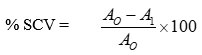Manuscript accepted on :03-06-2022
Published online on: 24-06-2022
Plagiarism Check: Yes
Reviewed by: Dr. Rajesh Dumpala, Dr. Vishnu Choudhari
Second Review by: Dr. Swastika Maity
Final Approval by: Dr. Patorn Piromchai
Md. Imran Nur Manik1* , Md. Hazrat Ali2
, Md. Hazrat Ali2 , Md. Monirul Islam3
, Md. Monirul Islam3 , Abu Zobayed1
, Abu Zobayed1 , Saadullah4
, Saadullah4 , Alam Khan5
, Alam Khan5 , Fatema Tabassum1
, Fatema Tabassum1 and Furhatun-Noor1
and Furhatun-Noor1
1Department of Pharmacy, Faculty of Health Science, Northern University Bangladesh, Dhaka-1205, Bangladesh
2Department of Pharmacy, Faculty of Science and Engineering, International Islamic University Chittagong, Chittagong-4318, Bangladesh
3Department of Pharmacy, Noakhali Science and Technology University, Noakhali-3814, Bangladesh
4Department of Pharmacy, Faculty of Science and Engineering, University of Information Technology and Sciences, Dhaka-1212, Bangladesh
5Department of Pharmacy, Faculty of Science, University of Rajshahi, Rajshahi-6205, Bangladesh
Corresponding Author E-mail: manikrupharmacy@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2426
Abstract
Context: Oxidative stress and pertaining counterbalance mechanism are actively working in the living organisms. The overproduction of reactive oxygen species (ROS) in the ongoing equipoising process requires to be compensated by strong antioxidants. Plants as a rich source of antioxidants not only reduce oxidative stress but also possess cytotoxic, thrombolytic and phytochemical potentials. Aims: To find out the antioxidant, cytotoxic, thrombolytic and phytochemical capabilities of the methanolic extracts of Ampelocissus barbata (Wall.) leaves. Methods and Material: Assessment of the in vitro antioxidant activity of extract was carried out using DPPH radical scavenging assay, determination of reducing power capacity and total phenolic content. The thrombolytic activity was assessed by disintegration of clot and prospective phytochemical activities were by standard qualitative analysis such as Mayer’s, Dragendroff’s Wagner’s and Hager’s Reagent test for alkaloids; Libermann-Burchared and Salkowski Reagent tests for steroid and terpenoids; Molish Reagent, Benedict’s Reagent, Fehling’s Solution A & B reagent test for carbohydrates; Ferric Chloride (5%) Solution, Potassium Dichromate (10%) Solution tests for tannins; Shinoda test and Alkaline reagent test for Flavonoids; Froth tests & Haemolysis test for Saponins. Statistical analysis used: The statistical analysis was carried out using GraphPad Prism and Microsoft excel Results: Appreciable DPPH radical scavenging activity of the extract was observed with the IC50 value of 107.47±1.46 µg/ml. A significant correlation was found between the standard ascorbic acid (AA) and the plant extracts at the p˂0.05 for the reducing power assay where, the activity increased with the concentration of the extracts and the highest absorbance value was 3.025±0.15 and 1.826±0.006 for the AA and the extracts respectively. The plant also accommodates a considerable amount of polyphenols, reflected in the value of gallic acid equivalent 277.397±0.419 mg/ml. Finally, the percentage (%) of clot lysis for the thrombolytic activity was revealed to be 7.031±0.697, 35.297±1.307, and 75.083±0.599 for the water (negative control), extract, and the standard Streptokinase respectively. The study revealed the presence of phytochemicals namely alkaloids, flavonoids, tannins and glycosides. Conclusions: The study disclosed the promising in vitro activity of the plant, which necessitates the further analysis for the isolation and evaluation of the active principles.
Keywords
Ampelocissus barbata; Antioxidant; Phytochemical Assay; Thrombolytic
Download this article as:| Copy the following to cite this article: Manik M. I. N, Ali M. H, Islam M. M, Zobayed A, Saadullah S, Khan A, Tabassum F, Noor N. In Vitro Antioxidant, Cytotoxic, Thrombolytic Activities and Phytochemical Evaluation of Methanol Extract of the Ampelocissus Barbata (Wall.) Leaves. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Manik M. I. N, Ali M. H, Islam M. M, Zobayed A, Saadullah S, Khan A, Tabassum F, Noor N. In Vitro Antioxidant, Cytotoxic, Thrombolytic Activities and Phytochemical Evaluation of Methanol Extract of the Ampelocissus Barbata (Wall.) Leaves. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/39KUrne |
Introduction
The significance of oxidative metabolism is indispensable for the existence of living cells and is extensively acknowledged. By-product of these processes includes the generation of free radicals and other reactive oxygen species (ROS) 1. In the biological systems formation of ROS over the antioxidant capability results in oxidative stress 2-4. Such species are associated with a large number of typical in vivo regulatory systems 5. For instance in the plants as the signaling molecules, ROS regulates defence, growth, abiotic stress acclimation, and development thus plants have regulatory systems to maintain appropriate level of ROS in the cell58. Similarly, imbalance of ROS may be harmful to the animals since ROS was identified as causative factors for diseases namely cancer , cardiovascular disease, rheumatoid arthritis, neurodegenerative disorders and diabetes59. Redundant production of free radicals can beat defensive enzymes such as peroxidase, catalase as well as superoxide dismutase (SOD), bringing in devastating and fatal consequences for cells leading towards oxidation of the cellular proteins, DNA, and membrane lipids, ultimately shutting cellular respiration down 6. In addition, the pathogenesis of different types of human diseases such as hypertension, atherosclerosis, inflammation diabetes mellitus, AIDS, and cancer have been found to be associated with free-radical mediated oxidative stress7. Moreover, the cell signalling pathways were also appeared to be influenced by reactive oxygen species in ways currently being unravelled8.
Thrombosis is the formation of a blood clot inside a blood vessel, obstructing blood flow in the systemic circulation 9. Blood clots formed within a vein may dislodge from their origin and travel (embolus)10. Thrombolysis (the breakdown) of blood clots is carried out by stimulating plasminogen, which produces cleaved product plasmin, a proteolytic enzyme that disintegrates cross-links between fibrin molecules resulting insoluble degradation products from insoluble fibrin. Thrombolysis basically encompasses the application of thrombolytic drugs, also called “plasminogen activators” and “fibrinolytic drugs.”11. These drugs solubilize thrombin in accurately obstructed coronary arteries, thereby normalizing blood supply to ischemic myocardium, reducing necrosis and advance prognosis 12.
There are three main categories of fibrinolytic agents, namely tissue plasminogen activator (tPA) factors, streptokinase (SK), and urokinase (UK); amongst these, the UK and SK are widely used 13,14. But they are associated with high hemorrhagic risk15and extensive anaphylactic reactions. Moreover, immunogenicity constrains treatment with SK16. Since all available thrombolytic agents suffer from significant deficits, for example, the requirement of large doses, definite specificity for fibrin, and bleeding tendency; therefore, better alternatives for the drugs with minimum side effects and maximum efficiency are badly needed 17.
For thousands of years, plants acted as the source of various traditional medicines worldwide and still serve as the basis of new remedies for humankind. Initially, medications from plants are dispensed as crude drugs 18.
In that connection, modern medicine is blessed with various active compounds isolated from plants, whereas for some of the essential drugs, the basic raw material continues to come from the plants 19.
Moreover, phytochemistry abridges plant biochemistry and organic chemistry from the natural product. Generally, plant deals with a large variety of chemical substances, along with their biosynthesis, natural distribution, and biological metabolism function20. Therefore, the plant extract with bioactive compounds such as tannins, alkaloids, phenolic compounds, and flavonoids is regarded as promising in terms of therapeutic activity 21.
For many developing countries, plants are the principal source of the bioactive tenets and medicine used explicitly in the traditional system of medicine 22,23.
Thus, it is clear that many plant species still possess medicinally important compounds that need to be discovered24.
As the plant remains an integral part in terms of the discovery of medicine thus the aim of the current work was to explore the phytochemical profiles of the plant Ampelocissus barbata for Antioxidant and thrombolytic activity.
Ampelocissus barbata is a species of liana that belongs to the grape family Vitaceae. Nathaniel Wallich described it from Sylhet (now in Bangladesh) and placed in the genus Vitis 25. The Ampelocissus genus of Vitaceae was recognized by Planchon in 1884 and encompassed about 95 species that are dispersed in the tropical regions of Asia, Australia, Central America and Africa 26.
Taxonomy 27
Kingdom: Plantae
Phylum: Tracheophyta
Class: Magnoliopsida
Order: Vitales
Family: Vitaceae
Genus: Ampelocissus Planch.
Species: Ampelocissus barbata (Wall.) Planch.
Synonyms : Vitis barbata Wall. ; Vitis latifolia Buch.-Ham.; Vitis latifolia Buch.-Ham. ex Wall.
Vernacular Name(s) : Jarila Lahari (Bangla); Khoissang (Chakma); Dang Gyae (Marma); Kanai Lak Mah (Tripura)28
Flowering & fruiting: June-October.
Ecology: Hilly forests and bushy thickets of foot hills.
Use: Fruits are edible. Paste prepared from roots is applied to boils.
Distribution: Bandarban, Chattogram, Cox’s Bazar, Khagrachari and Rangamati, Bangladesh.
Materials and Methods
Plant material
Fresh Stem of Ampelocissus barbata was collected from Chakaria, Cox’s Bazar; Chittagong, Bangladesh. Plant material was authenticated by Dr. Shaikh Bokhtear Uddin, Associate Professor, Department of Botany, University of Chittagong, Chittagong, Bangladesh, where voucher specimens have been deposited.
The novelty of the work and Literature search
The combined laboratory approaches to explore the plant in terms of phytopharmacology were not done before in the case of Ampelocissus barbata in Bangladesh. Therefore, this attempt was taken to evaluate the plant for active phytoconstituents with diverse implications as well as to widen the path for future screening of the plants for in vivo tests.
A previous study found dose-dependent analgesic activity and the presence of Phyto-constituents60 Apart from that there are some traditional applications found in the literature. Such as in the Sikkim province of India mouth and tongue sores of the milk-sucking baby, as well as cattle, are treated with plant juice61.
Preparation of crude extract
The stem was sun-dried for one week. After proper drying, it was ground into a fine powder using a Stainless Steel Herb Grinder Pulverizer Machine machine (R.S.Industries; India) . The ground Stem (500 g) was soaked in a sufficient amount of 95% methanol (Merck, Germany,CAS 67-56-1) for ten days at room temperature with occasional shaking and stirring manually. Afterward, the whole mixture was filtered through a cotton plug, followed by Whatman filter paper No. 1. Then the solvent evaporation was executed under reduced pressure at room temperature to yield a semisolid (5.9 %) mass and preserved in a refrigerator for further use.
In vitro antioxidant assay
DPPH Radical Scavenging Assay
Free radical scavenging ability of the test sample was carried out by the method described by Brand-Williams et al. 29, with slight modifications (modified in the university and standardized). The DPPH radical was used in the assay to quantify the ability of antioxidants to quench the DPPH radical. Upon scavenging the DPPH radical, the reaction mixture changes its colour from purple to yellow accompanied by decreasing absorbance at the wavelength 517 nm. One millilitre of the sample solution in methanol at various concentrations (25, 50, 100, 200 and 400 µg/ml) was mixed with three millilitres of 0.004% DPPH solution in methanol. After reaction for 30 min at room temperature in dark conditions, the absorbance values of the sample were measured by a UV spectrophotometer (Shimadzu, Kyoto, Japan) at 517 nm (λmax) against a corresponding blank. The calculation of Radical scavenging activity (%SCV) was accomplished by comparing the results of the test (sample/extract) with the control (not dealt with extract) applying the below formula:
Where SCV = Radical scavenging activity,
A0 = Absorbance of the control (containing all reagents except the test compound)
A1 = Absorbance of the test compounds (extracts / standard).
Extract concentration providing 50% inhibition (IC50) was calculated from the graph obtained by plotting % SCV versus concentration and subsequently verified using Graphpad prism. The assays were repeated three times, and ascorbic acid was used as standard.
Reducing power capacity
The reducing power of Ampelocissus barbata stem extracts was evaluated according to the method formerly described by Oyaizu 30 with slight modification (modified in the university and standardized)..
In the Reducing power assay, based on the reducing power of the sample, the test solution colour changes from yellow to several shades of green and blue 31. The presence of antioxidant substances in the sample converts the Fe3+ to Fe2+of the ferricyanide and thus the amount of Fe2+ complex monitored by taking absorbance at 700 nm.
Various concentrations of Ampelocissus barbata stem extracts (25mg/ml, 50mg/ml, 100mg/ml, 200mg/ml and 400mg/ml) were mixed with potassium ferricyanide [K3Fe(CN)6] (2.5 mL, 1%)and phosphate buffer (2.5 mL, 0.2 M, pH 6.6). To complete the reaction, the mixture was incubated for 20 min at 50°C. Afterward, 2.5 ml of trichloroacetic acid (10%) was added to the mixture, followed by centrifugation at 3000 rpm for 10 min. 2.5 ml supernatant solution was withdrawn from the mixture and mixed with 2.5 ml of distilled water and FeCl3 (0.5 mL, 0.1%), and finally the absorbance was measured at 700 nm against the blank, which contained the same solution mixture without plant extract or standard and was treated in the same manner as the samples solution. Ascorbic acid was used as standard. The correlation was determined between the absorbance of the Ascorbic acid and the plant extract. All analyses were carried out in triplicate, and results were averaged.
Determination of total phenolic content
The determination of total phenolic content for the methanol extract of Ampelocissus barbata was carried out by employing the method described by Singleton et al. with slight modification(modified in the university and standardized)., involving Gallic acid as standard and Folin-Ciocalteau reagent (FCR) as the oxidizing agent 32. The basis for this test lies in the oxidation of phenolic groups caused by phosphomolybdic and phosphotungstic acids (FCR).
In brief, a sample aliquot of 0.5 mL of extract (1 mg/mL) was added to a test tube containing 2.5 mL of Folin-Ciocalteau (Diluted 10 times with deionized water) reagent. Moreover, 2.0 mL of Na2CO3 (7.5% in water, w/v) was added, and the resulting solution was vortexed and left 30 minutes at 250C to complete the reaction. The absorbance of the consequential blue colour was taken at 760 nm against blank. Using gallic acid as standard, total phenolic content was calculated from a calibration curve using gallic acid and expressed as mg of gallic acid equivalent (GAE) per gram dry weight (dw) was calculated by the following formula:
A = (c x V)/m
Where,
A = total content of phenolic compounds, mg/g plant extract, in GAE;
c = the concentration of gallic acid established from the calibration curve, mg/ml;
V = the volume of extract, ml;
m = the weight of pure plant extracts, gm.
Data are reported as mean (SD) for at least three replications.
In vitro Thrombolytic activity
In vitro thrombolytic activity of Ampelocissus barbata chloroform extract was determined following the method described by Prasad et al.33
Streptokinase (SK)
For the in vitro thrombolytic analysis, lyophilized SK vial of 15, 00,000 I.U (Commercially available from Square Pharmaceuticals Ltd. Bangladesh) was used. The suspension was made by adding 5 ml sterile distilled water with proper mixing. From the resulted suspension, 100 μl (30,000 I.U.) was utilized for thrombolysis.
Specimen
Four (04) mL whole blood was drained from ten healthy human volunteers who have no history of anticoagulant therapy or an oral contraceptive. Before collecting the sample from volunteers, prior consent was taken as per the protocol approved by the Institutional Ethics Committee of Northern University Bangladesh. Approval number: DoP/RC/EC/2021/04/app/01.
From the collected blood, 500 μl (0.5 ml) of the blood was transferred to individual Eppendorf tubes weighed beforehand to form clots.
Herbal preparation
100 mg Ampelocisus babata methanol extract was suspended in 10mL distilled water and then agitated using a vortex mixer. The suspension was kept for overnight and was poured to eliminate soluble supernatant. It was then filtered by using a 0.22 micron syringe filter, and the filtrate was prepared for the assay.
Clot lysis
The collected blood specimen was incubated at 37°C for 45 minutes followed by complete careful removal of serum after clot formation. To find out the weight of the clot, the tube was weighed (clot weight = weight of clot containing tube – the weight of tube alone).
The tubes were labelled correctly. Then, 100 μl aqueous extract of Ampelocissus barbata was added to the Eppendorf tube with a pre-weighed clot, incubated for 90 minutes at 37°C to observe for clot lysis. The fluid discharged in incubation was eliminated, and the tubes were weighed one more time to detect the weight variation after disruption of the clot. The change in weight was stated as a percentage of clot lysis. 100 μl of SK applied as positive control and 100 μl of distilled water as a negative non-thrombolytic control, where all the tubes were treated in the same manner as described above.
% clot lysis = (Weight of the lysis clot /Weight of clot before lysis) × 100.
The experiment was repeated 10 times with the blood samples of 10 volunteers.
Phytochemical screening
The presence of different chemical groups in the methanol extract of A. barbarata W. leaves was screened as the preliminary step in the phytochemical studies. The chemical group tests were performed following standard test procedures 19,24, 34-40. In each test, 10% (W/V) solution of extract in Chloroform was taken unless otherwise mentioned in the individual test.
Statistical analysis
The statistical analysis (determination of IC50 values, the Pearson correlation coefficient at P<0.05) was performed using GraphPad Prism version 9.0.1 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com
Calculation of mean, standard deviation, and the constructions of graphs was carried out using Microsoft Excel version Office 2016.
Result
In vitro Antioxidants Activity
DPPH Radical Scavenging Assay
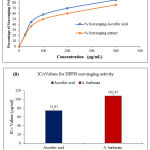
The complete procedure is previously delineated and the results obtained are given in Table 01 as well as elucidated in Figure 01. Test results exhibited concentration-dependent scavenging of the free radicals. The range of concentration for both the ascorbic acid (AA) and the plant extract was from 25-400 µg/ml, where the % inhibition was lower 22.21% (AA); 18.58% (A. barbarata) at 25 µg/ml and maximum 84.49% ( AA) ; 75.66 %( A. barbarata) ; at the highest concentration 400 µg/ml; for the standard and the extract respectively. The 50% inhibitory concentration of the antioxidant called IC50 was found as 73.97±03.37 µg/ml and 107.47±1.46 µg/ml for the standard and the plant extract, respectively.
Table 1: Percentage of DPPH radical scavenging activity of ascorbic acid and methanol extract of A. barbata stem at different concentrations. Results are mean± SD of three measurements.
|
Concentration (µg/ml) |
% inhibition by ascorbic acid ± SD | % inhibition by A. barbarata ± SD |
| 25 | 22.21 ±1.035 | 18.58±0.782 |
| 50 | 44.92±1.398 | 37.80±0.253 |
| 100 | 58.97±1.567 | 49.98±1.049 |
| 200 | 69.59±0.942 | 60.56±0.624 |
| 400 | 84.49±1.437 | 75.66±0.810 |
| IC50 =73.97±03.37 µg/ml |
IC50 =107.47±1.46 µg/ml |
Reducing Power Capacity
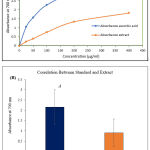
The plant’s methanolic extract (MeOH) showed the potential reducing capacity, outlined in Figure 02 and Table 02. The result for reducing power capacity indicates that the extract’s reducing power was enhanced in a concentration-dependent manner. The ascorbic acid (AA) was used as the standard reference compound. At the concentration of 25 µg/mL, the absorbance’s of the plant extract and AA were 0.232±0.028 1.051±0.041, respectively. But it was significantly increased at 200 µg/mL, depicting the absorbance as 1.338±0.065 by the extract about which is almost about half of the AA at the same concentration. The was a significant correlation observed between the reducing power capacity of standard ascorbic acid and the plant extract at P<0.05.
Table 2: Reducing power of ascorbic acid (Standard) & A. barbata at different concentrations. Results are mean± SD of three measurements.
| Concentration (µg/ml) | Absorbance (Mean ±SD) | |
| Ascorbic acid | A. barbata | |
| 25 | 1.051±0.041 | 0.232±0.028 |
| 50 | 1.571±0.135 | 0.391±0.048 |
| 100 | 2.276±0.124 | 0.753±0.029 |
| 200 | 2.884±0.135 | 1.338±0.065 |
| 400 | 3.025±0.154 | 1.826±0.006 |
Determination of total phenolic content
A standard Gallic acid curve was constructed to estimate the phenolic content from the sample, and the Gallic Acid Equivalent (GAE) was obtained by reference formula. The absorbance values for the Gallic acid are represented in Table 03 and the standard curve in Figure 03. Using the data (y = 0.0073x + 0.058 R² = 0.9948) from the standard curve, the total phenolic contents of the sample were calculated and expressed as GAE. The average phenolic content of A. barbata was found to be 277.397±0.419 mg Gallic acid/gm of extract, as shown in Table 04.
Table 3: Absorbance values of Gallic acid. Results are mean± SD of three measurements.
| Concentration(µg/ml) | Absorbance ±SD |
| 25 | 0.181±0.008 |
| 500 | 0.386±0.012 |
| 100 | 0.824±0.051 |
| 200 | 1.647±0.059 |
| 400 | 2.921±0.054 |
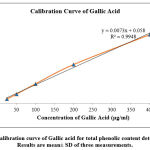 |
Figure 3: Calibration curve of Gallic acid for total phenolic content determination. Results are mean± SD of three measurements. |
Table 4: Data for the determination phenol content of A. barbata samples. Results are mean± SD of three measurements Thrombolytic activity.
| Concentration of Sample solution (µg/ml) | Mass of the extract per ml in gm | Absorbance | Gallic acid equivalent c (mg/ml) |
TPC as GAE (A) | Mean ±STD |
| 400 | 0.0004 | 1.678 | 0.2221 | 277.397 | 277.397±0.419 |
| 400 | 0.0004 | 1.675 | 0.2215 | 276.884 | |
| 400 | 0.0004 | 1.681 | 0.2223 | 277.911 |
The assessed results for the thrombolytic property of the plant extract are presented in Table 05, 06 & Figure 04. In the thrombolytic activity test, the methanolic extract of A.barbarata demonstrated 35.2972±3.9196% lysis of blood clot while 75.577±0.489% for the positive control (streptokinase-SK) as well as 7.667± 0.230% lysis were acquired for negative control(sterile distilled water).
Table 5: Thrombolytic Activity of A. barbata. Results are mean± SD of three measurements.
| Sl. Volunteer | Empty Weight of
tube (gm) |
Weight of tube with
clot (gm) |
Weight of Clot
(gm) |
Weight of Tube with
clot after lysis (gm) |
Weight of Lysis
(gm) |
% of clot
Lysis |
Mean% of clot Lysis ±SD |
| A | B | C=B-A | D | E=B-D | 35.2972±3.9196 | ||
| 01 | 0.8256 | 1.3211 | 0.4955 | 1.1321 | 0.1890 | 38.1433 | |
| 02 | 0.8197 | 1.0285 | 0.2088 | 0.9631 | 0.0654 | 31.3218 | |
| 03 | 0.8176 | 1.1321 | 0.3145 | 1.0285 | 0.1036 | 32.9412 | |
| 04 | 0.8235 | 1.2132 | 0.3897 | 1.1034 | 0.1098 | 28.1755 | |
| 05 | 0.8207 | 1.2662 | 0.4455 | 1.1087 | 0.1575 | 35.3535 | |
| 06 | 0.8023 | 1.2281 | 0.4258 | 1.0928 | 0.1353 | 31.7755 | |
| 07 | 0.8138 | 1.1284 | 0.3146 | 1.0054 | 0.1230 | 39.0973 | |
| 08 | 0.8311 | 1.0572 | 0.2261 | 0.9657 | 0.0915 | 40.4688 | |
| 09 | 0.7987 | 1.1243 | 0.3256 | 1.0071 | 0.1172 | 35.9951 | |
| 10 | 0.8138 | 1.3201 | 0.5063 | 1.1191 | 0.2010 | 39.6998 |
Table 6: Clot lysis by water and Ampelocissus barbata compared with streptokinase. Results are mean± SD of three measurements.
| Treatment | % clot lysis ±SD | P value when compared with negative control |
| Water | 7.667± 0.230 | |
| Ampelocissus barbata | 35.2972±3.9196 | P < 0.05 |
| Streptokinase | 75.577±0.489 | P < 0.05 |
 |
Figure 4: (A) Clot lysis by water and Ampelocissus barbata compared with streptokinase (B) Significance of clot lysis. Results are mean± SD of three measurements. |
Phytochemical analysis
The phytochemical screening indicates qualitative presence of carbohydrate, alkaloid, tannins, steroids , saponins and flavonoids (Table 07).
Table 7: Phytochemical elements recognised in the extracts of Ampelocissus barbata.
| Phytochemical class | Observation |
| Alkaloids | +++ |
| Triterpenoids and Steroids | – |
| Carbohydrates | – |
| Tannins | + |
| Flavonoids | ++ |
| Saponins | + |
| +++ = very prominent; ++ = moderate; += Minor ; – = Absent
|
|
+++ = very prominent; ++ = moderate; += Minor ; – = Absent
Discussion
DPPH Radical Scavenging Assay
The free radical scavenging activity was evaluated by DPPH where ascorbic acid was used as the reference standard. The assay is carried out to find out the potential antioxidant activity of plant extract applying DPPH (2, 2-diphenyl-1-picrylhydrazyl) as a radical donor. In vitro free radical scavenging activity assay of the methanolic extract for A. barbata (stem) demonstrated the presence of prospective antioxidant activity. The extract’s efficiency in scavenging DPPH free radical with respect to a standard depicts the significant potential of the extract as a natural antioxidant 41.
Reducing power capacity assay reveals the compound’s reducing capacity as an important marker of its probable antioxidant activity. The reducing power of the plant extract components might serve as a significant indicator of its potential antioxidant activity 42. Previous studies found a direct correlation has observed between the reducing power of certain plant extracts and antioxidant activities.
Nevertheless, there are several types of mechanisms by which the antioxidants exert their activity, such as radical scavenging, prevention of continued hydrogen abstraction & chain initiation, binding of transition metal ion catalysts, reductive capacity, and decomposition of peroxides 43,44.
For a compound, the value of reducing capacity may be an important index of its significant antioxidant activity 45 as the compounds with reducing power are active electron donors and consequently function as primary and secondary antioxidants.
Different studies have been indicated that the reducing properties of the compound are due to the presence of reductants 46, and the antioxidant activity is obtained by breaking the free radical chain through the donation of the hydrogen 47.
As a class of antioxidants, the phenolic compounds act as free radical scavengers. Phenols are one of the most important plant constituents because of their scavenging ability due to their hydroxyl group 48. The prominent amount of GAE of this plant indicates the high antioxidant profile of this plant. The presence of hydroxyl groups confers scavenging ability to the phenolic compounds, ultimately making them important plant constituents 49. In general, the phenolic compounds are treated as solid chain-breaking antioxidants 50. However, the connection between the antioxidant activity and phenolic content has been reported by many authors 51,52, indicating that the antioxidative action is attributed directly to the phenolic compounds 53.
As compared to the lysis percentage of SK and water, the thrombolytic activity of A.barbarata was incredibly significant. The effective decrease in the percentage of fat of clot by the extract solution is indicative of promising thrombolytic potential. The plant extracts can be used for the development of anti-thrombotic agents for the healing of related cardiovascular diseases due to their promising thrombolytic activity.
Conclusion
From the above discussion, it can be concluded that the methanolic extract of the plant has DPPH radical scavenging, reducing power and antioxidant activity as compared to standard ascorbic acid. Furthermore, significant thrombolytic activity was also demonstrated with respect to SK. Phenolic compounds are present in plants, have antioxidant activity due to their redox properties, and therefore play a vital role in counterbalancing the free radicals 54,55.
With regards to reducing capacity, higher reducing powers might be attributed to higher amounts of total phenolic and flavonoid, and the reducing power of a compound may reflect its antioxidant potential 56.
However, this result shows the plant A.barbarata has appreciable thrombolytic activity and may become a useful thrombolytic agent with respect to further processing, furnishing a sound cardiovascular system57.
Further comprehensive pharmacological and phytochemical study for the isolation and characterization of the specific compound is required to get a more potent agent with significant activity. Since the polyphenol compounds as well as other components with potent antioxidant activity are not known, thus advanced level of work should be performed for the isolation and identification of the antioxidant components in A. barbarata.
Acknowledgement
We are grateful to the Department of Pharmacy, International Islamic University Chittagong, and the Department of Pharmacy, Northern University Bangladesh provided the facilities for the accomplishment of the work.
Conflict of Interest
All authors declare no conflict of interest.
Funding Sources
No. (Self-funded by the authors)
References
- Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K. Methods for testing antioxidant activity. Analyst. 2002;127(1):183-98.
CrossRef - Gutteridge John MC, Halliwell, B. Oxidative stress. In: Antioxidants in Nutrition, Health and Disease, Illustrated reprinted. United Kingdom, UK: Oxford University Press. 1994, p.90- 102.
- Maxwell SR. Prospects for the use of antioxidant therapies. Drugs. 1995;49(3):345-61.
CrossRef - Sies H. Oxidative stress: Introduction. In: Sies H. (ed.) Oxidative Stress: Oxidants and Antioxidants. Academic Press; London: 1991. p. xv–xxii
- Winrow VR, Winyard PG, Morris CJ, Blake DR. Free radicals in inflammation: second messengers and mediators of tissue destruction. Br Med Bull. 1993;49(3):506-22.
CrossRef - Bauer V, Sotníková R, Machová J, Mátyás S, Pucovský V, Stefek M. Reactive oxygen species induced smooth muscle responses in the intestine, vessels and airways and the effect of antioxidants. Life Sci. 1999;65(18-19):1909-17.
CrossRef - Halliwell B, Gutteridge JM. Free radicals in biology and medicine. 2nd ed. United Kingdom, UK: Clarendon Press, Oxford;1989
- Bae GU, Seo DW, Kwon HK, Lee HY, Hong S, Lee Z-W, et al. Hydrogen peroxide activates p70(S6k) signaling pathway. J Biol Chem. 1999;274(46):32596-602.
CrossRef - Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008 ;359(9):938-49.
CrossRef - Islam MS (ed.). Thrombosis and Embolism: from Research to Clinical Practice.. Vol.1, Switzerland: Springer International Publishing, 2017.
CrossRef - Chowdhury TA, Kamal AM, Chowdhury KA, Jahan A, Hossain MS, Mamur A, et al. Cytotoxic & thrombolytic activity of methanolic extract of Macaranga denticulata Bark. Pharma Innovation. 2015 ;4(5): 36-39.
- Laurence DR, Bennett PN. Clinical Pharmacology.7th ed. New York, NY, USA: Churchill Livingstone an affiliate of Elsevier Inc. 1992.
- Collen D. Coronary thrombolysis: streptokinase or recombinant tissue-type plasminogen activator? Ann Intern Med. 1990 ;112(7):529-38.
CrossRef - Mucklow JC. Thrombolytic treatment. Streptokinase is more economical than alteplase. BMJ. 1995 ;311(7018):1506.
CrossRef - Rouf SA, Moo-Young M, Chisti Y. Tissue-type plasminogen activator: characteristics, applications and production technology. Biotechnol Adv. 1996;14(3):239-66.
CrossRef - Jennings K. Antibodies to streptokinase. BMJ. 1996;312(7028):393-4.
CrossRef - Ramjan A, Hossain M, Runa JF, Md H, Mahmodul I. Evaluation of thrombolytic potential of three medicinal plants available in Bangladesh, as a potent source of thrombolytic compounds. Avicenna J Phytomed. 2014;4(6):430-6.
- Samuelsson G. Drugs of Natural Origin: A Textbook of Pharmacognosy, 5th ed. Swedish Pharmaceutical Press, Stockholm. Taylor & Francis.2004.
- Ali M. Textbook of pharmacognosy. 2nd ed. CBS Publishers & Distributors, Darya Ganj, New Delhi. 1998.
- Harborne JB. Methods of plant analysis. In Phytochemical methods. 2nd ed. Springer, Dordrecht. 1984. pp. 1-36.
CrossRef - Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005;4(7):685-8.
CrossRef - Houghton PJ. The role of plants in traditional medicine and current therapy. J Altern Complement Med. 1995;1(2):131-43.
CrossRef - Sikri N, Bardia A. A history of streptokinase use in acute myocardial infarction. Tex Heart Inst J. 2007;34(3):318-27.
- Evans WC . Trease and Evans Pharmacognosy. 15th ed. W.B Sauders Company Ltd, London. 2002.
- Roxburgh W. Flora Indica Or Descriptions of Indian Plants. William Carey (ed.) (Vol. 1). Mission Press. Serampore, 1820. p. 478.
- Wen J. Vitaceae. In: Kubitzki K. (ed.) The families and genera of vascular plants. Vol. 9. Springer-Verlag, Berlin. 2007. p. 466 – 478.
- The Global Biodiversity Information Facility (GBIF) (2020): Catalogue of Life;[ page last accessed 14 December , 2021] Available from https://www.gbif.org/species/4054232
- Flora of Bangladesh: Ministry of Environment & Forest; Survey of Vascular Flora of Chittagong and the Chittagong Hill Tracts Project; Bangladesh National Herbarium; [Page last accessed on 14 December, 2021] Available from http://bnh-flora.gov.bd/species-description/?id=2001
- Brand-Williams W, Cuvelier ME, Berset CL. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 1995 ;28(1):25-30.
CrossRef - yaizu M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn J Nutr Diet.1986;44(6):307-315. Japanese
CrossRef - F.erreira ICFR, Baptista P, Vilas-Boas M, Barros L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: individual cap and stipe activity. Food Chem. 2007;100(4):1511–1516.
CrossRef - Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16(3):144-58.
- Prasad S, Kashyap RS, Deopujari JY, Purohit HJ, Taori GM, Daginawala HF. Effect of Fagonia Arabica (Dhamasa) on in vitro thrombolysis. BMC Complement Altern Med. 2007;7(1):1-6.
CrossRef - Kokate, C.K. Practical Pharmacognosy. 4th ed. Vallabh Prakashan, Delhi:1994. pp 107-111.
- Trease and Evans . In : Pharmacognosy, 10th ed, Balliere Timdal, London, 1972.pp. 107, 378.
- Trease, G.E. And Evans, W.C. Drugs Of Biological Origin. Pharmacognosy, 12th ed., Balliere Tindall, United Kingdom.1983. pp.309-540.
- Sofowora, A. Medicinal Plants and Traditional Medicinal in Africa. 2nd ed. Sunshine House, Ibadan, Nigeria: Spectrum Books Ltd; Screening Plants for Bioactive Agents. pp. 1993. 134-156.
- Kokate CK, Purohit AP, and Gokhal SB . Pharmacognosy. vol. 35, Nirali Prakashan, Pune, India:2006. pp. 133–525.
- Harborne JB . Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. 3rd ed. New York, NY, USA. Chapman & Hall. 1998
- Gul R, Jan SU, Faridullah S, Sherani S, Jahan N. Preliminary phytochemical screening, quantitative analysis of alkaloids, and antioxidant activity of crude plant extracts from Ephedra intermedia indigenous to Balochistan. ScientificWorldJournal. 2017;2017:5873648.
CrossRef - Gülçın İ, Oktay M, Kıreçcı E, Küfrevıoǧlu Öİ. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food chem. 2003;83(3):371-82.
CrossRef - Diplock AT. Will the ‘good fairies’ please prove to us that vitamin E lessens human degenerative disease? Free Radic Res. 1997 ;27(5):511-32.
CrossRef - Yıldırım A, Oktay M, Bilaloglu V. The antioxidant activities of the leaves of Cydonia vulgaris. Tr. J. Medical Sci. 2001;31(1): 23-27.
- Meir S, Kanner J, Akiri B, Philosoph-Hadas S. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J. Agric. Food Chem. 1995 ;43(7):1813-9.
CrossRef - Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995 ;43(1):27-32.
CrossRef - Meir S, Kanner J, Akiri B, Philosoph-Hadas S. Determination and involvement of aqueous reducing compounds in oxidative defense systems of various senescing leaves. J. Agric. Food Chem. 1995 ;43(7):1813-1819.
CrossRef - Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem.1992;40(6):945-8.
CrossRef - Shahidi F, Wanasundara PK. Phenolic antioxidants. Crit Rev Food Sci Nutr. 1992;32(1):67-103.
CrossRef - Hatano T, Edamatsu R, Hiramatsu M, MORI A, Fujita Y, Yasuhara T, et al. Effects of the interaction of tannins with co-existing substances. VI. effects of tannins and related polyphenols on superoxide anion radical, and on 1, 1-Diphenyl-2-picrylhydrazyl radical. Chem. Pharm. Bull(Tokyo). 1989 25;37(8):2016-21.
CrossRef - Amir M, Mujeeb M, Khan A, Ashraf K, Sharma D, Aqil M. Phytochemical analysis and in vitro antioxidant activity of Uncaria gambir. Int. J. Green Pharm. 2012;6(1). 67-72
CrossRef - Yen GC, Duh PD, Tsai CL. Relationship between antioxidant activity and maturity of peanut hulls. J. Agric. Food Chem. 1993;41(1):67-70.
CrossRef - Yang JH, Lin HC, Mau JL. Antioxidant properties of several commercial mushrooms. Food chem. 2002;77(2):229-35.
CrossRef - Duh PD, Tu YY, Yen GC. Antioxidant activity of water extract of harng jyur (chrysanthemum morifolium Ramat). Lebenson Wiss Technol. 1999;32(5):269–77.
CrossRef - Osawa T. Novel Natural Antioxidants for Utilization in Food and Biological Systems. Tokyo. Japan: Japan Scientific Scoieties Press; 1994. pp. 241–251
CrossRef - Siriwardhana N, Lee KW, Jeon YJ, Kim SH, Haw JW. Antioxidant activity of Hizikia fusiformis on reactive oxygen species scavenging and lipid peroxidation inhibition. Food Sci Tech Int. 2003;9(5):339-46.
CrossRef - Shon MY, Kim TH, Sung NJ. Antioxidants and free radical scavenging activity of Phellinus baumii (Phellinus of Hymenochaetaceae) extracts. Food chem. 2003;82(4):593-7.
CrossRef - Doggen CJ, Smith NL, Lemaitre RN, Heckbert SR, Rosendaal FR, Psaty BM. Serum lipid levels and the risk of venous thrombosis. Arterioscler Thromb Vasc Biol. 2004;24(10):1970-5.
CrossRef - Katano K, Honda K, Suzuki N. Integration between ROS regulatory systems and other signals in the regulation of various types of heat responses in plants. Int J Mol Sci. 2018;19(11):3370.
CrossRef - Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev. 2016 ;2016:1245049.
CrossRef - Khandokar L, Mim SR, Rana RB. Phytochemical Screening and In vivo Analgesic Activity of Ampelocissus barbata (Wall.) Planch. Banglad Pharm J.2021;24(2):117–24.
CrossRef - Uprety Y, Poudel RC, Gurung J, Chettri N, Chaudhary RP. Traditional use and management of NTFPs in Kangchenjunga Landscape: implications for conservation and livelihoods. J Ethnobiol Ethnomed. 2016 ;12(1):19.
CrossRef