Manuscript accepted on :20-05-2022
Published online on: 13-06-2022
Plagiarism Check: Yes
Reviewed by: Dr. Mohsen Tabasi, Dr. Ahmed Salah
Second Review by: Dr. Ana Golez
Final Approval by: Dr. Juei-Tang Cheng
Efraín Navarro-Olivos1 , Francisco J. Magos-Vázquez2
, Francisco J. Magos-Vázquez2 , María del Rosario Sánchez-Navarro3
, María del Rosario Sánchez-Navarro3 , Daniel A. Díaz-Martínez2
, Daniel A. Díaz-Martínez2 , Elia Lara-Lona4
, Elia Lara-Lona4 , María de Jesús Gallardo-Luna5
, María de Jesús Gallardo-Luna5 , Gilberto Flores-Vargas5
, Gilberto Flores-Vargas5 and Nicolás Padilla-Raygoza5*
and Nicolás Padilla-Raygoza5*
1Directorate of Teaching and Research, Institute of Public Health from Guanajuato State, Guanajuato, México.
2Directorate of Health Services, Institute of Public Health from Guanajuato State, Guanajuato, México
3State Laboratory of Public Health, Institute of Public Health from Guanajuato State, Leon, México.
4Department of Medicine and Nutrition, Division of Health Sciences, Campus Leon, University of Guanajuato, León, Mexico.
5Department of Research and Technological Development, Directorate of Teaching and Research, Institute of Public Health from Guanajuato State, Guanajuato, Mexico.
Corresponding author E-mail: npadillar@guanajuato.gob.mx
DOI : https://dx.doi.org/10.13005/bpj/2435
Abstract
The Severe Acute Respiratory Syndrome Coronavirus 2, first detected in Wuhan, China, in 2019, had spread all over the world. It has caused the COVID-19 pandemic. Nowadays, there are effective and safe vaccines proven against this virus. The goal of this study was to verify it among health-care workers from the Institute of Public Health from Guanajuato State who received the BioNTech/Pfizer vaccine. For this purpose, we designed a quantitative cross-sectional study. The database was obtained from a previous strategy program by the Institute of Public Health from Guanajuato State called ENSERO-COVID. The available data consisted of two chemiluminescence measures of the IgG anti-Spike antibodies after one and six months of the BioNTech/Pfizer vaccine two doses application. The survey also included self-reported reactions to this vaccine. Frequency tables are presented for descriptive purposes. We performed the chi-square test, the z test for proportions, and the t-test for comparisons. Also, two linear regression models were fitted between the first and second chemiluminescence levels stratifying by prior infection by SARS-CoV-2. The database consisted of 177 records. Of them, 45 (25.4%) were positive for SARS-CoV-2 before vaccination. Only one person did not react to the two doses of vaccine application. Most of the self-reported reactions ceased in a short period -less than three days-. The differences observed, regarding chemiluminescence levels, between those with and without prior infection by SARS-CoV-2 were not statistically significant. More analyses are required to assess the long-term effects of the BioNTech/Pfizer ® vaccine.
Keywords
BioNTech/Pfizer vaccine; COVID-19; Health-Care professional vaccination; IgG antibodies; SARS-CoV-2
Download this article as:| Copy the following to cite this article: Olivos E. N, Vázquez F. J. M, Navarro M. D. R. S, Martínez D. A. D, Lona E. L, Luna M. D. J. G, Vargas G. F, Raygoza N. P.Reactivity and Safety of BioNTech/Pfizer ® Vaccine Anti-SARS-CoV-2, in Health Personnel from the Mexican State of Guanajuato. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Olivos E. N, Vázquez F. J. M, Navarro M. D. R. S, Martínez D. A. D, Lona E. L, Luna M. D. J. G, Vargas G. F, Raygoza N. P.Reactivity and Safety of BioNTech/Pfizer ® Vaccine Anti-SARS-CoV-2, in Health Personnel from the Mexican State of Guanajuato. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3mEZfxh |
Introduction
In December 2019, several cases of pneumonia of unknown cause were reported in Wuhan, Hubei Province, China 1. On January 10, 2020, the first death from this disease was reported 2. On February 7, 2020, it was concluded that the disease had originated from a new coronavirus nowadays called SARS-CoV-2 and was named COVID-19 3,4.
Worldwide, by December 29, 2021, 281,808,931 confirmed cases with 5,411,759 deaths had been reported, with a Case Fatality Ratio (CFR) of 1.9%, with America with the highest number of case reports and followed by Europe. In Mexico, by December 31, 2021, 3,979,723 confirmed cases and 299,428 deaths were reported, with a CFR of 7.52%. The states with the highest frequency of reported cases were Mexico City, Mexico State, Nuevo León, Guanajuato, and Jalisco 5.
In Mexico, with the high number of confirmed cases and a case fatality rate that exceeds the global fatality rate, one of the hopes to achieve herd immunity is the application of the vaccine so that there are no longer so many susceptible people and reduce the new cases.
The S protein is crucial for virus-cell receptor binding and virus-cell membrane fusion, suggesting that it can be an effective target for CoV vaccine design 6. Studies have shown that antibodies against the S protein are long-lasting and immunodominant in recovered SARS patients 7,8. In addition, several studies have demonstrated that the anti-S antibody can neutralize SARS-CoV and MERS-CoV and provides protective effects in animals and humans 9-11. Moreover, many S protein-based vaccines against SARS-CoV and MERS-CoV have elicited potent immune responses and protective effects in preclinical models 12-16.
SARS-CoV S protein is an ideal vaccine target to induce neutralizing antibodies and protective immunity. Besides S protein, other structural proteins have also been tested as vaccine targets. N protein-based vaccines usually cannot produce neutralizing antibodies, likely because N protein is not displayed on the CoV surface 17.
BioNTech and Pfizer’s mRNA vaccine is the BNT162b2 vaccine. It is 95% effective against COVID-19 18. This result was based on the analysis of 170 confirmed COVID-19 cases. From them, 162 cases of COVID-19 were placed in the placebo group, while 8 cases were in the BNT162b2 group 18. Among ten severe COVID-19 cases observed in this trial, nine were in the placebo group, and only one was in the BNT162b2 group 18. The observed efficacy in the advanced age people was over 94%, which would help protect the most vulnerable population against COVID-19 [18]. There were no serious safety concerns among 43,000 enrolled participants [18]. These data indicated that BNT162b2 is another well-tolerated and efficacious COVID-19 vaccine 18.
In response to the COVID-19 pandemic, many countries have implemented large-scale public health and social measures attempting to reduce community transmission and minimize the outbreak impact 19. The benefits derived from implementing these measures are evident in terms of incidence reduction of SARS-CoV-2 cases and associated deaths; thus, countries are looking for ways to resume economic and social activities 19. Ideally, to carry this out, it must be evaluated and determined if the population has acquired sufficient herd immunity: to the point that any reintroduction of the virus does not trigger a new epidemic wave; this requires a deeper understanding of the kinetics of antibodies acquired after SARS-CoV-2 infection 19.
In Guanajuato State, the vaccination of the healthcare professionals in the first line from the units of the Institute of Public Health from Guanajuato State (IPHGS) began on February 17, 2021. The applied vaccine was the BioNTech-Pfizer ® vaccine.
As part of the actions to better understand the pandemic behavior among health workers, it is necessary to know the efficacy and safety of the BioNTech-Pfizer ® vaccine in IPHGS health personnel from the first line of care who have received the second dose. It is one of the chief reasons to conduct this study.
The objective was to analyze the reactivity and safety of the anti-SARS-CoV-2 vaccine in IPHGS health personnel because healthcare workers were infected commonly by SARS-CoV-2.
We hypothesize that those previously infected by SARS-CoV-2 will show a higher reactivity measured indirectly by chemiluminescence levels (CLS). Regarding adverse reactions, we do not have a prior belief about differences between those previously infected and non-infected.
Material and methods
The study was designed as a cross-sectional one. It is based on the registries from the ENSERO-COVID strategy program by the IPHGS.
The universe was registries from healthcare workers who participated in the ENSERO-COVID strategy program and are in the first line for the care of COVID-19 patients.
The data analysis was conducted between October 2021 and January 2022.
The sampling method was by availability. We included all registries from health workers participating in the strategy in the analysis.
The selection criteria were men and women, 18 years old or higher, who are first-line health personnel, who agreed to participate in the strategy to determine the efficacy and safety of the anti-SARS-CoV-2 vaccine, and registered in ENSERO-COVID.
There were no exclusion criteria, and elimination criteria were registries incompletes.
Besides age and sex, the variables considered for the analysis were, presented and defined by type, the following:
Independent variables
RT-PCR test result. It is a dichotomous categorical variable. It is the reactivity for SARS-CoV-2. Its values are positive or negative. It is presented with frequencies and percentages.
SARS-CoV-2 antigen result. It is a dichotomous categorical variable. It is the reactivity for SARS-CoV-2. Its values are positive or negative. It is presented with frequencies and percentages.
Dependent variables
1st determination of antibodies. It is a dichotomic variable. It is the presence of IgG antibodies -determined by laboratory tests one month after the second dose of the BioNTech-Pfizer® vaccine application-. It is considered reactive with 1.0 or greater or non-reactive with <1.0. It is presented with mean and standard deviation.
Adverse events. It is a nominal categorical variable. It is the presence of any effect related or not to the administration of the vaccine. It accounts for pain in the application arm, fatigue, headache, fever, myalgia, arthralgia, etc. Also, a blank space was available for reporting adverse events not included in the catalog. The results are presented with frequencies and percentages.
2nd determination of antibodies. It is a dichotomic variable. It is the presence of IgG antibodies -determined by laboratory tests six months after the second dose of the BioNTech-Pfizer® vaccine application-. It is considered reactive with 1.0 or greater or non-reactive with <1.0. It is presented with mean and standard deviation.
SARS-CoV-2 IgG antibodies quantitative determination. It is a continuous quantitative variable. It is the chemiluminescence level of anti-SARS-CoV-2 IgG antibodies. It was determined one and six months after the second dose of the BioNTech-Pfizer ® vaccine. It is truncated at two decimals. The mean and standard deviation of this variable are presented.
Procedures
The first determination of SARS-CoV-2 IgG antibodies was measured one month after the second dose of the vaccine, and a second determination was measured six months after.
The results were saved in the database from the ENSERO-COVID strategy program. From there, vaccinated people who participated in the strategy were sought in the National Epidemiological Surveillance System to know if at any time they had an RT-PCR or antigen test result to detect SARS-CoV-2 infection.
The vaccines were applied according to the logistics established by the Federal Health Secretariat and IPHGS.
A peripheral venous blood sample for each participant was taken one month and six months after receiving the second dose of the vaccine.
At six months after the second dose of the vaccine, a survey for each participant was self-filled to detect symptoms and perceived side effects.
The technique for determining antibodies IgG against the SARS-CoV-2 is as follows:
Anti-SARS-CoV-2 IgG detection test was performed using the VITROS Anti-SARS-CoV-2 IgG Reagent Pack. The VITROS Anti-SARS-CoV-2 IgG Calibrator and Anti SARS-CoV-2 IgG lab controls allow the qualitative measurement of total IgG anti-spike antibodies to SARS-CoV-2. The procedure was carried out using the VITROS 5600 Integrated Systems Ortho Clinical Diagnostics.
An immunometric technique is used, which involves a two-step reaction. In the first step, the SARS-CoV-2 IgG antibodies in the sample bind to the S1 antigen of the SARS-CoV-2 spike protein coated on the wells. The unfixed sample is removed by washing. For the second step, the conjugate with the recombinant S1 antigen of the SARS-CoV-2 spike protein is added -labeled as horseradish peroxidase (HRP)-. The conjugate binds to any anti-SARS-CoV-2 antibody captured in the well in the first step. Unbound conjugate is removed in the subsequent wash step.
The bounded HRP conjugate is measured by a luminescent reaction. A reagent containing luminogenic substrates (a luminol derivative and a peracid salt) and an electron transfer agent are added to the wells. The HRP in the bound conjugate catalyzes the oxidation of the luminol derivative and produces light. The electron transfer agent (substituted acetanilide) increases the light level and prolongs its emission. Then, the system reads the signals. The bounded HRP conjugate indicates the amount of SARS-CoV-2 IgG antibodies present. The signal value is proportional to the SARS-CoV-2 IgG antibodies amount in the sample 20.
The results allow the SARS-CoV-2 IgG antibodies detection; they are automatically computed by the VITROS 5600 Integrated Systems Ortho Clinical Diagnostics immunodiagnostic system. The result is calculated as the signal of the test sample divided by the one at the cut-off point (limit value).
If the value reported is under 1.00, it is considered a non-reactive sample for anti-SARS-CoV-2. If the results are equal to or greater than 1.00, the sample is reactive for anti-SARS-CoV-2.
Once the Research (CI) and Research Ethics (CEI) Committees approved the research protocol, a database was processed in STATA 13.0 (Stata Corp., College Station, TX, USA), obtaining the variables indicated in the operationalization of variables. Privacy was safeguarded by deleting all personal identification variables.
Sample size calculation
If those infected by SARS-CoV-2 have 99% reactivity for antibodies and 85% of those not infected, the minimum sample size is 161: 53 previously infected and 106 without previous infection, with 95% precision and 80 % power (EpiInfo 7.2.2.16, 2018. CDC, Atlanta, GA, USA).
Statistical analysis
Descriptive statistics were used to present all the variables. The chi-squared test was performed to assess the relationship between categorical variables and previous infection of SARS-CoV-2. On the other hand, the t Student test was computed for quantitative variables. Also, two linear regression models were fitted to assess the relationship between prior chemiluminescence levels decay and previous infection.
Results and discussion
The database consisted of 177 records from health professionals in the first line of care for COVID-19 patients who agreed to participate in the strategy program.
The participants were classified as positive or negative to SARS-CoV-2, in accordance with registered prior infection. Of them, 45 were positive for SARS-CoV-2, and 132 were negative. It is worth noting that those who never underwent an RT-PCR test were included in the latter group. The data about RT-PCR tests were obtained from the National Epidemiological Surveillance System.
Table 1 shows the characteristics of the participants. Age means between positive and negative SARS-CoV-2 infected did not statistically significantly differ (P> .05). Regarding chemiluminescence levels, one month and six months after the second dose, there was no difference between those with and without previous infection by SARS-CoV-2(P> .05). The female sex predominated in positives and negatives with 71% and 76%, respectively. One person did have a non-reactive qualitative test result for anti-SARS-CoV-2 for each sample.
Table 1: Distribution of variables by SARS-CoV-2 positive and negative in first line health workers.
| Quantitative variables | SARS-CoV-2 +
n=45 |
SARS-CoV-2 –
n=132 |
t Student test (DF) P-value | |||
| Age (years)
Range Mean Standard deviation |
22 – 55 34.76 8.98 |
20 – 58 35.20 10.36 |
0.25 (175) .80 | |||
| Anti-spike S1 IgG antibodies after 1 month of vaccine 2nd dose
Range Mean Standard deviation |
.01 – 24.7
20.71 4.16 |
10.3 – 25.1
20.73 2.33 |
0.04 (175) .97 | |||
| Anti-spike S1 IgG antibodies after 6 months of vaccine 2nd dose
Range Mean Standard deviation |
0 -18.3
12.15 3.13 |
5.19 – 18.6
11.86 2.30 |
– 0.66 (175) .51 | |||
| Qualitative variables | n | % | n | % | Z for two proportions, P-value | Chi squared test (DF) P-value |
| Sex
Female Male |
32 13 |
71.11 28.89 |
101 31 |
76.52 23.48 |
0.73, .47 | 0.51 (1) .47 |
| Antibodies reactivity after 1 month from vaccine 2nd dose
Yes No |
44
1 |
97.77
0.22 |
132
0 |
100
0 |
||
| Antibodies reactivity 6 months after vaccine 2nd dose
Yes No |
44
1 |
97.77
0.22 |
132
0 |
100
0 |
||
Source: ENSERO-COVID, IPHGS
Table 2 shows the distribution of adverse events derived from the application of the first dose of the vaccine and the averages of its duration in days; the most frequent was a pain in the application area (82% in positive subjects and 87% in negative subjects), and its duration was two days on average. Body pain occurred more frequently among those previously infected by SARS-CoV-2 (48%) than among those not infected (30%), the difference being significant (P <.05), and the duration was less than two days on average. Fatigue was another frequent adverse event among the participants, with 55% and 46%, but without a significant difference.
Table 2: Distribution of adverse events from vaccine and their duration in days, after first dose, by infected and non-infected for SARS-CoV-2
| Adverse events | SARS-CoV-2 positive
n = 45 n % |
SARS-CoV-2 negative
n = 132 n % |
Z for two proportions P-value
t Student (DF) P-value |
| Pain in area of injection
Yes No Duration (days) mean ± SD |
37 82.22
8 17.78 2.40 ± 1.81 |
116 87.88
16 12.12 1.87 ± 1.70 |
0.96 P=.34
-1.66 (151) .10 |
| Redness in application area
Yes No Duration (days) mean ± SD |
4 8.89 41 91.11 2.25 ± 0.96 |
10 7.58 122 92.42 2.95 ± 3.96 |
-0.28 P= .78
0.34 (12) .74 |
| Myoarthralgia
Yes No Duration (days) mean ± SD |
22 48.89 23 51.11 1.62 ± 1.47 |
40 30.30 92 69.70 1.82 ± 2.15 |
-2.26 P= .02
0.41 (67) .68 |
| Swelling in application area
Yes No Duration (days) mean ± SD |
6 13.33 39 86.67 3.04 ± 2.30 |
27 20.45 105 79.55 2.31 ± 2.61 |
1.06 P=.29
-0.63 (31) .5 |
| Fatigue
Yes No Duration (days) mean ± SD |
25 55.56 20 44.44 2.76 ± 5.96 |
61 46.21 71 53.79 2.43 ± 4.13 |
-1.10 P= .27
-0.30 (82) .77 |
| Headache
Yes No Duration (days) mean ± SD |
21 46.67 24 53.33 4.26 ± 8.72 |
48 36.36 84 63.64 3.94 ± 8.62 |
-1.22 P= .22
-0.14 (67) .89 |
| Fever
Yes No Duration (days) mean ± SD |
6 13.33 39 86.67 0.69 ± 0.49 |
18 13.64 114 86.36 1.08 ± 0.71 |
0.05 P= .96
1.33 (22) .2 |
| Chills
Yes No Duration (days) mean ± SD |
5 11.11 40 88.89 0.43 ± 0.52 |
18 13.64 114 86.36 1.12 ± 0.80 |
0.44 P=.66
1.81 (21) .08 |
| Cough
Yes No Duration (days) mean ± SD |
2 4.44 43 95.56 4.5 ± 3.54 |
3 2.27 129 97.73 5.00 ± 2.00 |
-0.76 P= .45
-0.21 (3) .85 |
| Rhinorrhea
Yes No Duration (days) mean ± SD |
5 11.11 40 88.89 2.75 ± 1.5 |
6 4.55 126 95.45 8.67 ± 10.56 |
1.57 P= .12
-1.09 (8) .31 |
| Nausea
Yes No Duration (days) mean ± SD |
4 8.89 41 81.11 1.26 ± 0.95 |
9 6.82 123 93.18 1.12 ± 0.58 |
-0.46 P=.65
0.33 (11) .75 |
(DF) Degree of freedom
Source: ENSERO-COVID, IPHGS
Table 3 shows the adverse events reported after the second dose of the vaccine -which results are like those after the administration of the first dose-. There were no significant differences between those previously infected by SARS-CoV-2 and those not infected.
Table 3: Distribution of adverse events from vaccine and their duration in days, after second dose, by infected and non-infected for SARS-CoV-2
| Adverse events | SARS-CoV-2 positive
n = 45 n % |
SARS-CoV-2 negative
n = 132 n % |
Z for two proportions P-value
t Student (DF) P-value |
| Pain in application area
Yes No Duration (days) mean ± SD |
31 68.89 14 31.11 5.47 ± 16.04 |
105 79.55 27 20.45 1.72 ± 1.36 |
1.46 P=.14
-2.39 (133) .02 |
| Redness in application area
Yes No Duration (days) mean ± SD |
4 8.89 41 91.11 3.25 ± 2.63 |
11 8.33 121 91.67 2.26 ± 1.76 |
0.12 P= .91
-0.87 (14) .40 |
| Myoarthralgia
Yes No Duration (days) mean ± SD |
17 37.78 28 62.22 2.65 ± 2.40 |
42 31.82 90 68.18 2.43 ± 3.23 |
-0.61 P= .54
-0.25 (56) .80 |
| Swelling in application area
Yes No Duration (days) mean ± SD |
5 11.11 40 88.89 2.62 ± 2.68 |
25 18.94 107 81.06 1.83 ± 1.49 |
1.21 P=.23
-0.93 (27) .36 |
| Fatigue
Yes No Duration (days) mean ± SD |
24 53.33 21 46.67 2.62 ± 3.32 |
59 44.70 73 55.30 1.87 ± 2.14 |
-1.00 P= .32
-1.21 (80) .23 |
| Headache
Yes No Duration (days) mean ± SD |
19 42.22 26 57.78 4.34 ± 7.13 |
49 37.12 83 62.88 1.57 ± 1.40 |
-0.61 P= .54
-2.55 (63) .01 |
| Fever
Yes No Duration (days) mean ± SD |
5 11.11 40 88.89 0.69 ± 0.49 |
18 13.64 114 86.36 1.08 ± 0.71 |
0.44 P= .66
1.33 (22) .2 |
| Chills
Yes No Duration (days) mean ± SD |
5 11.11 40 88.89 1.01 ± 0.69 |
23 17.42 109 82.58 1.18 ± 1.11 |
1.00 P=.32
0.33 (26) .75 |
| Cough
Yes No Duration (days) mean ± SD |
2 4.44 43 95.56 11.50 ± 12.02 |
2 1.52 130 98.48 6.50 ± 7.78 |
-0.33 P= .74
-0.49 (2) .67 |
| Rhinorrhea
Yes No Duration (days) mean ± SD |
1 2.22 44 97.78 2.00 ± 0.00 |
4 3.03 128 96.97 4.00 ± 5.36 |
0.28 P= .78
NA |
| Nausea
Yes No Duration (days) mean ± SD |
5 11.11 40 88.89 1.14 ± 1.30 |
5 3.79 127 96.21 0.82 ± 0.41 |
-1.84 P=.07
-0.36 (8) .73 |
Source: ENSERO-COVID, IPHGS
Figure 1 shows the correlation and linear regression between the chemiluminescence levels one month and six months after the second dose of the vaccine. A good correlation of SARS-CoV-2 IgG antibodies is found among those previously infected by SARS-CoV-2 (r = 0.71) with a statistically significant linear relationship (P <.05). A mild correlation was found among those without previous infection. Nevertheless, the linear relation was statistically significant (P <.05).
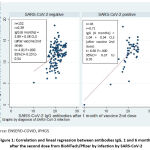 |
Figure 1: Correlation and lineal regression between antibodies IgG, 1 and 6 months after the second dose from BioNTech/Pfizer by infection by SARS-CoV-2. |
Figure 2 shows the comparison of medians and percentiles of the SARS-CoV-2 IgG antibodies between those previously infected by SARS-CoV-2 and those not infected. A slightly higher amount of SARS-CoV-2 IgG antibodies among those infected is observed, although the difference was not significant.
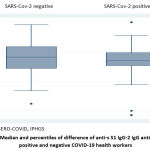 |
Figure 2: Median and percentiles of difference of anti-s S1 IgG-2 IgG antibodies by positive and negative COVID-19 health workers. |
Table 4 compares the mean difference between the first and the second measurement of chemiluminescence levels, stratifying those previously infected and those who were not. The differences were positive, indicating a higher CLS in the first measurement. The difference was statistically significant in both cases (P <.05).
Table 4: Mean differences by SARS-CoV-2 positive and negative status in health workers.
| Mean of difference in amount of SARS-CoV-2 IgG antibodies ± standard deviation (95%CI) | t Student (DF) P-value | |
| SARS-CoV-2 positive (n=45) | 8.57 ± 0.44 (7.69 to 9.45) | 19.62 (44) .0000 |
| SARS-CoV-2 negative (n=132) | 8.87 ± 0.22 (8.43 to 9.31) | 39.81 (131) .0000 |
DF Degree of freedom
Source: ENSERO-COVID, IPHGS
Only one person was not reactive to the two doses of anti-SARS-CoV-2 vaccines. Of the 177 participants, 45 (25.42%) had a positive RT-PCR or SARS-CoV-2 antigen before the second dose of the BioNTech-Pfizer vaccine. Women proportion predominated both the positive and negative RT-PCR. Between those previously positive and negative for RT-PCR, there were no differences in mean age, gender distribution, or reactivity after two doses of BioNTech-Pfizer® vaccines at one month and six months. Regarding adverse events, only myoarthralgia showed a statistically significant difference (P<.05) after the first dose between positive and negative RT-PCR; after the second, significant differences were reported between the two groups for pain in the arm and headache (P<.05). A statistically significant linear relationship and high correlation between chemiluminescence levels were found among those with a previous positive RT-PCR test result. Comparing the first measurement of CLS with the second, significant differences were found between the positive and the negative ones.
Polak et al. 21 reported in a multicenter study with the BioNTech-Pfizer® vaccine that the secondary reaction most reported among patients older than 55 years was mild to moderate pain at the application site after the first (71%) and second dose (66%). In Guanajuato, pain at the application site was more than 80%, in previously positive and negative for RRT-PCR, and lasted 1-2 days (Table 2), like those reported by Polack21.
Among the IPHGS health personnel, 14 persons (7.91%) reported redness in the area of application (1-2 days) and 33 (18.64%) accompanied by swelling (2-3 days) (Table 2), while in another study, from 18,860 participants, 12 reported redness and 14 accompanied by swelling after the first dose of the vaccine 21.
In the study by Polack et al. 21, nearly 50% of the participants reported fatigue and headache after the first dose of the vaccine. On the other hand, fever was reported in about 17% of the participants. In the sample from Guanajuato, 24 (13.56%) reported fever (1 day), 86 (48.59%) reported fatigue (2.5 days), and 14 (7.91%) reported headache (4 days) (Table 2).
After the second dose of the vaccine, the effects were milder both among the participants from Guanajuato (Table 3) and among the participants in Polack’s multicenter study 21.
Rastawicki et al. 22 reported that 136 of 137 persons vaccinated against SARS-CoV-2 with the BNT162b2 vaccine had higher levels of antibodies with preexisting SARS-CoV-2 infections, measured with IgM antibodies against the S-protein of SARS-CoV-2, detected by ELISA. In the sample of Guanajuato, there is no difference in reactivity to the BioNTech-Pfizer® vaccine between the positive and negative groups (Table 1, Figure 1,2).
Ali al. 23 reported higher levels of IgG (157 BAU/ml) in non-previous infected with SARS-CoV-2 and in previous infected (195 BAU/ml) after the first dose of the BNT162b2 vaccine. After the second dose of the vaccine, the IgG levels were 137 BAU/ml and 188 BAU/ml, respectively.
Weaknesses
In the Guanajuato sample, antibody levels against SARS-CoV-2 spike protein S were not quantified; it was only a qualitative test (reactive, not reactive); what was quantified was the chemiluminescence for reading the samples.
The sample consisted of 177 health professionals who agreed to participate. Nevertheless, the number of IPHGS employees is above 5,000.
Conclusion
As expected, most participants showed reactivity to the BioNTech-Pfizer® vaccine -only one did not present it-. There were no severe adverse events. Most of the reported reactions resumed in a short period. Although the qualitative nature of the antibody tests used, those positive for SARS-CoV-2 before the vaccine application showed a higher correlation between the first and second chemiluminescence levels. In future work, it should be assessed the robustness of mRNA vaccines against infection by SARS-CoV-2 variants. Particularly for those with mutations in the S protein.
The observed CLS decay pattern suggests that, among those previously infected, the antibodies generated by the vaccine application last longer than those without prior infection. Nevertheless, this was not statistically significantly supported by the data.
It is concluded that the BioNTech-Pfizer® vaccine produced the expected reactivity and that it does not pose severe safety concerns to the IPHGS healthcare workers.
Ethics Committee
The protocol was approved by Committee of Research Ethics Hospital General San Luis de la Paz, September 1, 2021.
Conflict of Interest
There are no conflict of interest.
Funding Source
There is no funding source
References
- Lu H, Stratton CW, Tang YW.Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol. 2020; 92: 401-402 doi: https://doi.org/10.1002/jmv.25678
CrossRef - Yoo JH, Hong ST. The outbreak cases with the novel coronavirus suggest upgraded quarantine and isolation in Korea. J Korean Med Sci. 2020; 35(5):e62 Doi: https://doi.org/10.3346/jkms.2020.35.e62
CrossRef - World Health Organization Rolling updates on coronavirus disease (COVID-19). Updated 7 May 2020. Disponible en: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/
- Carlos WG, De la Cruz C, Cao B, Pasnick S, Jamil S. Novel Wuhan (2019 -CoV) coronavirus. Am J Respi Crit Care Med. 2020; 201(4):7-8. Doi: https://doi.org/10.1164/rccm.2014P7
CrossRef - Subsecretaría de Prevención y Promoción de la Salud. Secretaría de Salud. Informe Técnico DiarioCOVID-19 México. 31 diciembre 2021. Disponible en: https://www.gob.mx/cms/uploads/attachment/file/689974/Comunicado_Tecnico_Diario_COVID-19_2021.12.31.pdf
- Du L, He Y, Zhou Y, Liu S, Zheng BJ, Jiang S. The spike protein of SARS-CoV–a target for vaccine and therapeutic development. Nat Rev Microbiol. 2009;7(3):226–236. Doi: https://doi.org/10.1038/nrmicro2090
CrossRef - Cao Z, Liu L, Du L, Zhang C, Jiang S, Li T, He Y. Potent and persistent antibody responses against the receptor-binding domain of SARS-CoV spike protein in recovered patients. Virol J. 2010;7:299. Doi: https://doi.org/10.1186/1743-422X-7-299
CrossRef - Zhong X, Yang H, Guo ZF, Sin WY, Chen W, Xu J, et al. B-cell responses in patients who have recovered from severe acute respiratory syndrome target a dominant site in the S2 domain of the surface spike glycoprotein. J Virol. 2005;79(6):3401–3408. Doi: https://doi.org/10.1128/JVI.79.6.3401-3408.2005
CrossRef - Qiu M, Shi Y, Guo Z, Chen Z, He R, Chen R, et al. Antibody responses to individual proteins of SARS coronavirus and their neutralization activities. Microbes Infect. 2005;7(5–6):882–889. Doi: https://doi.org/10.1016/j.micinf.2005.02.006
CrossRef - Tang XC, Agnihothram SS, Jiao Y, Stanhope J, Graham RL, Peterson EC, et al. Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution. Proc Natl Acad Sci USA. 2014;111(19):E2018–2026. Doi: https://doi.org/10.1073/pnas.1402074111
CrossRef - Li Y, Wan Y, Liu P, Zhao J, Lu G, Qi J, et al. A humanized neutralizing antibody against MERS-CoV targeting the receptor-binding domain of the spike protein. Cell Res. 2015;25(11):1237–1249. Doi: https://doi.org/10.1038/cr.2015.113
CrossRef - Li J, Ulitzky L, Silberstein E, Taylor DR, Viscidi R. Immunogenicity and protection efficacy of monomeric and trimeric recombinant SARS coronavirus spike protein subunit vaccine candidates. Viral Immunol. 2013;26(2):126–132. Doi: https://doi.org/10.1089/vim.2012.0076
CrossRef - He Y, Li J, Heck S, Lustigman S, Jiang S. Antigenic and immunogenic characterization of recombinant baculovirus-expressed severe acute respiratory syndrome coronavirus spike protein: implication for vaccine design. J Virol. 2006;80(12):5757–5767. Doi: https://doi.org/10.1128/JVI.00083-06
CrossRef - Tai W, Wang Y, Fett CA, Zhao G, Li F, Perlman S, et al. Recombinant receptor-binding domains of multiple Middle East respiratory syndrome coronaviruses (MERS-CoVs) induce cross-neutralizing antibodies against divergent human and camel MERS-CoVs and antibody escape mutants. J Virol. 2017;91(1): e01651-16. Doi: https://doi.org/10.1128/JVI.01651-16
CrossRef - Tai W, Zhao G, Sun S, Guo Y, Wang Y, Tao X, et al. A recombinant receptor-binding domain of MERS-CoV in trimeric form protects human dipeptidyl peptidase 4 (hDPP4) transgenic mice from MERS-CoV infection. Virology. 2016;499:375–382. Doi: https://doi.org/10.1016/j.virol.2016.10.005
CrossRef - Wang Y, Tai W, Yang J, Zhao G, Sun S, Tseng CK, et al. Receptor-binding domain of MERS-CoV with optimal immunogen dosage and immunization interval protects human transgenic mice from MERS-CoV infection. Hum Vaccin Immunother. 2017;13(7):1615–1624. Doi: https://doi.org/10.1080/21645515.2017.1296994
CrossRef - Wang N, Shang J, Jiang S, Du L. Subunit vaccines against emerging pathogenic human coronaviruses. Front Microbiol. 2020;11:298. Doi: https://doi.org/10.3389/fmicb.2020.00298
CrossRef - Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints. Pfizer, 2020. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine
- Fafi-Kremer S, Bruel T, Madec Y, Grant R, Tondeur L, Grzelak L, et al. Serologic responses to SARS-CoV-2 infection among hospital staff with mild disease in eastern France. EBioMedicine. 2020; 59: 102915. Doi: https://doi.org/10.1016/j.ebiom.2020.102915
CrossRef - Summers MR, Booth TM, Brockas T, Edgar H, Edwards J, Nunnerley CS, et al.: Luminogenic Reagent Using 3-Chloro 4-Hydroxy Acetanilide to Enhance Peroxidase/Luminol Chemiluminescence. Clinical Chemistry. 1995; 41: S73.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N engl J Med. 2020; 383(27):2603-2615. Doi: https://doi.org/10.1056/NEJMoa2034577
CrossRef - Rastawicki W, Plaza W. The level of protective post-vaccination antibodies in NIPH-NIH employees after administration of Pfizer vaccine against COVID-19. Przegl Epidemiol. 2021;75(1):3-13. Doi: https://doi.org/10.32394/pe.75.01
CrossRef - Ali H, Alahmad B, Al-Shammari AA, Alterki A, Hammond M, Cherian P, et al. Previous COVID-19 infection and antibody levels after vaccination. Front Public Health. 2021;9:778243 Doi: https://doi.org/10.3389/fpubh.2021.77243
CrossRef







