Manuscript accepted on :07-04-2022
Published online on: 18-05-2022
Plagiarism Check: Yes
Reviewed by: Dr. Ahmar Rauf
Second Review by: Dr. Bhavana Gundavarapu
Final Approval by: Dr. Ian James Marti
Tapasya K , Ashmitha Suresh Kumar
, Ashmitha Suresh Kumar , Arunasalam Dharmarajan and Venkatachalam Deepa Parvathi*
, Arunasalam Dharmarajan and Venkatachalam Deepa Parvathi*
Department of Biomedical Sciences, Faculty of Biomedical Sciences and Technology, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai, India.
Corresponding Author E-mail: deepakoushik305@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2416
Abstract
Nanotechnology recently gained attention for the novel and successful tools it has thus far provided for cancer diagnosis and treatment. Some of them include lipid-based carriers such as liposomes and metal-based particles such as nanoshells (NSs), used for anti-cancer drug delivery for the most part. Each one of these systems has been carefully designed in order to bypass the obstacles brought forward by conventional diagnosis and treatment strategies. These challenges include non-specificity, premature drug release and toxicity. From research conducted over the years it is clear that nanocarriers ameliorate bioavailability, specificity and accumulation of the drugs at the target site. These improvements can be explained by their easily adjustable physical and chemical properties. Alterations to their size and surface structure are often made to enhance their accumulation at the target sites and overall targeting capabilities respectively. Some nanocarriers such as quantum dots (QDs) and carbon nanotubes (CNTs) display excellent fluorescent properties and are useful candidates for imaging techniques and fluorescence-guided surgery. Another group of promising nanoparticles is biomimetic nanoparticles that mimic the functionality of biological components. These NPs are designed to mimic basic cellular and physical features of the source cells and their surface. This type of NPs construct is exploited for its unique characteristics that aid in effective interaction with complex biological systems, consequently enhancing therapeutic outcomes After establishing them as adequate tools for drug delivery and imaging, nanocarriers are now being tested in combined cancer treatment strategies. This review provides an understanding of the salient nano-devices and their applications in oncology.
Keywords
Drug delivery; Enhanced permeability and retention (EPR) effect; Near-Infrared (NIR); Nanocarriers; Stimuli-Responsive
Download this article as:| Copy the following to cite this article: Tapasya K, Kumar A. S, Dharmarajan A, Parvathi V. D. Nanocarriers: The Promising Future to Cancer Diagnostics and Treatment. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Tapasya K, Kumar A. S, Dharmarajan A, Parvathi V. D. Nanocarriers: The Promising Future to Cancer Diagnostics and Treatment. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3z49SAW |
Introduction
Cancer is a ubiquitous disease characterized by the abnormal and uncontrolled proliferation of cells within the body, combined with metastasis. Despite extensive research on the various types of cancer and technological advances, it still remains the leading cause of death globally. According to GLOBOCAN 2018, the number of new cases is estimated to reach 29.5 million by the year 2040 1. With the intention of improving prognosis and cancer patient survival rates, various analysis and imaging techniques have been employed for their detection and diagnosis. Such examples include Magnetic Resonance Imaging (MRI), mammography, Computed Tomography (CT), cytology, and histopathology 2. However, these methods prove to be competent when there are significant changes to the related tissues. In other words, by the time the cancer is detected, there are abundant abnormal cells and the tumour has metastasized 3. Additionally, the latter two techniques make it difficult to diagnose low grade lesions including gastrointestinal, urinary, respiratory, and pancreatic lesions, ultimately giving rise to a false positive diagnosis 4,5. Hence, the aforementioned tools demonstrate their inefficiency in detecting cancers at an early stage 2,6. The need to overcome this challenge has thus urged scientists and researchers to explore the field of nanotechnology, which appears to be promising as far as cancer diagnosis and treatment are concerned. Efficacy in cancer detection and therapy by nanoparticles (NPs) and nano carriers, for the most part, is often attributed to their sensitivity to biomarkers in relation to the cancer, targeted drug delivery, and increased accumulation of drugs in affected tissues 7. An example is the use of lipid-based NPs, such as liposomes, for controlled delivery to selected cells while reducing side effects of the pharmaceutical agents 8. Other nano-based technologies have also been applied in clinical practice, including CNTs and QDs for targeted imaging, and gold nanoparticles GNPs for phototherapy for the treatment of malignancies 2. This review provides a brief insight into some of the important nano-based tools and techniques used for early detection and treatment of cancers, as well as the recent advancements as far as NPs are concerned. We also discuss the interaction of these NPs and nano carriers with the immune system, and discuss the superiority of nano-oncology.
Nano-Based Tools for Cancer Diagnosis and Therapy
Liposomes
Liposomes are an example of colloidal vesicles that have the ability to modify the distribution of the substances they carry. They were one of the first products of nanotechnology to be studied as a potential carrier of drugs for cancer treatment. Liposomes are spherical vesicles composed of a phospholipid bilayer and are capable of transporting water soluble and insoluble anticancer medications, while protecting the drugs from degradation by host enzymes and chemicals 9. From extensive research over the years, it is clear that decreased toxicity, and enhanced drug accumulation at the target site as a result of the enhanced permeability and retention (EPR) effect, make these lipid carriers an attractive delivery platform 10. EPR is a phenomenon that is strongly dictated by the size of the molecules. Particles that are small enough tend to accumulate in tumour tissues more easily compared to normal tissues 11. Initially, conventional liposomes presented short circulating times in the blood due to interference by the reticuloendothelial system (RES) 12. This is when certain cells belonging to the immune system remove foreign substances from the body 13. To overcome this, polyethylene glycol (PEG) was incorporated onto their surface. PEG is known for having low immunogenicity and antigenicity properties as well as high stability 9,14. PEGylation helps liposomes escape mononuclear phagocytic system cells by forming a hydrophilic layer on the lipid surface, thus allowing for longer circulation times 15,16. Despite being able to overcome drawbacks such as short circulation times, researchers are facing a perpetual challenge, which is the liposomal drugs’ failure to increase anticancer efficacy. This is possibly due to interactions of liposomes with the host’s immune system 10. According to a meta-analysis from 2016 that compared the anticancer efficacy of liposomal doxorubicin to standard doxorubicin treatment, it was found that the former did not show any significant improvement in objective response rates, progression-free survival, or overall survival in cancer patients 17. However, when used in combination with other drugs or gene agents, with no regard to PEGylation, liposomal drug therapy is efficacious, as demonstrated by other clinical studies 18-20. Currently, liposomes with targeting surface moieties are being assessed. The surfaces of these ‘smart’ nanocarriers are grafted with antibody or antigen fragments, proteins, and glycoproteins in order to actively target the malignancies 21. Such nanocarriers are also known as theragnostic liposomes, which perform the function of both therapy and imaging. For instance, vesicles in combination with radio-ligands and therapeutic agents can be used to diagnose the tumour and cancer stage based on the distribution of the liposomes, while simultaneously delivering the anticancer agent 12.
Micelles
Micelles form another class of colloidal particles. They are dynamic spherical arrangements of amphiphiles 22. Apart from having a monolayer of phospholipids, they are very similar to liposomes in terms of function. The lipid based nanocarrier is commonly used for therapeutic agents that are close to insoluble in water 8-21. Micelles are closely studied due to the added advantages over their predecessor, liposomes. For instance, supplementation of organic solvents and surfactants, for drugs taken intravenously, is now almost inessential 21. Moreover, their small size in comparison to liposomes allows maximum penetration into the tumour tissues as a result of the EPR effect, resulting in better efficacy 23. However, efficacy cannot be determined merely by penetrating capacity. The key element to stability, and thus efficacy, is the critical micelle concentration, below which micelles will fall apart, resulting in premature release of the drug 24,25. This ultimately leads to negative biodistribution of the drug 26. Another reason for low efficacy is slow drug release, which results in drug inactivation 27. This happens because the drug spends most of its time in the blood making it susceptible to degradation by enzymes and the RES, before it reaches the target site. In order to avoid this, micelles are being developed in such a way that drug release is triggered only by stimulation from intracellular components of the cancerous cells. For example, the high levels of glutathione in tumour cells act as a reducing agent and cleave the disulphide bonds present in the micelles 28-30. These are known as reduction-responsive polymers. From a recent study conducted by Dong Wan et al., it was inferred that such polymers promote fast drug release and are more effective as opposed to standard ‘free’ drug formulations 28. Similarly, other studies have concluded that reduction-responsive based polymers are a promising solution for targeted drug delivery, as well as bioimaging of cancers 31,32. In addition to intracellular stimuli, researchers are also exploiting the components of the tumour microenvironment (TME) for triggered drug release that is specific to the type of malignancy 28. Aside from that, micelles, particularly PEG-polylactic acid micelles have been considered as one of the most propitious, as far as colloidal carriers are concerned. This is particularly due to PEG’s non-toxic nature as well as its ability to prevent adsorption of proteins and phagocytes. The latter property ensures weakened uptake by the RES 33. After establishing the main features contributing to the success of micelles, scientists are now looking into smart strategies, mainly the co-delivery of drugs, for cancer therapy 34,35. Gong et al. recently conducted a study that merged the two concepts, active targeting and combined delivery of antitumour medication. They investigated triggered release of co-delivered drugs in response to contact with fibronectin in the TME, using PEGylated micelles, for therapy of metastatic breast cancer in mice. The drugs doxorubicin and vinorelbine were encapsulated in nanocarriers functionalized with fibronectin-targeting CREKA peptides. The results suggested that inhibition of metastatic invasion was attributed to efficient co-delivery of drugs, and accumulation of these drugs via active targeting 34. However, in order for this strategy and other smart strategies to be considered reliable, and hence transition into clinical settings, more studies using cancer patients as subjects need to be conducted.
Nanoshells
NSs are spherical structures that range from 1nm to 20nm in size. They consist of a hollow or filled core, typically made from dielectric silica, coated with a thin metal layer, usually gold or silver. NSs possess tunable physical properties that make them a suitable candidate for imaging and therapeutic applications. One such property is Surface Plasmon Resonance (SPR). Adjusting the radius and thickness of the NSs’ core and shell respectively will result in varying optical resonances ranging from the ultraviolet to infrared region 36. This includes the near-infrared (NIR) region. Wavelengths in this ‘therapeutic window’ (800nm – 2500nm) exhibit maximum tissue penetration, compared to other wavelengths. This is due to less absorption and scattering by compounds present in biological fluids such as haemoglobin 37. A popular example of a treatment method that exploits this particular property of NSs is photothermal therapy (PTT). In NS-mediated PTT, the nanostructures are tuned to strongly absorb light in the NIR region, and are placed in deep tissues. Exposing NSs to light in the NIR region causes them to reach optimal temperatures required for irreversible thermal ablation of cancerous tissues. By utilizing magnetic resonance as guide, Hirsch et al. were able to form a strong positive correlation between high temperatures and irreversible thermal damage. According to their findings, the average maximum temperature rise was about 37° C, enough to ablate the cells 38. Similarly, a later study by JM Stern et al. revealed that laser-activated gold NSs were able to selectively destroy prostate tumors induced in mice models. The maximum temperature recorded was 65.4° C, and complete tumour destruction was also observed 39. In terms of delivery, NSs can either be injected directly into the tumour tissue, or allowed to accumulate via the EPR effect when supplied intravenously. However, the latter method is limited by the sites that the NSs can travel to. For instance, nanocarriers injected intravenously may not be able to traverse the blood brain barrier (BBB). To overcome this, macrophages have served as important vectors to transport the NSs to the target sites 40. They seem to be a promising alternative in delivering NSs, and other nano-based carriers, for cancer treatments such as PTT and chemotherapy. This statement is supported by the results of an in-vitro study where macrophage-delivered NSs caused significant damage to the human glioma cells after NIR exposure 41. Aside from this, the chemical properties of NSs can be modified to further enhance specificity. As seen with other nanocarriers, biologically active moieties such as antibodies and organic molecules such as PEG have been incorporated. PEGylated nanoshells have been designed with the purpose of reducing absorption of proteins while maintaining their solubility in the blood 42. As of recent, they are being exercised in studies testing the stimuli-responsive drug release phenomenon for combined cancer treatments 43-44. A way to ensure that these NSs only target the affected cells, and are successfully taken up by them is to meet the demands of those cells. Cancer cells divide at a fast rate, which equates to rapid glucose uptake owing to increased cellular metabolism. Therefore, attaching glucose molecules to their surfaces will result in better accumulation and internalization of NSs. This is backed by a recent study conducted by Nouri et al., where glycosylated gold NSs (GGNSs) were used to thermally ablate melanoma cancer cells. Their results showed a significant uptake of GGNSs by the cancer cells, and that GGNSs caused higher amounts of toxicity in the cancer cells compared to standard GNSs 45. Another approach to achieve specificity is to conjugate the NSs with biomolecules such as antibodies, as mentioned previously. The antibodies bind to target antigens expressed on cancer cells. In cases of drug-loaded NSs, the anticancer agent will be released upon binding. This has proven to be useful in combined imaging and therapy, as demonstrated by Loo et al. They found that the NSs designed to target the human epidermal growth factor receptor-2, found on breast cancer cells, provided better optical contrast, and caused cell death after NIR exposure 36. This immuno-targeting technique was also tested on medulloblastoma and glioma cell lines by Ronald J et al. in a later study, and provided similar results 46.
Carbon Nanotubes
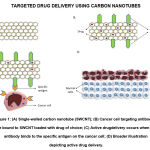
CNTs are graphene sheets rolled into cylindrical structures, with their outer diameters ranging from 3nm to 30nm. CNTs can be classified as single-walled (SWCNTs) and multi-walled (MWCNTs) 47. Apart from being extremely light-weight, CNTs possess other favourable properties such as large surface area, high aspect ratios, high stability, low toxicity, and low immunogenicity, which make them a suitable nanocarrier for antitumour drugs 48. The idea of using CNTs to facilitate drug delivery and imaging sprouted after its tissue penetrating properties were demonstrated 49. Similar to other nanocarriers, PEGylated CNTs demonstrate reduced toxicity, better capability in avoiding uptake by RES, and faster drug release rates. Initially, the major drawbacks of this nanocarrier were its inadequate solubility in aqueous solutions and tendency to form aggregates. This was quickly overcome by conjugating hydrophilic material, i.e., PEG, on the walls of the CNTs 50. PEGylation of SWCNTs allows for better localization within the cancer cells’ compartments 12. As for MWCNTs, PEGylation promotes faster drug release while simultaneously reducing the drug loading capacity. This was demonstrated by a study conducted by Dinan et al. to investigate the cytotoxicity and kinetics of doxorubicin, delivered by folate-targeted PEGylated MWCNTs. It was found that faster drug release and inefficient drug loading was due to increased hydrophilicity, and low affinity of doxorubicin to the CNTs respectively. Additionally, low cytotoxicity was observed 51. Furthermore, PEGylation combined with other modifications have proven to be useful for metronomic drugs. In a recent in vitro study, Sharma et al. designed enteric-coated PEGylated nanocarriers that were pH-responsive, for delivery of cisplatin to breast cancer cells 52. From the results, it can be inferred that the nanocarriers are much more stable and improve drug bioavailability, thus establishing this design as a potential candidate for efficient delivery of metronomic drugs. On another note, apart from delivering drugs, CNTs have been studied as potential non-viral vectors for transferring genetic material in gene therapy [53]. Mohseni-Dargah and colleagues were able to display the enhanced chemotherapeutic effects when MWCNTs, were used to carry the iC9 suicide gene to kill breast cancer cells in vitro [54]. However, supplementary research should be conducted to test this strategy in vivo. With respect to active targeting, surfaces of CNTs can be easily functionalized via attachment of moieties, such as glycoproteins, to enhance specificity of drug delivery and overcome drug resistance, ultimately improving the efficacy of the treatment as shown in Figure 1. [55,56]. Ozgen and peers were able to exhibit the same using a unique approach. They used carboxylic acid-modified CNTs coated with glycopolymers and folic acid for doxorubicin delivery, as an attempt towards dual receptor-mediated breast cancer therapy [57]. Incidentally, owing to their unique physical properties, CNTs are also used in bioimaging for the detection and monitoring of cancerous cells and tumours. These nanostructures display fluorescence in the NIR window, allowing them to be used as non-photobleaching fluorophores for in vivo NIR imaging [53] [58-61]. By devising M13 phage-functionalized SWCNTs, Yi et al. were able to successfully perform NIR fluorescence imaging of targeted tumours [62]. From this, it is clear that CNT-based imaging is highly efficient as a diagnostic technique. This claim is backed by the equally successful results of a second study, conducted by Ghosh et al. that utilized identically functionalized SWCNTs to target and remove tumour nodules in a mouse model of human ovarian cancer [63]. Other CNT based imaging techniques include ultrasonography, photoacoustic imaging, and MRI [64].
Quantum Dots
QDs are man-made nanocrystals composed of semiconducting elements from groups II to VI, or III to V of the periodic table. The diameter of these crystals can range from 2nm to 10nm. With respect to biomedical applications, QDs can be defined as NPs with a fluorescent core, semiconductor shell, and a functionalized surface that allows them to solubilize in water and integrate with other biomolecules, in order to target specific proteins expressed on the surface of cancer cells (Figure 2.). Additionally, QDs have the ability to emit fluorescence as a result of excitation of molecules when they are exposed to a light source. The majority of the studies on the core material of QDs have mostly focused on elements such as cadmium, lead, and mercury. The reason being that, these elements are known to express a significant amount of toxicity in the body, even at low levels [65,66]. Over the last decade, researchers’ interest in biomedical applications of QDs has switched to biocompatible QDs that are heavy metal-free compositions made from transition elements, for the most part, with a few from groups III and VI. Examples include: Copper (Cu), Silver (Ag), Indium (In), Zinc (Zn), Sulfur (S), and Selenium (Se), to improve biocompatibility for in vivo applications [65]. Studies have shown that the fluorescence signals of NIR-QDs can be detected in deep tissues, making them a preferable choice for in vivo imaging. However, in the case of QDs with signal emission in the visible range, their applications are limited to in vitro studies due to the high level of visible light absorbance by tissues, resulting in low resolution images. In contrast to this, NIR light passes more readily through biological tissue, with lower absorption and scattering, enabling NIR-QDs to maintain high resolution even when imaging deeper structures in vivo. Hence, a vast majority of work has focused on developing emission-tunable and heavy-metal free QDs as in vivo imaging probes that excite and emit strongly in the NIR region. This will significantly increase the contrast, sensitivity, and the extent of penetration while avoiding optical damage to the body [65,67]. Additionally, QDs have unique optical photophysical and chemical properties that include resistance to photobleaching, tunable fluorescence intensity, and high brightness over other organic fluorescent dyes, and proteins used for imaging [67,68]. Overall, QDs have better imaging capabilities in terms of higher specificity, capacity to bind to biomarkers, and prolonged imaging duration (Figure 3.) in comparison to conventional imaging systems such as MRI, X-ray, and CT [69].
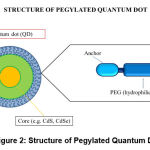 |
Figure 2: Structure of Pegylated Quantum Dot. |
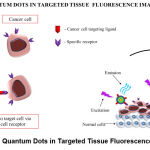 |
Figure 3: Quantum Dots in Targeted Tissue Fluorescence Imaging. |
Gold Nanoparticles
GNPs as drug carriers, radiosensitizers, and photothermal agents for cancer therapy are promising agents. In general, biological synthesis of GNPs is preferred over chemical and physical synthesis. The reason is that the latter two require extreme pH and temperature conditions, as well as microwave and ultraviolet radiation. Moreover, biological methods for synthesis are eco-friendly, with lower impacts on the environment and humans [70]. GNPs also possess a property known as SPR. In response to an incoming radiation of a specific wavelength, electrons present in the gold atoms begin to resonate, resulting in absorption and scattering of light by the NPs. Owing to their tunable physical properties, GNPs of specific size and shape can be synthesized, resulting in a plasmonic resonance shift from 520nm to 800–1200 nm (therapeutic window) [71]. And since the body tissues are relatively transparent to NIR light, this resonance range makes GNPs useful in PTT and photoimaging, especially for tumours residing in deeper tissues. The surface area of GNPs plays a key role in cancer therapy. For instance, in PTT, smaller GNPs are preferred as they are more capable of transforming light energy to heat, thus raising the temperature of the TME beyond the threshold level necessary for irreversible cell destruction. Whereas in photoimaging, larger GNPs are preferred due to their higher scattering capacity. The ability to conjugate GNPs with biologically active moieties, particularly thiol and amine groups, creates a wide range of design strategies with respect to targeted drug delivery, cancer diagnostics, and imaging. This has motivated researchers to explore tailor-made GNPs and conduct more studies using different designs [70,72]. Usually, when administering chemotherapeutic drugs orally or intravenously, only a fraction of the drug reaches the tumour tissue. This has been avoided by targeting these drugs actively and passively to the site of the tumour by attaching them to the GNPs. The typical TME has a very leaky vasculature, which allows NPs to conveniently accumulate in the surrounding tissues. This type of passive targeting is the EPR effect. However, this phenomenon cannot be attained at all tumour sites. Therefore, active targeting is preferred, which utilizes NPs that have tumour specific markers bound to them [73]. A study conducted by Chen et al. investigated the outcomes of using GNPs conjugated with methotrexate on lung tumour cells. They recorded greater cytotoxicity levels towards the cancer cells and faster accumulation rates, which together inhibited tumour growth, as opposed to ‘free’ methotrexate [74]. Incidentally, another approach to killing cancerous cells is photodynamic therapy. This type of therapy utilizes light, sensitizers such as pyrophin (photosensitizing agent), and oxygen available in the cancer tissue. The photosensitizer is intravenously injected and excited using specific wavelengths, resulting in energy transfer that generates reactive oxygen species. This causes cell death by apoptosis [75]. Other studies have shown that these photosensitizing agents are not easily soluble, therefore inhibiting their uptake by the TME. To add on, multi-drug resistance (MDR) in cancer cells has been a great setback for favourable outcomes of chemotherapy. All cells are tightly regulated by P-glycoprotein (P-gp), a transmembrane efflux pump that removes foreign bodies. Overexpression of this protein on the membranes is the key explanation to MDR in these cells. Several studies have been carried out to subdue MDR either by inhibiting P-gp expression or, by avoiding P-gp mediated efflux. A system that overcomes MDR and probes intracellular drug release in response to acidic organelles was designed by Wang et al. The system comprises doxorubicin tethered onto the surface of PEGylated GNPs along with an acid labile linkage. From the results, it is inferred that the system can inhibit the proliferation of multidrug-resistant breast cancer cells owing to the sufficient absorption of the drug through endocytosis, and the acidic stimulus-responsive release of the drug [76]. In light of the environmental concerns GNPs have raised, eco-friendly methods and technologies are sought after. Different plant extracts are being tested as potential candidates to help synthesize GNPs. Common examples include ginger and tea leaves [77]. Microorganisms are also used as alternatives to plant extracts. It is believed that using biocompatible materials and non-toxic solvents will help reduce toxic side effects compared to conventional methods, while maintaining their stability. In a study conducted by Clarance et al., GNPs were synthesized using an endophytic fungus isolated from a plant, and were used to study their cytotoxic effects on cervical cancer and breast cancer cells. The GNPs induced cytotoxicity and apoptosis through generation of reactive oxygen species (ROS) [78]. In another study conducted by Jumah et al., GNPs (synthesized from neem tree extracts) were observed for their cytotoxicity, genotoxicity, and anticancer potential in both cancerous and normal liver cells. Similarly, the cytotoxicity and apoptotic activity observed was a result of the fact that the GNPs induced ROS in both types of cells. It was also observed that the cancerous cells were moderately more sensitive to the GNPs than to the normal cells [79]. Overall, these biosynthesized GNPs have proven to be effective anticancer nanotools.
Biomimetic Nanoparticles
Another group of promising nanoparticles is biomimetic nanoparticles that mimic the functionality of biological components. The overall structure of these NPs consists of functional attributes of biologic particles in combination with tunable synthetic materials. These NPs are designed to mimic basic cellular and physical features of the source cells and their surface. This type of NPs construct is exploited for its unique characteristics that aid in effective interaction with complex biological systems, consequently enhancing therapeutic outcomes [80].
When compared to standard approaches, NPs camouflaged with cell membranes such as immune cell membrane, cancer cell membrane, red blood cell (RBC) membrane and platelet membrane display prolonged systemic circulation and evasion of immune system clearance [81].
To give an instance, cancer cell membrane-coated NPs assist in targeting cells of similar features and structure [82]. In the case of NPs coated with immune cell membranes such as neutrophils or monocyte, prolonged circulation is achieved owing to the immune cell’s self-recognition mechanism [83].
Cancer cell membrane camouflaged NPs are employed as diagnostic imaging tools as well. In a study conducted by Kumar et al, for imaging purposes, a polymeric nanoparticle (mPEG-PLGA) coated with a brain metastatic breast cancer cell membrane (MDA-MB-831) was loaded with NIR dye IR780. The study was performed both In-vivo and Ex- Vivo. The results showed that the imaging in mice exhibited prolonged circulation of the coated NPs and the NPs were capable of crossing the BBB [84]. In another study conducted by Kroll et al [85] biomimetic NPs were used to develop anticancer vaccines, where a cancer cell membrane coated NPs along with an immunological adjuvant induces an anticancer immune response.
For a detailed understanding of cancer cell membrane coated biomimetic NPs, Jin et al and Wang et al discuss and elaborate on the coated NPs ability in cancer targeting, use and their application in cancer theranostics [86], [87].
Considering the unique and beneficial characteristics of biomimetic NPs, they possess great potential for cancer diagnostics and treatment. Further research and study are needed specifically for the clinical translation and production of biomimetic NPs [88].
Table 1: Liposomes, Micelles and GNPS used for Cancer Treatment [89-91]
| No. | Nanocarrier | Commercial Name | Compound | Company | Indication | Approval Status |
|
1. |
PEGylated Liposome |
Doxil/ Caelyx |
Doxorubicin |
Schering-Plough; Ortho Biotech |
Ovarian cancer Breast cancer Kaposi’s sarcoma |
Approved |
| 2. | Liposome | Myocet | Doxorubicin |
Sopherion; Cephalon
|
Metastatic breast cancer
|
Approved |
| 3. | Liposome | Marqibo |
Vincristine sulfate |
Talon Therapeutics | Acute lymphoblastic leukaemia | Approved |
| 4. | Liposome | DaunoXome | Daunorubicin | Galen | Kaposi’s sarcoma | Approved |
| 5. | Polymeric micelles | Genexol-PM | Paclitaxel | Samyang Biopharm | Head and neck cancer
Ovarian cancer Breast cancer Lung cancer |
Approved |
| 6. | PEG-PAA
Micelles |
NK105 | Paclitaxel | Nippon Kayaku | Gastric cancer
Breast cancer |
Phase II/III of clinical trials |
| 7. | Micelles | Paclical | Paclitaxel | Oasmia | Ovarian cancer | Phase III of clinical trials |
| 8. | Colloidal Gold Nanoparticles | Aurimmune | TNF | CytImmune Sciences | Solid tumours |
Phase II of clinical trials |
| 9. | PEGylated
Liposome |
Onivyde | Irinotecan, fluorouracil and folinic acid | Ipsen | Pancreatic cancer | Approved |
| 10. | Liposome | Vyxeos | Daunorubicin and
Cytarabine |
Jazz | Acute myeloid leukaemia | Approved |
Interactions with the Immune System
The physiological effects of NPs are determined by their physio-chemical properties such as size, surface area, and composition, as well as their interaction with the immune system. Special attention is given to the mechanisms under innate immunity, which are crucial in recognizing and eliminating foreign matter entering the living system [92]. With increasing interest in nanotechnology and its biomedical applications, studies have shown that NPs can be toxic and can even modify the immune system by either stimulating or suppressing the immune responses. If these immunomodulatory responses are intentional and positive, then they do not pose any health risks. Hence, it is imperative that we understand the properties of NPs and assess their interaction with the immune system. This will be useful when designing and constructing compatible and safe nanocarriers for drug delivery [93]. On another front, layers of adsorbed proteins resulting from the interaction of biological fluids and the NPs form an envelope known as protein corona (PC) on their surfaces. The formation of PC depends on the NPs’ physical properties, the NP to protein ratio, media composition, and presence of ions or other molecules that impede or influence the interaction between the NPs and proteins. This envelope imparts a biological identity to the NPs for the immune system to detect and initiate a signalling pathway. This is also useful in determining their toxicity and biological response levels [94]. Several studies have focused on the impact of PC on immunotoxicity and cytotoxicity. For example, protein aggregates influenced by the NPs’ surface can induce an autoimmune reaction as well as trigger the components of the complement system to cause an immune response [94-96]. NPs can be engineered by manipulating their physio-chemical factors in order to achieve desirable outcomes that either promote interaction with the proteins and immune cells, or completely avoid these proteins and cells [97]. The toxicity of NPs can be attributed to chemical and biological impurities, which are the by-products of NP synthesis and biological endotoxins respectively. Hence, it is crucial when determining the cause of immunotoxicity, as it can be triggered by chemical and biological impurities as well as by the NPs themselves. Additionally, metals such as iron and nickel, when used as a catalyst in the synthesis of CNTs, are seen to trigger inflammatory reactions when exposed to these nanotubes [98]. Bacterial endotoxins are the most common biological impurities that affect some pre-clinical grade nanomaterials. Therefore, it is important to remove these endotoxins during nano formulations as these impurities can affect both efficacy and immunotoxicity. Some carbon and silica-based nanomaterials have also been shown to trigger endotoxin-mediated inflammation in the lungs [99-101].
Recent Advancements
Over the years, several studies have been carried out in order to expand our knowledge and understanding of NPs, and their biomedical applications in the field of cancer biology. Conventional drug delivery systems are associated with drawbacks such as non-specific drug delivery, drug toxicity, and uncontrolled release of drugs. This has eventually led to the development of smart nanocarrier-based drug delivery systems or smart drug delivery systems, which comparatively release drugs at controlled dosage levels [12]. Of late, small interfering RNA (siRNA) has also been widely studied as a potential strategy for cancer treatment, since its substantial gene silencing ability has been observed in the treatment of solid cancers [102]. However, major drawbacks of using siRNA include nuclease degradation, rapid clearance from the bloodstream, and incapability of crossing the cell membrane successfully [103]. To address this issue, suitable nanocarriers have been constructed to efficiently deliver therapeutic siRNA. These nano sized products protect the siRNAs from enzymatic degradation and clearance, as well as enhance the pharmacokinetics, and promote controlled release and distribution of the drug to the specific target site. All in all, delivery of siRNA by nanocarriers such as liposomes and micelles, presents a promising future in siRNA-based cancer treatment [104-106]. On a separate note, the cyclic peptide iRGD is a tumour penetrating peptide that is either covalently bound to the nanocarriers or co-administered with them. This is done to ameliorate the active-targeting ability of these carriers to the tumour sites. In most cases, solid tumours, such as pancreatic cancer, prevent the drugs from reaching the leaky tumour vasculature, therefore diminishing the EPR effect and ultimately resulting in poor bioavailability. In order to avoid the EPR phenomenon without compromising on bioavailability, an alternative mechanism of transcytosis was investigated. Transcytosis can be observed at the blood brain barrier, where only selected substances are allowed to pass through. Sugahara et al. found that this pathway could be therapeutically accessed by iRGD [107]. Ever since, the primary objectives of research concerning nanocarriers in cancer therapy have been to understand this mechanism in contrast to EPR, and provide reliable results that can eventually allow this strategy to transition into clinical settings. The idea of performing combinational therapy using NPs is underway [108]. Majority of the experiments include combining PTT with chemotherapy, imaging techniques and even gene therapy. In a study conducted by Chen et al., functionalized thermosensitive Copper Sulphide-based (CuS) micelles were used for combined treatment which included chemotherapy, PTT and photoacoustic imaging on triple negative breast cancer models. When subjected to NIR irradiation, temperature elevation of the CuS-based NP core induced PTT and a chemical phase shift of the polymers on the micelles, which led to rapid drug release. The micelles also acted as contrast agents for photoacoustic imaging and exhibited absorptions greater than 900 nm. Ultimately it was proven that the combinational therapy was much more effective than performing PTT or chemotherapy alone [109]. In a different study, PTT was combined with gene therapy and immunotherapy as a potential treatment for gastric cancer. Results of the in-vitro and in-vivo tests conducted in this study strongly suggested that combined treatment strategies are better than monotherapy [110]. Another aspect of drug release is stimulus-based response. As described earlier, stimuli-responsive polymeric nanocarriers can deliver anticancer drugs in a controlled manner to the target site, in reaction to endogenous or exogenous stimuli [111]. Studies on dual stimuli-responsive nanocarriers have been performed in an attempt to increase the bioavailability and response rate at the target site. [44] [102,113]. Zhang et al. designed triple-stimuli-responsive (temperature, pH, and reduction) inner-layer crosslinked micelles which could be used as a nanocarrier for doxorubicin. These micelles seem to be a promising nanocarrier for drug delivery as they exhibited accelerated release of doxorubicin against HepG2 cancer cell lines, low toxicity, high drug loading capacity and adequate biocompatibility [114]. On a different note, treatment and diagnosis of brain tumors are impeded by the presence of the BBB, which limits the potential of drug delivery to the targeted tumor sites in the brain. Given the high ROS activity in the brain TME, Oddonne et al. designed a self-assembling micelle composed of Melphalan linked with methoxy polyethylene glycol. It responds to ROS stimulus through a ROS cleavable component called thioketal. The results of the study revealed that ROS stimulus-controlled delivery of drugs was selective to cancer cells [115]. Alternatively, Tang et al. created aptamer 32-conjugated QDs, capable of penetrating the BBB and accumulating in the tumor site by selectively binding to Epidermal growth factor variant III found on the glioma cells’ surface, and producing significant amounts of fluorescence. The fluorescence aided in clear visualization of the glioma margins, making it useful for accurate resection during fluorescence-guided surgery, as well as for pre- and post-operative examination of gliomas [116]. On a different note, another method of delivering anticancer agents is by coating them on the membranes of cells. This is possible because of the physical similarities they share with liposomes. Binding the drugs directly to the cell membranes increases their overall bioavailability [117]. Based on this strategy, Kim et al. constructed red blood cell and platelet membrane-coated gold nano stars containing curcumin (R/P-cGNS). The self-antigens provided by the red blood cell coating aided in the evasion of macrophages and the platelet coating improved targeting. In conclusion, R/P-cGNS promoted controlled drug release and better targeting, while being able to evade the immune system. This biomimetic membrane coated strategy can be a good approach for cancer therapy in the future [118].
Limitations
The majority of studies conducted in the past support the outstanding performance of nanocarriers, including drug efficacy and side effects, when compared to conventional formulations. Yet, toxicity remains a major concern. Owing to the large surface area of these NPs, the chances of chemical interactions taking place in the body are increased, which in turn enhances their toxicity [89]. NPs may interact with biological components such as plasma proteins and immune cells, or accumulate in the tissues, leading to organ failure. Cationic liposomes are more likely to be taken up by malignant and normal cells due to the electrostatic-attraction between the liposomes and the negatively charged cell membrane, which leads to greater toxicity. Studies conducted on HepG2 cell lines, macrophages, and U937 cell lines (lymphoid leukaemia) have demonstrated the same [12]. Apart from chemical properties, toxicity can also be attributed to other factors, including route of administration, dosage, and presence of contaminants. For example, as a result of inadequate purification processes, CNTs exhibit augmented toxic effects caused by metal catalysts and other impurities [64]. Following toxicity is the steric-hindrance seen with PEGylated nanocarriers. This is explained by the formation of globular structures resulting from the long chains of the PEG polymer, e.g., PEG (5000). Steric-hindrance limits the penetration property of nanocarriers into target tissues. In order to facilitate active targeting, nanocarriers are modified by attaching ligands to their surfaces [21]. However, if the ligand density surpasses the optimum concentration, the nanocarriers will begin to aggregate [119]. This may enhance their toxic effects on body tissues. Another limitation is the overall high cost of raw materials and nanomedicine. To overcome this, large-scale production of nanocarriers can be encouraged. Nonetheless, it poses a challenge since physical and chemical properties may vary from one type of nanocarrier to another [120]. Further to this, the most crucial drawback is the lack of reference standards needed for immunotoxicity evaluations. Examining properties such as biocompatibility and overall function becomes more difficult with shortage in reference standards. Although evidence connecting certain nanocarriers to immunotoxic conditions exists, such as cationic dendrimers being thrombogenic, the lack of reference standards make it difficult to view these links as definitive [99].
Conclusion
Scientists are looking into nano-based tools and techniques as potential candidates for the early detection and treatment of cancer. Extensive research has been conducted to shine a spotlight on strategies that can improve targeted drug delivery and imaging, while maintaining efficacy. Carefully engineered nanocarriers such as PEGylated micelles and CNTs, functionalized with biologically active moieties, have helped overcome some of the drawbacks seen in ‘free’ drug administrations. Common problems include non-specific drug delivery, low bioavailability, drug toxicity, and drug resistance. As for targeted imaging, GNPs, CNTs, and QDs are promising solutions in their own ways. The overall structure of biomimetic nanoparticles consists of functional attributes of biologic particles in combination with tunable synthetic materials. These NPs are designed to mimic basic cellular and physical features of the source cells and their surface. When compared to standard approaches, NPs camouflaged with cell membranes such as immune cell membrane, cancer cell membrane, red blood cell (RBC) membrane and platelet membrane display prolonged systemic circulation and evasion of immune system clearance. In order to have these novel strategies tagged as efficacious and reliable approaches, more research on human subjects is highly espoused.
Acknowledgement
None
Conflict of Interest
There is no conflict of interest.
Funding Source
There is no funding source.
References
- Cancer Tomorrow.” [Online]. Available: https://gco.iarc.fr/tomorrow/graphic-bar. [Accessed: 17-Jan-2021].
- E. Choi, J. W. Kwak, and J. W. Park, “Nanotechnology for early cancer detection,” Sensors. 2010, doi: 10.3390/s100100428.
CrossRef - Zhang, M. Li, X. Gao, Y. Chen, and T. Liu, “Nanotechnology in cancer diagnosis: Progress, challenges and opportunities,” Journal of Hematology and Oncology. 2019, doi: 10.1186/s13045-019-0833-3.
CrossRef - Conrad, S. Castelino-Prabhu, C. Cobb, and A. Raza, “Role of cytopathology in the diagnosis and management of gastrointestinal tract cancers,” Journal of Gastrointestinal Oncology. 2012, doi: 10.3978/j.issn.2078-6891.2012.023.
- Al-Abbadi, “Basics of cytology,” Avicenna J. Med., vol. 1, no. 1, p. 18, 2011, doi: 10.4103/2231-0770.83719.
CrossRef - B. Chinen, C. M. Guan, J. R. Ferrer, S. N. Barnaby, T. J. Merkel, and C. A. Mirkin, “Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence,” Chemical Reviews, vol. 115, no. 19. American Chemical Society, pp. 10530–10574, 2015, doi: 10.1021/acs.chemrev.5b00321.
CrossRef - R. Zocchi, F. Tosetti, R. Benelli, and A. Poggi, “Cancer nanomedicine special issue review anticancer drug delivery with nanoparticles: Extracellular vesicles or synthetic nanobeads as therapeutic tools for conventional treatment or immunotherapy,” Cancers (Basel)., vol. 12, no. 7, pp. 1–32, Jul. 2020, doi: 10.3390/cancers12071886.
CrossRef - K. Chaturvedi, A. Singh, V. K. Singh, and M. P. Singh, “Cancer Nanotechnology: A New Revolution for Cancer Diagnosis and Therapy,” Curr. Drug Metab., vol. 20, no. 6, pp. 416–429, Sep. 2018, doi: 10.2174/1389200219666180918111528.
CrossRef - Bozzuto and A. Molinari, “Liposomes as nanomedical devices,” International Journal of Nanomedicine. 2015, doi: 10.2147/IJN.S68861.
CrossRef - M. La-Beck and A. A. Gabizon, “Nanoparticle interactions with the immune system: Clinical implications for liposome-based cancer chemotherapy,” Front. Immunol., 2017, doi: 10.3389/fimmu.2017.00416.
CrossRef - Y. Yhee, S. Son, S. Son, M. K. Joo, and I. C. Kwon, “The EPR effect in cancer therapy,” in Cancer Targeted Drug Delivery: An Elusive Dream, vol. 9781461478768, Springer New York, 2013, pp. 621–632.
CrossRef - Hossen, M. K. Hossain, M. K. Basher, M. N. H. Mia, M. T. Rahman, and M. J. Uddin, “Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review,” Journal of Advanced Research. 2019, doi: 10.1016/j.jare.2018.06.005.
CrossRef - Baas, N. Senninger, H. Elser, and C. H. Herfarth, “Dynamic liver scintigraphy: A new way of measuring the function of the reticulo-endothelial system of the liver,” Eur. Surg. Res., vol. 27, no. 3, pp. 137–144, 1995, doi: 10.1159/000129392.
CrossRef - M. Veronese and A. Mero, “The impact of PEGylation on biological therapies,” BioDrugs, vol. 22, no. 5. BioDrugs, pp. 315–329, 2008, doi: 10.2165/00063030-200822050-00004.
CrossRef - Heyes, K. Hall, V. Tailor, R. Lenz, and I. MacLachlan, “Synthesis and characterization of novel poly(ethylene glycol)-lipid conjugates suitable for use in drug delivery,” J. Control. Release, vol. 112, no. 2, pp. 280–290, May 2006, doi: 10.1016/j.jconrel.2006.02.012.
CrossRef - Mok, K. H. Bae, C. H. Ahn, and T. G. Park, “PEGylated and MMP-2 specifically DePEGylated quantum dots: Comparative evaluation of cellular uptake,” Langmuir, vol. 25, no. 3, pp. 1645–1650, Feb. 2009, doi: 10.1021/la803542v.
CrossRef - H. Petersen, S. K. Alzghari, W. Chee, S. S. Sankari, and N. M. La-Beck, “Meta-analysis of clinical and preclinical studies comparing the anticancer efficacy of liposomal versus conventional non-liposomal doxorubicin,” J. Control. Release, vol. 232, pp. 255–264, Jun. 2016, doi: 10.1016/j.jconrel.2016.04.028.
CrossRef - Gibson, S. Alzghari, C. Ahn, H. Trantham, and N. M. La‐Beck, “The Role of Pegylated Liposomal Doxorubicin in Ovarian Cancer: A Meta‐Analysis of Randomized Clinical Trials,” Oncologist, vol. 18, no. 9, pp. 1022–1031, Sep. 2013, doi: 10.1634/theoncologist.2013-0126.
CrossRef - A. S. Samson, S. Park, S. Y. Kim, D. H. Min, N. L. Jeon, and J. M. Song, “Liposomal co-delivery-based quantitative evaluation of chemosensitivity enhancement in breast cancer stem cells by knockdown of GRP78/CLU,” J. Liposome Res., vol. 29, no. 1, pp. 44–52, Jan. 2019, doi: 10.1080/08982104.2017.1420081.
CrossRef - B. Sutradhar and M. L. Amin, “Nanotechnology in Cancer Drug Delivery and Selective Targeting,” ISRN Nanotechnol., vol. 2014, pp. 1–12, 2014, doi: 10.1155/2014/939378.
CrossRef - Oerlemans, W. Bult, M. Bos, G. Storm, J. F. W. Nijsen, and W. E. Hennink, “Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release,” Pharmaceutical Research, vol. 27, no. 12. Springer, pp. 2569–2589, Dec-2010, doi: 10.1007/s11095-010-0233-4.
CrossRef - Zhou, L. Zhang, T. H. Yang, and H. Wu, “Stimuli-responsive polymeric micelles for drug delivery and cancer therapy,” International Journal of Nanomedicine, vol. 13. Dove Medical Press Ltd., pp. 2921–2942, 18-May-2018, doi: 10.2147/IJN.S158696.
CrossRef - Keskin and A. Tezcaner, “Micelles As Delivery System for Cancer Treatment,” Curr. Pharm. Des., vol. 23, no. 35, May 2017, doi: 10.2174/1381612823666170526102757.
CrossRef - Lu, E. Zhang, J. Yang, and Z. Cao, “Strategies to improve micelle stability for drug delivery,” Nano Research, vol. 11, no. 10. Tsinghua University Press, pp. 4985–4998, 01-Oct-2018, doi: 10.1007/s12274-018-2152-3.
CrossRef - A. N. Hanafy, M. El-Kemary, and S. Leporatti, “Micelles structure development as a strategy to improve smart cancer therapy,” Cancers, vol. 10, no. 7. MDPI AG, 20-Jul-2018, doi: 10.3390/cancers10070238.
CrossRef - Blanco, H. Shen, and M. Ferrari, “Principles of nanoparticle design for overcoming biological barriers to drug delivery,” Nature Biotechnology, vol. 33, no. 9. Nature Publishing Group, pp. 941–951, 08-Sep-2015, doi: 10.1038/nbt.3330.
CrossRef - PottanamChali and B. J. Ravoo, “Polymer Nanocontainers for Intracellular Delivery,” Angew. Chemie Int. Ed., vol. 59, no. 8, pp. 2962–2972, Feb. 2020, doi: 10.1002/anie.201907484.
CrossRef - Wan, C. Li, and J. Pan, “Polymeric Micelles with Reduction-Responsive Function for Targeted Cancer Chemotherapy,” ACS Appl. Bio Mater., 2020, doi: 10.1021/acsabm.9b01070.
CrossRef - F. Monteiro, A. Travanut, C. Conte, and C. Alexander, “Reduction-responsive polymers for drug delivery in cancer therapy—Is there anything new to discover?,” Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. Wiley-Blackwell, 2020, doi: 10.1002/wnan.1678.
CrossRef - Qiaoet al., “Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy,” Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology, vol. 11, no. 1, p. e1527, Jan. 2019, doi: 10.1002/wnan.1527.
CrossRef - Zhao, C. Simon, M. Daoud Attieh, K. Haupt, and A. Falcimaigne-Cordin, “Reduction-responsive molecularly imprinted nanogels for drug delivery applications,” RSC Adv., vol. 10, no. 10, pp. 5978–5987, Feb. 2020, doi: 10.1039/c9ra07512g.
CrossRef - Bai et al., “Reduction-responsive dithiomaleimide-based polymeric micelles for controlled anti-cancer drug delivery and bioimaging,” Polym. Chem., vol. 8, no. 46, pp. 7160–7168, Dec. 2017, doi: 10.1039/c7py01675a.
CrossRef - Wang et al., “Poly(ethylene glycol)-polylactide micelles for cancer therapy,” Frontiers in Pharmacology. 2018, doi: 10.3389/fphar.2018.00202.
CrossRef - Gong, M. Chen, Q. Ren, X. Yue, and Z. Dai, “Fibronectin-targeted dual-acting micelles for combination therapy of metastatic breast cancer,” Signal Transduct. Target. Ther., 2020, doi: 10.1038/s41392-019-0104-3.
CrossRef - Yu, Q. Ning, Z. Mo, and S. Tang, “Intelligent polymeric micelles for multidrug co-delivery and cancer therapy,” Artif. Cells, Nanomedicine, Biotechnol., vol. 47, no. 1, pp. 1476–1487, Dec. 2019, doi: 10.1080/21691401.2019.1601104.
CrossRef - Loo, A. Lowery, N. Halas, J. West, and R. Drezek, “Immunotargeted nanoshells for integrated cancer imaging and therapy,” Nano Lett., vol. 5, no. 4, pp. 709–711, Apr. 2005, doi: 10.1021/nl050127s.
CrossRef - Kosaka, M. Ogawa, P. L. Choyke, and H. Kobayashi, “Clinical implications of near-infrared fluorescence imaging in cancer,” Future Oncology, vol. 5, no. 9. NIH Public Access, pp. 1501–1511, 2009, doi: 10.2217/fon.09.109.
CrossRef - R. Hirsch et al., “Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance,” Proc. Natl. Acad. Sci. U. S. A., vol. 100, no. 23, pp. 13549–13554, Nov. 2003, doi: 10.1073/pnas.2232479100.
CrossRef - M. Stern, J. Stanfield, W. Kabbani, J. T. Hsieh, and J. A. Cadeddu, “Selective prostate cancer thermal ablation with laser activated gold nanoshells,” J. Urol., vol. 179, no. 2, pp. 748–753, 2008, doi: 10.1016/j.juro.2007.09.018.
CrossRef - Baghaban-Eslaminejad, A. Oryan, A. Kamali, and A. Moshiri, “The role of nanomedicine, nanotechnology, and nanostructures on oral bone healing, modeling, and remodeling,” in Nanostructures for Oral Medicine, Elsevier Inc., 2017, pp. 777–832.
CrossRef - J. Madsen, S. K. Baek, A. R. Makkouk, T. Krasieva, and H. Hirschberg, “Macrophages as cell-based delivery systems for nanoshells in photothermal therapy,” Ann. Biomed. Eng., vol. 40, no. 2, pp. 507–515, Feb. 2012, doi: 10.1007/s10439-011-0415-1.
CrossRef - Leung, “Gold-polyethylene glycol nanoshells.,” National Center for Biotechnology Information (US), 2004.
- Dabbagh, R. Mahmoodian, B. J. J. Abdullah, H. Abdullah, M. Hamdi, and N. H. Abu Kasim, “Low-melting-point polymeric nanoshells for thermal-triggered drug release under hyperthermia condition,” Int. J. Hyperth., vol. 31, no. 8, pp. 920–929, Nov. 2015, doi: 10.3109/02656736.2015.1094147.
CrossRef - Huang, Z. Xue, and S. Zeng, “Hollow Mesoporous Bi@PEG-FANanoshell as a Novel Dual-Stimuli-Responsive Nanocarrier for Synergistic Chemo-Photothermal Cancer Therapy,” ACS Appl. Mater. Interfaces, vol. 12, no. 28, pp. 31172–31181, Jul. 2020, doi: 10.1021/acsami.0c07372.
CrossRef - Nouri et al., “NIR triggered glycosylated gold nanoshell as a photothermal agent on melanoma cancer cells,” Artif. Cells, Nanomedicine, Biotechnol., vol. 47, no. 1, pp. 2316–2324, Dec. 2019, doi: 10.1080/21691401.2019.1593187.
CrossRef - J. Bernardi, A. R. Lowery, P. A. Thompson, S. M. Blaney, and J. L. West, “Immunonanoshells for targeted photothermal ablation in medulloblastoma and glioma: An in vitro evaluation using human cell lines,” J. Neurooncol., vol. 86, no. 2, pp. 165–172, Jan. 2008, doi: 10.1007/s11060-007-9467-3.
CrossRef - Saifuddin, A. Z. Raziah, and A. R. Junizah, “Carbon nanotubes: A review on structure and their interaction with proteins,” J. Chem., 2013, doi: 10.1155/2013/676815.
CrossRef - H. Son, J. H. Hong, and J. W. Lee, “Carbon nanotubes as cancer therapeutic carriers and mediators,” International Journal of Nanomedicine, vol. 11. Dove Medical Press Ltd., pp. 5163–5185, 07-Oct-2016, doi: 10.2147/IJN.S112660.
CrossRef - Kumari, B. Ghosh, and S. Biswas, “Nanocarriers for cancer-targeted drug delivery,” Journal of Drug Targeting, vol. 24, no. 3. Taylor and Francis Ltd, pp. 179–191, 15-Mar-2016, doi: 10.3109/1061186X.2015.1051049.
CrossRef - Q. Wu, X. W. Wei, M. W. Shao, and J. S. Gu, “Synthesis of zinc oxide nanorods using carbon nanotubes as templates,” J. Cryst. Growth, vol. 265, no. 1–2, pp. 184–189, Apr. 2004, doi: 10.1016/j.jcrysgro.2004.01.052.
CrossRef - M. Dinan, F. Atyabi, M. R. Rouini, M. Amini, A. A. Golabchifar, and R. Dinarvand, “Doxorubicin loaded folate-targeted carbon nanotubes: Preparation, cellular internalization, in vitro cytotoxicity and disposition kinetic study in the isolated perfused rat liver,” Mater. Sci. Eng. C, vol. 39, no. 1, pp. 47–55, Jun. 2014, doi: 10.1016/j.msec.2014.01.055.
CrossRef - Sharma, S. Naskar, and K. Kuotsu, “Metronomic chemotherapy of carboplatin-loaded PEGylated MWCNTs: synthesis, characterization and in vitro toxicity in human breast cancer,” Carbon Lett., vol. 30, no. 4, pp. 435–447, Aug. 2020, doi: 10.1007/s42823-019-00113-0.
CrossRef - Hwang, S. H. Park, and J. W. Lee, “Applications of functionalized carbon nanotubes for the therapy and diagnosis of cancer,” Polymers, vol. 9, no. 1. MDPI AG, 2017, doi: 10.3390/polym9010013.
CrossRef - Mohseni-Dargah, S. Akbari-Birgani, Z. Madadi, F. Saghatchi, and B. Kaboudin, “Carbon nanotube-delivered iC9 suicide gene therapy for killing breast cancer cells in vitro,” Nanomedicine, vol. 14, no. 8, pp. 1033–1047, Apr. 2019, doi: 10.2217/nnm-2018-0342.
CrossRef - Zhang, L. Meng, Q. Lu, Z. Fei, and P. J. Dyson, “Targeted delivery and controlled release of doxorubicin to cancer cells using modified single wall carbon nanotubes,” Biomaterials, vol. 30, no. 30, pp. 6041–6047, Oct. 2009, doi: 10.1016/j.biomaterials.2009.07.025.
CrossRef - Li, R. Wu, L. Zhao, M. Wu, L. Yang, and H. Zou, “P-glycoprotein antibody functionalized carbon nanotube overcomes the multidrug resistance of human leukemia cells,” ACS Nano, vol. 4, no. 3, pp. 1399–1408, Mar. 2010, doi: 10.1021/nn9011225.
CrossRef - S. Omurtag Ozgen, S. Atasoy, B. Zengin Kurt, Z. Durmus, G. Yigit, and A. Dag, “Glycopolymer decorated multiwalled carbon nanotubes for dual targeted breast cancer therapy,” J. Mater. Chem. B, vol. 8, no. 15, pp. 3123–3137, Apr. 2020, doi: 10.1039/c9tb02711d.
CrossRef - Hong et al., “Multifunctional in vivo vascular imaging using near-infrared II fluorescence,” Nat. Med., vol. 18, no. 12, pp. 1841–1846, Dec. 2012, doi: 10.1038/nm.2995.
CrossRef - T. Robinson, G. Hong, Y. Liang, B. Zhang, O. K. Yaghi, and H. Dai, “In vivo fluorescence imaging in the second near-infrared window with long circulating carbon nanotubes capable of ultrahigh tumor uptake,” J. Am. Chem. Soc., vol. 134, no. 25, pp. 10664–10669, Jun. 2012, doi: 10.1021/ja303737a.
CrossRef - Diaoet al., “Chirality enriched (12,1) and (11,3) single-walled carbon nanotubes for biological imaging,” J. Am. Chem. Soc., vol. 134, no. 41, pp. 16971–16974, Oct. 2012, doi: 10.1021/ja307966u.
CrossRef - Welsher, S. P. Sherlock, and H. Dai, “Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window,” Proc. Natl. Acad. Sci. U. S. A., vol. 108, no. 22, pp. 8943–8948, May 2011, doi: 10.1073/pnas.1014501108.
CrossRef - Yi et al., “M13 phage-functionalized single-walled carbon nanotubes as nanoprobes for second near-infrared window fluorescence imaging of targeted tumors,” Nano Lett., vol. 12, no. 3, pp. 1176–1183, Mar. 2012, doi: 10.1021/nl2031663.
CrossRef - Ghosh, A. F. Bagley, Y. J. Na, M. J. Birrer, S. N. Bhatia, and A. M. Belcher, “Deep, noninvasive imaging and surgical guidance of submillimeter tumors using targeted M13-stabilized single-walled carbon nanotubes,” Proc. Natl. Acad. Sci., vol. 111, no. 38, pp. 13948–13953, Sep. 2014, doi: 10.1073/pnas.1400821111.
CrossRef - Sanginario, B. Miccoli, and D. Demarchi, “Carbon Nanotubes as an Effective Opportunity for Cancer Diagnosis and Treatment,” Biosensors. 2017, doi: 10.3390/bios7010009.
CrossRef - J. McHugh et al., “Biocompatible Semiconductor Quantum Dots as Cancer Imaging Agents,” Advanced Materials, vol. 30, no. 18. Wiley-VCH Verlag, p. 1706356, 03-May-2018, doi: 10.1002/adma.201706356.
CrossRef - K. Harris, P. M. Allen, H. S. Han, B. J. Walker, J. Lee, and M. G. Bawendi, “Synthesis of cadmium arsenide quantum dots luminescent in the infrared,” J. Am. Chem. Soc., vol. 133, no. 13, pp. 4676–4679, Apr. 2011, doi: 10.1021/ja1101932.
CrossRef - Fang, C. W. Peng, D. W. Pang, and Y. Li, “Quantum dots for cancer research: current status, remaining issues, and future perspectives,” Cancer Biol. Med., vol. 9, no. 3, pp. 151–163, Sep. 2012, doi: 10.7497/j.issn.2095-3941.2012.03.001.
- Zhang, D. Yee, and C. Wang, “Quantum dots for cancer diagnosis and therapy: Biological and clinical perspectives,” Nanomedicine, vol. 3, no. 1. pp. 83–91, Feb-2008, doi: 10.2217/17435889.3.1.83.
CrossRef - X. Zhao, B. J. Zhu, W. J. Yao, and D. F. Chen, “Therapeutic effect of quantum dots for cancer treatment,” RSC Adv., vol. 6, no. 114, pp. 113791–113795, 2016, doi: 10.1039/c6ra24063a.
CrossRef - Singh, S. Pandit, V. R. S. S. Mokkapati, A. Garg, V. Ravikumar, and I. Mijakovic, “Gold nanoparticles in diagnostics and therapeutics for human cancer,” International Journal of Molecular Sciences, vol. 19, no. 7. MDPI AG, 06-Jul-2018, doi: 10.3390/ijms19071979.
CrossRef - Aldewachi, T. Chalati, M. N. Woodroofe, N. Bricklebank, B. Sharrack, and P. Gardiner, “Gold nanoparticle-based colorimetric biosensors,” Nanoscale, vol. 10, no. 1. Royal Society of Chemistry, pp. 18–33, 07-Jan-2018, doi: 10.1039/c7nr06367a.
CrossRef - Zhang, M. Yang, Y. Zhu, and C. Mao, “Metallic Nanoclusters for Cancer Imaging and Therapy,” Curr. Med. Chem., vol. 25, no. 12, pp. 1379–1396, Apr. 2018, doi: 10.2174/0929867324666170331122757.
CrossRef - Bahrami et al., “Nanoparticles and targeted drug delivery in cancer therapy,” Immunology Letters, vol. 190. Elsevier B.V., pp. 64–83, 01-Oct-2017, doi: 10.1016/j.imlet.2017.07.015.
CrossRef - H. Chen et al., “Methotrexate conjugated to gold nanoparticles inhibits tumor growth in a syngeneic lung tumor model,” Mol. Pharm., vol. 4, no. 5, pp. 713–722, Sep. 2007, doi: 10.1021/mp060132k.
CrossRef - Vankayala, C. C. Lin, P. Kalluru, C. S. Chiang, and K. C. Hwang, “Gold nanoshells-mediated bimodal photodynamic and photothermal cancer treatment using ultra-low doses of near infra-red light,” Biomaterials, vol. 35, no. 21, pp. 5527–5538, 2014, doi: 10.1016/j.biomaterials.2014.03.065.
CrossRef - Wang, Y. C. Wang, S. Dou, M. H. Xiong, T. M. Sun, and J. Wang, “Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells,” ACS Nano, vol. 5, no. 5, pp. 3679–3692, May 2011, doi: 10.1021/nn200007z.
CrossRef - Mofrad, R. Hadi, B. Tahmasebi, S. Farhoudian, M. Mehravar, and R. Nasiri, “Green synthesis of gold nanoparticles using plant extract: Mini-review,” Nanochem Res, vol. 2, no. 1, pp. 8–19, Jan. 2017, doi: 10.22036/ncr.2017.01.002.
- Clarance et al., “Green synthesis and characterization of gold nanoparticles using endophytic fungi Fusarium solani and its in-vitro anticancer and biomedical applications,” Saudi J. Biol. Sci., vol. 27, no. 2, pp. 706–712, Feb. 2020, doi: 10.1016/j.sjbs.2019.12.026.
CrossRef - N. Bin-Jumah, M. Al-Abdan, G. Al-Basher, and S. Alarifi, “Molecular Mechanism of Cytotoxicity, Genotoxicity, and Anticancer Potential of Green Gold Nanoparticles on Human Liver Normal and Cancerous Cells,” Dose-Response, vol. 18, no. 2, Apr. 2020, doi: 10.1177/1559325820912154.
CrossRef - A. Meyer, J. C. Sunshine, and J. J. Green, “Biomimetic Particles as Therapeutics,” Trends Biotechnol., vol. 33, no. 9, p. 514, Sep. 2015, doi: 10.1016/J.TIBTECH.2015.07.001.
CrossRef - Dehaini et al., “Erythrocyte-Platelet Hybrid Membrane Coating for Enhanced Nanoparticle Functionalization,” Adv. Mater., vol. 29, no. 16, Apr. 2017, doi: 10.1002/ADMA.201606209.
CrossRef - Rao et al., “Cancer Cell Membrane-Coated Upconversion Nanoprobes for Highly Specific Tumor Imaging,” Adv. Mater., vol. 28, no. 18, pp. 3460–3466, May 2016, doi: 10.1002/ADMA.201506086.
CrossRef - Parodi et al., “Synthetic nanoparticles functionalized with biomimetic leukocyte membranes possess cell-like functions,” Nat. Nanotechnol., vol. 8, no. 1, pp. 61–68, 2013, doi: 10.1038/NNANO.2012.212.
CrossRef - Kumar, T. Van Treuren, A. P. Ranjan, P. Chaudhary, and J. K. Vishwanatha, “In vivo imaging and biodistribution of near infrared dye loaded brain-metastatic-breast-cancer-cell-membrane coated polymeric nanoparticles,” Nanotechnology, vol. 30, no. 26, p. 265101, Apr. 2019, doi: 10.1088/1361-6528/AB0F46.
CrossRef - V. Kroll et al., “Nanoparticulate Delivery of Cancer Cell Membrane Elicits Multiantigenic Antitumor Immunity,” Adv. Mater., vol. 29, no. 47, Dec. 2017, doi: 10.1002/ADMA.201703969.
CrossRef - Jin and Z. M. Bhujwalla, “Biomimetic Nanoparticles Camouflaged in Cancer Cell Membranes and Their Applications in Cancer Theranostics,” Front. Oncol., vol. 9, p. 1560, Jan. 2020, doi: 10.3389/FONC.2019.01560/BIBTEX.
CrossRef - Wang et al., “Cell membrane biomimetic nanoparticles for inflammation and cancer targeting in drug delivery,” Biomater. Sci., vol. 8, no. 2, pp. 552–568, Jan. 2020, doi: 10.1039/C9BM01392J.
CrossRef - Sushnitha, M. Evangelopoulos, E. Tasciotti, and F. Taraballi, “Cell Membrane-Based Biomimetic Nanoparticles and the Immune System: Immunomodulatory Interactions to Therapeutic Applications,” Front. Bioeng. Biotechnol., vol. 8, p. 627, Jun. 2020, doi: 10.3389/FBIOE.2020.00627/BIBTEX.
CrossRef - Liao, S. W. Wong, H. L. Yeo, and Y. Zhao, “Smart nanocarriers for cancer treatment: Clinical impact and safety,” NanoImpact, vol. 20. Elsevier B.V., p. 100253, 01-Oct-2020, doi: 10.1016/j.impact.2020.100253.
CrossRef - Fan and Q. Zhang, “Development of liposomal formulations: From concept to clinical investigations,” Asian J. Pharm. Sci., vol. 8, no. 2, pp. 81–87, Apr. 2013, doi: 10.1016/j.ajps.2013.07.010.
CrossRef - T. Jinet al., “Recent Trends in Nanocarrier-Based Targeted Chemotherapy: Selective Delivery of Anticancer Drugs for Effective Lung, Colon, Cervical, and Breast Cancer Treatment,” Journal of Nanomaterials, vol. 2020. Hindawi Limited, 2020, doi: 10.1155/2020/9184284.
CrossRef - Nel, T. Xia, L. Mädler, and N. Li, “Toxic potential of materials at the nanolevel,” Science, vol. 311, no. 5761. American Association for the Advancement of Science, pp. 622–627, 03-Feb-2006, doi: 10.1126/science.1114397.
CrossRef - Najafi-Hajivar et al., “Overview on experimental models of interactions between nanoparticles and the immune system,” Biomedicine and Pharmacotherapy, vol. 83. Elsevier Masson SAS, pp. 1365–1378, 01-Oct-2016, doi: 10.1016/j.biopha.2016.08.060.
CrossRef - Barbero et al., “Formation of the Protein Corona: The Interface between Nanoparticles and the Immune System,” Seminars in Immunology, vol. 34. Academic Press, pp. 52–60, 01-Dec-2017, doi: 10.1016/j.smim.2017.10.001.
CrossRef - Goy-López et al., “Physicochemical characteristics of protein-NP bioconjugates: The role of particle curvature and solution conditions on human serum albumin conformation and fibrillogenesis inhibition,” Langmuir, vol. 28, no. 24, pp. 9113–9126, Jun. 2012, doi: 10.1021/la300402w.
CrossRef - Han et al., “Toxic and adjuvant effects of silica nanoparticles on ovalbumin-induced allergic airway inflammation in mice,” Respir. Res., vol. 17, no. 1, May 2016, doi: 10.1186/s12931-016-0376-x.
CrossRef - M. Smith, J. K. Simon, and J. R. Baker, “Applications of nanotechnology for immunology,” Nature Reviews Immunology, vol. 13, no. 8. Nature Publishing Group, pp. 592–605, 25-Aug-2013, doi: 10.1038/nri3488.
CrossRef - Y. Madani, A. Mandel, and A. M. Seifalian, “A concise review of carbon nanotube’s toxicology,” Nano Rev., vol. 4, no. 1, p. 21521, Jan. 2013, doi: 10.3402/nano.v4i0.21521.
CrossRef - A. Dobrovolskaia, M. Shurin, and A. A. Shvedova, “Current understanding of interactions between nanoparticles and the immune system,” Toxicol. Appl. Pharmacol., vol. 299, pp. 78–89, May 2016, doi: 10.1016/j.taap.2015.12.022.
CrossRef - Vallhov et al., “The importance of an endotoxin-free environment during the production of nanoparticles used in medical applications,” Nano Lett., vol. 6, no. 8, pp. 1682–1686, Aug. 2006, doi: 10.1021/nl060860z.
CrossRef - I. Inoue, “Promoting effects of nanoparticles/materials on sensitive lung inflammatory diseases,” Environmental Health and Preventive Medicine, vol. 16, no. 3. Environ Health Prev Med, pp. 139–143, May-2011, doi: 10.1007/s12199-010-0177-7.
CrossRef - E. Zuckerman and M. E. Davis, “Clinical experiences with systemically administered siRNA-based therapeutics in cancer,” Nature Reviews Drug Discovery. 2015, doi: 10.1038/nrd4685.
CrossRef - Wittrup and J. Lieberman, “Knocking down disease: A progress report on siRNA therapeutics,” Nature Reviews Genetics, vol. 16, no. 9. Nature Publishing Group, pp. 543–552, 18-Aug-2015, doi: 10.1038/nrg3978.
CrossRef - Zhang, K. An, X. Duan, H. Xu, F. Li, and F. Xu, “Recent advances in siRNA delivery for cancer therapy using smart nanocarriers,” Drug Discovery Today, vol. 23, no. 4. Elsevier Ltd, pp. 900–911, 01-Apr-2018, doi: 10.1016/j.drudis.2018.01.042.
CrossRef - Gallas, C. Alexander, M. C. Davies, S. Puri, and S. Allen, “Chemistry and formulations for siRNA therapeutics,” Chem. Soc. Rev., vol. 42, no. 20, pp. 7983–7997, Sep. 2013, doi: 10.1039/c3cs35520a.
CrossRef - J-M. Williford, J. Wu, Y. Ren, M. M. Archang, K. W. Leong, and H.-Q. Mao, “Recent Advances in Nanoparticle-Mediated siRNA Delivery,” Annu. Rev. Biomed. Eng., vol. 16, no. 1, pp. 347–370, Jul. 2014, doi: 10.1146/annurev-bioeng-071813-105119.
CrossRef - K-N. Sugahara et al., “Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs,” Science (80-. )., vol. 328, no. 5981, pp. 1031–1035, May 2010, doi: 10.1126/science.1183057.
CrossRef - Liu, R. C. Anderson, X. Lan, P. S. Conti, and K. Chen, “Recent advances in the development of nanoparticles for multimodality imaging and therapy of cancer,” Medicinal Research Reviews, vol. 40, no. 3. John Wiley and Sons Inc., pp. 909–930, 01-May-2020, doi: 10.1002/med.21642.
CrossRef - G. Chen et al., “CuS-Based Theranostic Micelles for NIR-Controlled Combination Chemotherapy and Photothermal Therapy and Photoacoustic Imaging,” ACS Appl. Mater. Interfaces, vol. 9, no. 48, pp. 41700–41711, Dec. 2017, doi: 10.1021/acsami.7b14083.
CrossRef - J. Zhang, T. Zhao, F. Han, Y. Hu, and Y. Li, “Photothermal and gene therapy combined with immunotherapy to gastric cancer by the gold nanoshell-based system,” Nanobiotechnology, vol. 17, no. 1, p. 80, Jul. 2019, doi: 10.1186/s12951-019-0515-x.
CrossRef - N.Vijayakameswara Rao, H. Ko, J. Lee, and J. H. Park, “Recent progress and advances in stimuli-responsive polymers for cancer therapy,” Frontiers in Bioengineering and Biotechnology, vol. 6, no. AUG. Frontiers Media S.A., p. 110, 13-Aug-2018, doi: 10.3389/fbioe.2018.00110.
CrossRef - M.Alsehli, “Polymeric nanocarriers as stimuli-responsive systems for targeted tumor (cancer) therapy: Recent advances in drug delivery,” Saudi Pharmaceutical Journal, vol. 28, no. 3. Elsevier B.V., pp. 255–265, 01-Mar-2020, doi: 10.1016/j.jsps.2020.01.004.
CrossRef - M. Wang et al., “Gold nanoshell coated thermo-pH dual responsive liposomes for resveratrol delivery and chemo-photothermal synergistic cancer therapy,” Mater. Chem. B, vol. 5, no. 11, pp. 2161–2171, 2017, doi: 10.1039/c7tb00258k.
CrossRef - K. Zhang et al., “Temperature, pH, and reduction triple-stimuli-responsive inner-layer crosslinked micelles as nanocarriers for controlled release,” Appl. Polym. Sci., vol. 135, no. 40, p. 46714, Oct. 2018, doi: 10.1002/app.46714.
CrossRef - N.Oddoneet al., “Synthesis, Characterization, and In Vitro Studies of a Reactive Oxygen Species (ROS)-Responsive Methoxy Polyethylene Glycol-Thioketal-Melphalan Prodrug for Glioblastoma Treatment,” Pharmacol., vol. 11, p. 574, May 2020, doi: 10.3389/fphar.2020.00574.
CrossRef - J. Tang et al., “Aptamer-conjugated PEGylated quantum dots targeting epidermal growth factor receptor variant III for fluorescence imaging of glioma,” J. Nanomedicine, vol. 12, pp. 3899–3911, May 2017, doi: 10.2147/IJN.S133166.
CrossRef - R. H. Fang, A. V. Kroll, W. Gao, and L. Zhang, “Cell Membrane Coating Nanotechnology,” Mater., vol. 30, no. 23, p. 1706759, Jun. 2018, doi: 10.1002/adma.201706759.
CrossRef - [118] M. W. Kim, G. Lee, T. Niidome, Y. Komohara, R. Lee, and Y. Il Park, “Platelet-Like Gold Nanostars for Cancer Therapy: The Ability to Treat Cancer and Evade Immune Reactions,” Bioeng. Biotechnol., vol. 8, p. 133, Feb. 2020, doi: 10.3389/fbioe.2020.00133.
CrossRef - M. K. Riaz et al., “Surface functionalization and targeting strategies of liposomes in solid tumor therapy: A review,” International Journal of Molecular Sciences. 2018, doi: 10.3390/ijms19010195.
CrossRef - P. N. Navya, A. Kaphle, S. P. Srinivas, S. K. Bhargava, V. M. Rotello, and H. K. Daima, “Current trends and challenges in cancer management and therapy using designer nanomaterials,” Nano Convergence. 2019, doi: 10.1186/s40580-019-0193-2.
CrossRef