,J.M.,B.B.Keele and I.Fridovich,1976.An enzyme based theory of obigate anaerobiosis, the physiological functions of superoxide dismutase. Pro Nati Acad Sci. USA ; 68: 1024.
CrossRef - Sozmen,B.Y.,B.Sozmen, Y.Delen and T.Onat,2001.Catalase/superoxide dismutase (SOD) and catalase/paraoxonase (PON) ratios may implicate poor glycemic control. Ara Med Res.; 32: 283.
CrossRef - Chance,B.,D.S.Green Stein and R.J.Roughton,1952.The mechanism of catalase action 1-steady state analysis. Arch Biochem Biophys ; 37: 301.
CrossRef - Bruce,A.,D.Freeman and C .James,1982.Biology of disease. Free radicals and tissue injury. Lab Invest ; 47: 412.
- O’Brien,P.,M.Slaughter,A.Swain, J. Birmingham,R.Greenhill,F.Elcock and P.Bugelski,2000.Repeated acetaminophen dosing in rats: adaptation of hepatic antioxidant system. Hum. Exp. Toxicol.; 19:277.
CrossRef - Ahmed,M.B. and M.R. Khater,2001.Evaluation of the protective potential of ambrosia maritime extract on acetaminophen-induced liver damage, J. Ethnopharmacol.; 75: 169.
CrossRef - Abou-Taleb NI, Elblasy OA, Elbesoum EA, Basuny HI, Elhamadi EA, Nasr eldin MS, Emara AA, Ali AA, Salem MA, Ahmed FM, Hussein MA. 2021. Mechanism of Antiangiogenic and Antioxidant Activity of Newly Synthesized CAMBA in Ehrlich Ascites Carcinoma-Bearing Mice. Asian Journal of Chemistry. 33(10):2465-2471.
CrossRef - Abdel Maksoud HA, Elharrif MG, Mahfouz MK, Omnia MA, Abdullah MH, Eltabey ME. 2019. Biochemical study on occupational inhalation of benzene vapours in petrol station. Respiratory Medicine Case Reports. 27. DOI: 10.1016/j.rmcr.2019.100836.
CrossRef - Borik RM, Hussein MA. 2021. Synthesis, molecular docking, biological potentials, and structure activity relationship of new quinazoline and quinazoline-4-one derivatives. Asian Journal of Chemistry. 33: 423 – 438.
CrossRef - Hussein MA, Ismail NEM, Mohamed AH, Borik RM, Ali AA, Mosaad YO. Plasma Phospholipids: A Promising Simple Biochemical Parameter to Evaluate COVID-19 Infection Severity. Bioinform Biol Insights. 2021;15:11779322211055891.doi: 10.1177/11779322211055891.
CrossRef - Welsh,S.J.,W.T.Bellamy,M.M.Briehl and G.Powis,2002.The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1alpha protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Res.; 62: 5089–5095.
- Eriksson,S.,E.A.Prigge Talago,E.S.J.Arnér and E.E.Schmidt,2015. Dietary methionine can sustain cytosolic redox homeostasis in the mouse liver. Nature Communications , 6: 6479- 85. doi: 10.1038/ncomms7479
CrossRef - Huo,Y.,S.Yin and M.Yan,2017.Protective role of p53 in acetaminophen hepatotoxicity. Free Radic Biol Med.;106:111-117. doi:10.1016/j.freeradbiomed.2017.02.028.
CrossRef - Kruiswijk,F.,C.F.Labuschagne and K.H.Vousden,2015.p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat Rev Mol Cell Biol.; 16(7):393-405.
CrossRef - Singh,B.,P.G.Reddy,A.Goberdhan,C.Walsh,S.Dao,I.Ngai,T.C. Chou,P.Charoenrat,A.J.Levine, P.H.Rao and A.Stoffel,2002.p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev.; 16(8):984-93.
CrossRef - Klaassen,C.D. and S.A.Reisman,2010.Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol Appl Pharmacol.; 244(1):57-65.
CrossRef - Okawa,H.,H.Motohashi, A.Kobayashi, H.Aburatani,T.W.Kensler and M. Yamamoto,2006.Hepatocyte-specific deletion of the keap1 gene activates Nrf2 and confers potent resistance against acute drug toxicity. Biochem Biophys Res Commun. ; 339(1):79-88.
CrossRef - 77.Stamper,B.D.,M.L.Garcia,D.Q.Nguyen,R.P.Beyer,T.K.Bammler,F.M.Farin,T.J. Kavanagh and S.D.Nelson,2015. p53 Contributes to Differentiating Gene Expression Following Exposure to Acetaminophen and Its Less Hepatotoxic Regioisomer Both In Vitro and In Vivo. Gene Regul Syst Bio.; 9:1-14.
CrossRef - Boulares,A.H.,A.J.Zoltoski,B.A.Stoica,O.Cuvillier and M.E. Smulson,2002.Acetaminophen induces a caspase-dependent and Bcl-XL sensitive apoptosis in human hepatoma cells and lymphocytes. Pharmacol Toxicol.; 90(1):38–50.
CrossRef - El-Boghdady,N.A.,N.F.Abdeltawab and M.M.Nooh,2017.Resveratrol and Montelukast Alleviate Paraquat-Induced Hepatic Injury in Mice. Modulation of Oxidative Stress, Inflammation, and Apoptosis. Oxid Med Cell Longev. ;(10):9396425.
CrossRef - Pan,S.,S.Li,Y.Hu,H.Zhang,Y.Liu and H.Jiang, 2016. Resveratrol post-treatment protects against neonatal brain injury after hypoxia-ischemia. Oncotarget.;7(48):79247–61.
CrossRef







