Manuscript accepted on :21-04-2022
Published online on: 27-05-2022
Plagiarism Check: Yes
Reviewed by: Dr. Feng Xue
Second Review by: Dr. Umargani Jamal Mohamed
Final Approval by: Dr. H Fai Poon
R Sumitha1, M Dharshana1, N. Banu2* and S Vijayalakshmi3
and S Vijayalakshmi3
1Department of Biomedical Sciences, Sri Ramachandra Institute of Higher Education and Research, Porur, Chennai- 600116, India.
2Department of Botany, Bharathi Women's College, Prakasam Salai, Broadway, Chennai - 600108, Tamil Nadu, India.
3Department of Biotechnology, Vels Institute of Science technology and Advanced Studies (VISTAS) Pallavaram, Chennai - 600117, Tamil Nadu, India
Corresponding Author E-mail: sumithamadhu79@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2427
Abstract
Natural biomedical metabolites procured from marine sources have been the sole source of recent research. The antimicrobial resistance to human infections has made a mounting need for novel natural antibiotics. Much explored marine invertebrates largely the echinoderms (sea stars) tend to pose a natural innate mechanism to safeguard them against predators. The sea star secretes antimicrobial peptides which are naturally occurring secondary metabolites that possess a broad spectrum of antimicrobial susceptibility against bacteria, fungi, and viruses. The present study centers on the aspects of isolation and evaluation of active bioactive metabolite from the echinoderm Stellaster equestris from the Chennai coast. The whole body was utilized for the extraction using non-polar to polar solvents. The active crude extracts were investigated by qualitative assay for their chemical composition and were purified by column chromatography. The purity of the compound was further analyzed and checked for purity and quantified by the High-pressure liquid chromatography. The defined concentration of the isolated and purified compound from sea star Stellaster equestris (50,100, 150, 200, 250 and 300µg/ml) were subjected for antimicrobial sensitivity by well diffusion method and Tube dilution method. The outcome of the present study indicated the active crude extract from the sea star Stellaster equestris was rich in sterols. The evaluation of antimicrobial susceptibility by tube dilution and well diffusion assay indicated that the isolated purified compound from the sea star Stellaster equestris was reported to be evident for all the above-mentioned concentrations by a marked zone of clearance. A dose-dependent increase was observed in the tube dilution method. Therefore compounds possess antimicrobial activity and can be further subjected for developing the compound as a potent antimicrobial drug.
Keywords
Antimicrobial Resistance; Sea stars; Stellaster equestris; Well diffusion method and Tube dilution method
Download this article as:| Copy the following to cite this article: Sumitha R, Dharshana M, Banu N, Vijayalakshmi S. In vitro Antimicrobial Evaluation of an Isolated Compound from Sea Star Stellaster equisteris. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Sumitha R, Dharshana M, Banu N, Vijayalakshmi S. In vitro Antimicrobial Evaluation of an Isolated Compound from Sea Star Stellaster equisteris. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3MUWPpG |
Introduction
The rising global crisis in the field of medicine is the microbial resistance towards synthetic and semisynthetic drugs. Antimicrobial resistance has rapidly endangered the efficacy of generic medicines since the pharmaceutical industry introduced many newer synthetic drugs to solve the resistance problem; there is always a search for newer novel natural antibiotics from natural sources. On assessment with the natural products attained from terrestrial sources, marine origin equally produces massive resources of novel compounds with possible pharmaceutical importance. Marine echinoderms namely sea star generate an array of compounds with various pharmacological activities this is generated mainly due to their survival instinct as they live in a very intimidating environment and hence need to be competitive for their survival. Sea stars are intertidal squandering echinoderms which are rich sources of bioactive metabolites. These metabolites secreted by the sea stars are small molecular weight proteins that are vital effector molecules synthesized and secreted as secondary metabolite protects them from predators. Sea stars represent an incomparable resource of polar steroids of immense structural diversity, showing a range of biological activities. The cell membrane of the sea star is composed of polar steroids which is the integral basic component of the sea star. The major source of compounds abundantly found in sea stars is steroidal components namely saponins, asterosaponins, and astropectenol. These compounds isolated from the marine sea stars have good recourses to fight against deadly diseases in humans exhibiting cytotoxic, hemolytic, antiviral, antifungal, and antimicrobial activities [1,2,3]. The present study focuses on the antimicrobial metabolite from the isolated partially purified sterol compound of sea star Stellestar equisteris.
Materials and Method
Sample collection Extraction and active extract identification
The sea star Stellaster equestris samples were collected from the coastal fishing port of north Chennai (Latitude 13º 06’N, Longitude 80º 18’E). Collected samples were transported to the laboratory by freezer storage thermal resistant box aseptically. The samples were further processed for whole body extraction by cold percolation method [3]. The extracts were further subjected to check the IC50 value against PA1 cell line the active extracts were subjected for qualitative analysis.
Qualitative analysis of the active crude extracts
The crude active extract was subjected to check for the presence of organic compounds by qualitative analysis. The evaluation was done with the active crude extract was tested for the presence of phenol, reducing sugars, flavones, glycosides, saponins, alkaloids, anthraquinone, quinines, proteins amino acids, tannins and sterols were performed [4].
Purification of Active Extract
The active crude extracts were subjected for purification by column chromatography before which the solvent choice was crucial for separation of components. Hence the selection of solvent system was determined by Thin-layer chromatography [5]. The ideal solvent system of separation was subjected to further isolation by column chromatography. The purity of the active guided fractions obtained from column chromatography was verified. After identifying the active components from the guided fractions, the fractions were pooled based on the further confirmation by Thin- layer chromatography which was followed by further purification by sub-column chromatography [6].
High-Performance Liquid Chromatography (HPLC)
High- performance liquid Chromatography is done to extricate organic compounds. The automated HPLC machine is flexible enough to enhance reproducibility and quantification of the purified compounds. The purified marine sample 100 mg was subjected for further analysis using PROMINENCE SHIMADZU High-Performance Liquid Chromatograph in Tamil Nādu Test house facility Pvt Ltd Chennai. All the solvents used for HPLC analysis were analytical grade obtained from Merck, India.
Antimicrobial Susceptibility
Antibacterial and antifungal activity of the sea star Stelletar equestris purified sample (1mg/ml) was analyzed by well diffusion method [7]. Antibacterial and fungal evaluation was performed against five medically important bacterial species and two medically important fungal species. For antibacterial activity, sterile Muller Hinton agar, five selected bacterial strains were used viz., Escherichia coli ATCC 12435, Staphylococcus aureus ATCC 6538, Streptococcus pneumonia., ATCC 700669, Pseudomonas aeruginosa., ATCC 9027, Klebsiella pneumonia., ATCC 700721 and two fungal organisms namely Candia albicans ATCC 10231 and Aspergillus niger ATCC 16888 were procured from Himedia. These strains were tested against the pure compound from the sea star Stellestar equisteris. Commercially available antibiotics for antibacterial were (Ciprofloxacin, Levofloxacin, and Amoxicillin) and antifungal agents as (Itraconazole) were used control triplicates were performed for each organism.
Tube dilution method
Antibacterial and antifungal activity of the sea star Stelletar equestris purified sample was performed against five medically important bacterial species and two medically important fungal species by broth dilution method. This quantitative method was done to check the minimum inhibitory concentration of the compound against the medicinally important bacterial and fungal pathogen. Defined concentrations of the purified sample from sea star Stelletar equestris (50, 100, 150, 200, 250,300 µg/ ml) was determined. The bacterial strains were inoculated into Muller Hinton broth and the fungal in the yeast extract mannitol agar. A total of 0.5 ml of each concentration was transferred to test tube containing 2 ml of Muller Hinton broth and yeast extract mannitol broth aseptically all of it was done in triplicates. The test organism adjusted to a concentration of 105 cells/ml was then introduced. A set of test tubes containing broth alone was used as control. The least concentration of inhibition was checked by peak points determined using UV- Visible Spectroscopy at a range of 570 nm . Triplicates were performed for each organism.
Agar well diffusion method
Muller Hinton agar and yeast extract mannitol agar was prepared, autoclaved aseptically transferred to sterile Petri plates. The stock solution of the purified samples was prepared for further process. The cultures of the medicinally important bacterial organisms were spread over the Mueller-Hinton agar medium and Yeast extract mannitol agar with a sterile cotton swab. Wells were cut using a cork borer for 8mm. 100 µl of defined concentrations from the of the purified sample from sea star Stellaster equestris (50, 100, 150, 200, 250,300 µg/ ml) was added to the wells respectively. Commercially available antibiotics were taken as control (1mg/ml) triplicates were performed for each organism. Plates were kept for incubation at 37º C for the bacterial culture plates for 24 hours and 25º C for fungal culture plates for 7- 14 days. After, incubation the plates were observed for the zone of clearance against the tested bacterial and the fungal species. Triplicates were done for all the pathogens.
Results and Discussion
Sample collection Extraction and active extract identification
The sea star samples were collected from a coastal fishing port in north Chennai (Latitude 13º 06’N, Longitude 80º 18’E). A part of the collected sample was sent for species identification to Marine Biology Regional Center, Zoological Survey of India, Chennai. The collected sample was identified as Stellaster equestris. The whole body was subjected to extraction with solvents namely (hexane, dichloromethane, chloroform, dichloromethane, and methanol). These extracts were further subjected for cytotoxicity against PA1 cell lines by MTT assay, AO/PI staining, and DNA fragmentation assay. Chloroform extract showed good inhibitory effect against the PA1 cell lines.
Qualitative analysis of the active crude extracts
The dried active extracts were subjected to the presence of potential organic compounds by performing a preliminary qualitative screening by using the following standard methods. The results were tabulated as shown in (Table 1)
Table 1: Qualitative Analysis of Crude Extract.
| S. No | Qualitative test | Observation | Present/Absent |
| 1. | Phenolic compound- Fecl3 | No Bluish black was observed | Absent |
| 2. | Reducing sugar –
Fehling’s test |
The red color was observed | Present |
| 3. | Flavones – Lead acetate test | No yellow colorprecipitate | Absent |
| 4. | Glycosides – Anthrone test | dark green color | Present |
| 5. | Saponins | copious latter formation | Present |
| 6. | Alkaloids – Wagner’s test | Reddish – brown precipitate | Absent |
| 7. | Anthraquinone – Borntrager’s test | No pink, red or violet colour | Absent |
| 8. | Quinone | The Red color (color)was formed | Absent |
| 9. | Protein – Biuret test
|
No blue color was observed | Absent
|
| 10. | Tannin – Lead acetate test | No orange – red precipitate was observed | Absent |
| 11. | Test for sterols –
Libermann-(B) Burchard Test |
Bluish – green indicated | Present |
Qualitative analysis of the crude chloroform extract of Stellaster equestris contained the presence of reducing sugar, glycosides, saponins, and sterols.
Purification of Active Extract
TLC was used to separate the various compounds present in the active crude extract of Stellaster equestris using an ideal solvent system of Hexane: Ethyl acetate (7:3, v/v) was performed which indicated many numbers of bands with different Rf values ranging from (0.3 – 0.7) which was ideal separation. The column chromatography was performed based on the solvent system and the guided fractions were further subjected for sub-column chromatography. The isolated and purified sample was documented was further subjected for characterization.
High- Performance Liquid Chromatography (HPLC)
The purity of the fractioned sample was checked and quantified by the PROMINENCE SHIMADZU High-Performance Liquid Chromatograph in Tamil Nādu Test house facility Pvt Ltd Chennai. The sharp peak as shown in (Fig 1) was observed for the presence of sterol with retention time of 7.999 and a purity of 98.5% of sterol content present in the sample as shown in (Table 2) which was subjected respectively.
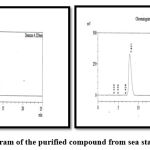 |
Figure 1: Chromatogram of the purified compound from sea star Stellestar equisteris |
Table 2: Peak Table Chromatogram of the Purified Compound from Stellestar equisteris
| Peak No | Name | Retention time | Area % |
| 1 | – | 3.560 | 0.022 |
| 2 | – | 4.580 | 0.018 |
| 3 | – | 6.482 | 0.345 |
| 4 | Sterol | 7.999 | 99.575 |
| 5 | – | 13.516 | 0.040 |
| total | 100 |
Antimicrobial Susceptibility
Tube dilution method
The tube dilution method for the five pathogenic bacteria and two pathogenic fungal strains against the defined concentration of the compound from sea star Stellaster equestris was recorded. The clearance of growth in each tube with the defined concentration of the compound was compared with the positive control which was incorporated with a commercially available antimicrobial agent. The least concentration of inhibition was checked by peak point’s determined using UV- Visible Spectroscopy at a range of 570 nm. The minimum amount of the compound which completely inhibited the growth of bacteria and the fungi was recorded to be at 50 µg/ml for exposed five pathogenic bacteria and two pathogenic fungal strains.
Agar well diffusion method
Antibacterial activities of a purified sample of sea star Stellaster equestris showed the zone of inhibition as shown in (Table 3) and (Fig.2)
Table 3: Purified Sample of Sea Star Stellestar equestris against Human Pathogen at various Concentrations and the zone of inhibition (mm) with mean and SD.
| S. No | Pathogens | Zone of the inhibition (mm)
(concentration µg/ml) |
Positive control (Antibiotic) | Negative control | |||||
| 50 | 100 | 150 | 200 | 250 | 300 | ||||
| 1. | E.coli | 19±0.15 | 21±0.05 | 24±0.26 | 26±0.05 | 29±0.17 | 30±0.11 | 35 ±0.1 | 0 |
| 2. | Klebsiella sp | 12±0.4 | 13±0.1 | 14±0.1 | 15±0.1 | 18±0.2 | 27±0.1 | 39 ±0.1 | 0 |
| 3. | Pseudomonas sp. | 9±0.1 | 12±0.1 | 14±0.28 | 16±0.26 | 19±0.1 | 21±0.25 | 43 ±0.05 | 0 |
| 4. | Streptococcus sp | 21±0.25 | 22±0.26 | 24±0.1 | 26±0.05 | 27±0.05 | 28±0.1 | 15±0.05 | 0 |
| 5. | Staphylococcus sp | 2±0.05 | 4±0.05 | 6±0.26 | 9±0.05 | 10±0.05 | 11±0.1 | 13±0.1 | 0 |
| 6. | Candida albicans | 3±0.15 | 7±0.25 | 8±0.43 | 10±0.17 | 11±0.1 | 12±0.15 | 3±0.15 | 0 |
| 7. | Aspergillus sp | 5±0.15 | 10±0.05 | 10±0.05 | 12±0.26 | 17±0.1 | 25±0.1 | 13±0.1 | 0 |
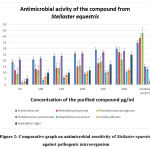 |
Figure 2: Comparative graph on antimicrobial sensitivity of Stellaster equestris against pathogenic microorganism. |
The defined concentration of the isolated compound from sea star Stellaster equestris (50, 100, 150, 200, 250, and 300µg/ml) were subjected for antimicrobial sensitivity by well diffusion method against five medically important bacterial species and two medically important fungal species. The zone of inhibition [(30±0.11) mm] for E.coli was good in comparison with the commercially used (Ciprofloxacin) antibiotics. The zone of inhibition [(27±0.10) mm] for Klebsiella sp was moderate in comparison with the commercially used (Levofloxacin) antibiotics.
The zone of inhibition [(21±0.25) mm] for Pseudomonas sp. was moderate in comparison with the commercially used (Ciprofloxacin) antibiotics. The maximum inhibition zone [(21±0.25) mm] for Streptococcus Sp. was observed which was most sensitive than the commercially used (Amoxicillin) antibiotics. The zone of inhibition [(12±0.15) mm] and for Staphylococcus sp was good in comparison with the commercially used (Amoxicillin) antibiotics.
The maximum inhibition zone [(21±0.25) mm] and [(25±0.1) mm] for Candida albicans and Aspergillus clavatus was observed which was most sensitive than the commercially used (Itraconazole) antifungal agent.
The marine echinoderms are rich resources of natural bioactive compounds that have prospective biomedical properties. The present study was to focus on the in vitro evaluation of the compound from sea star Stellaster equestris. Qualitative assessment of the crude metabolite from the active extract was considered effective in discovering bioactive profiles from plant or animal sources. Hence preliminary screening before the isolation of the active compound is done by two methods qualitative analysis which is used to classify the constituents and the quantitative method used to measure or determine the number of active constituents present. The bioactive constituents such as phenol, saponins, alkaloids, flavonoids, sterol, and tannins serve as the secondary metabolites secreted during the defense mechanism of these invertebrates to protect themselves from the external sources [9].
In the present investigation different qualitative chemical tests were performed for ascertaining information of the extract for its chemical composition; the following tests for phenolic compound, flavones, glycosides, saponins, alkaloids, quinone, proteins, amino acids, tannin, and test for sterols were performed as shown in (Table 1) on active crude chloroform and methanol extracts of sea star Stellaster equestris. The metabolites present in the crude extracts showed positive results for reducing sugars, glycosides, saponins, and sterols and were reported negative for phenols, flavones, alkaloids, anthraquinone, proteins, amino acids, and tannins.
Previous studies on sea stars have established major metabolites namely steroidal glycosides, astero saponins, and polyhydroxylated steroids from marine sea stars [10]. Polyhydroxylated sterols are the predominant metabolite of the sea star which was being reported from sea star Tremaster novae caledoniae of New Caledonia, Mediterranean Sea star Coscinasterias tenuispina, Antarctic Sea star Acodontaster conspicuous, and Japanese sea star Asterina pectinifera [11]. Few studies have also been authenticated on the presence of saponins from sea star Asterias amurensis, from the pacific coast, Asterina pectinifera Japanese sea star Gulf of California sea star Patiria miniata [12, 13].
Subsequently, the present study correlates the presence of the glycosides, saponins, and sterols to be major metabolites isolated from the crude chloroform and methanolic extract of sea star Stellaster equestris. Since both the extract showed similar results and methanol is considered to be the universal solvent that even non-polar molecules can be picked up in methanol extraction hence chloroform extract was considered for further purification.
The extracted active crude chloroform extract of the sea star Stellaster equestris was then purified by column chromatography with a different solvent system based on the results from thin layer chromatography as mentioned in the results. Hexane: Ethyl acetate (7:3, v/v) was marked to be an ideal solvent system which showed a better separation than the other solvent system ratio. Hence the solvent system was used as the mobile phase for the separation of compound from the active chloroform crude extract. The active guided fractions eluted from the column chromatography presented a promising yield of compound. The present study is promoted by another investigation done on active crude ethanolic, methanolic, and bioactive fractioned extracts from sea star Culcita novaeguineae, Ctenodiscus crispatus and Acanthaster planci which were purified by column and sub-column chromatography resulting in the elucidation of newer potent compounds like sterols, asterosaponins, and steroidal biglycoside indicating promising yield of bioactive compounds from the sea stars. [14,15,16].
The elucidated active fractioned sample from the sub column chromatography was further checked for purity and quantified by the High- Performance Liquid Chromatography. The sharp peak as indicated in (Fig 1) was observed which quantified the presence of sterol with the retention time of 7.999 and a purity of 98.5% of sterol content present in the sample which reported the purity content of sterol present in the compound respectively. Since there were abundant studies related to the extraction array of sea stars using the various solvent systems for the elution of active compounds. The present study is the first report on the extraction, isolation of bioactive metabolite from the active crude extract of chloroform from less studied sea star Stellaster equestris.
Screening for an antimicrobial agent is being lead research in the field of medicine. With this aim, the present study was focused on isolating and identifying a potent antimicrobial agent from the isolated compound of sea star Stellaster equestris. The defined concentration of the isolated compound from sea star Stellaster equestris (50, 100, 150, 200, 250, and 300 µg/ml) were subjected for antimicrobial sensitivity by well diffusion and tube dilution method against five medically important bacterial species and two medically important fungal species. Five selected human pathogenic bacterial strains were tested against the purified compound of Stellaster equestris in which three-gram negative bacilli viz., Escherichia coli ATCC 12435, Pseudomonas aeruginosa ATCC 9027, Klebsiella pneumonia ATCC 70072, and two-gram positive cocci Staphylococcus aureus ATCC 6538, Streptococcus sp., ATCC 31577, with commercially available antibiotics (Ciprofloxacin, Levofloxacin, and Amoxicillin) as positive control were evaluated. For antifungal activity, sterile yeast extract mannitol agar was used as the medium of choice, and two fungal organisms were tested against the purified sample (Candia albicans ATCC 10231 & Aspergillus niger ATCC 16888) with commercially available antifungal agents (Itraconazole) as a positive control.
The compound showed promising activity against the gram- positive bacterial species and the fungal pathogens as shown in (Fig. 2) than the gram- negative bacteria which was reported to be evident for all the above-mentioned concentrations by a marked zone of clearance. Dose-dependent increase was observed in the agar diffusion method. The tube dilution method indicated the minimum inhibitory concentration recorded to be at 50 µg/ml for exposed five pathogenic bacteria and two pathogenic fungal strains hence the minimum inhibitory concentration of the compound that completely inhibits the growth of microorganisms was comparable to the standard antibiotics used as the positive control.
Antimicrobial drugs have been isolated, characterized, and studied extensively over the past three decades, particularly from the vast domain of marine origin. Significant data has been subjected to the compounds isolated and evaluated from the marine invertebrates. The competition for survival and the environmental pressure has made this sea anemone produce secondary metabolites which target the microbes that attack them. A survey of compounds isolated from marine invertebrates namely sea stars has often showed the greatest percentage of active samples that posse’s antimicrobial activity.
Mostly the bacterial and fungal pathogens are the predominant source of microbes that act like the predators of marine invertebrates. This is mainly due to their cell walls. The bacteria are rich in peptidoglycan and the fungus is predominant with the ergosterol which is highly adapted for the high salinity environment [17]. This enhances them to invade the surfaces of the marine invertebrates. To evade and protect an innate immunity is being naturally present in the marine organism those are the antimicrobial peptides.
Previous studies have reported the antibacterial activity on compounds like polyhydroxylated steroid glycosides, polyhydroxylated sterol, and disulfate sterol from sea stars and brittle stars possessing activity against the gram-negative bacteria and were reported to be inactive against gram-positive bacterium [18]. Few other studies have also been reported on antimicrobial activity against the crude methanol and ethyl acetate extract of sea star Astropecten indicus, brittle sea star Ophiocnemis marmorata and on the purified coelomic fluid of sea star Protoreaster linckii. Dose dependant increase of antimicrobial activity was reported from these extracts and the coelomic fluid of the starfish against human bacterial, and fungal pathogens [19, 20, 21]. Hence these studies reported substantiate the present study on the compound isolated from the sea star Stellaster equestris which has been reported to show promising antimicrobial activity in a dose-dependent manner.
Conclusion
In conclusion, the present study on In vitro susceptibility evaluation of isolated compound from sea star Stellaster equestris correlates the finding that the extracted compound possesses antimicrobial activity and hence can be further subjected for developing the compound as a potent antimicrobial drug.
Acknowledement
We would like to thank the Department of Biomedical Sciences, Sri Ramachandra Institute of Higher Education and Research for the necessities and facilities provided for the conduct of the study. We take this opportunity to thank the Department of Microbiology, Sri Ramachandra Institute of Higher Education and Technology (DU), Porur, Chennai for providing us with the bacterial strains.
Authors’ Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that there is no conflict of interest. All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding Sources
There is no funding source.
Reference
- Guang C, Xiang Z, Hai F.T, Yun Z and Xin . Asterosaponin 1, a cytostatic compound from the starfish Culcita novaeguineae, functions by inducing apoptosis in human glioblastoma U87MG cells. J. Neur.Oncol., 2006; 79:235 -241.
CrossRef - Tran H.Q, Lee D, Han S, Kim C and Yim J.H. Steroids from the cold water starfish Ctenodiscus crispatus with cytotoxic and apoptotic effects on human hepatocellular carcinoma and glioblastoma. B. Korean. Chem. Soc., 2014;35:2335 – 2341.
CrossRef - Malyarenko T.V, Kicha A.A, Ivanchina N.V, Kalinovsky A.I and Popov R.S. Asterosaponins from the Far Eastern starfish Leptasterias ochotensis and their anticancer activity. Steroids., 2014; 87: 119 – 127.
CrossRef - Sumitha R, Banu N and Deepa Parvathi V. Novel natural products from marine sea stars. Curr. Tren. Biomed. Eng. Bio., 2017; 2: 555592 – 555595.
CrossRef - Prashant T, Bimlesh K, Mandeep K, Gurpreet PK and Harleen K et al. Phytochemical screening and Extraction: A Review. Inter. Pharma. Sciencia., 2011; 1: 98 – 106.
- Martson A. Thin-layer chromatography with biological detection in phytochemistry. J. Chromatography., 2011; 1218: 2676 – 2683.
CrossRef - Sumitha R, Deepa Parvathi V and Banu N. Effect of sterol isolate from echinoderm Stellaster equestris on zebrafish (Danio rerio). Inter. J. Pharma. Sci. Research., 2019; 10: 3234 – 3240.
- Reinheimer JA, Demkow MR and Candioti MC. Inhibition of coliform bacteria by lactic cultures. Australian. J. Dairy. Tech., 1990; 45: 5 – 9.
- Cowan, MM. Plant products as antimicrobial agents. Clin . Micro. Rev., 1999; 12: 564 – 582.
CrossRef - Maier, M.S. Biological activities of sulphated glycosides from echinoderms studies. Nat. Prod. Chem., 2008; 35: 311 – 354.
CrossRef - Dong G, Xuc T, Yanga B, Lina X, Zhoua X, Yanga X and Liu Y. Chemical constituents and bioactivities of star fish. Chem. Biodiv., 2011; 8: 740 – 791.
CrossRef - Raffaele R, Maria I, Luigi M, Yasukatsu O and Takeshi Yasumoto. Starfish saponins novel steroidal glycoside sulphates from the starfish Asterias amurensis. J. chem. Soc., 1988; 6: 1337 – 1347.
CrossRef - Valentina L, Vincenzo A, Claudio L, Manuela M and Mirella V. Bright spots in the darkness of cancer: A review of starfishes-derived compounds and their anti-tumor action. Mar. Drugs., 2019; 17: 1 – 30.
CrossRef - Tang H.F, Yi Y.H, Li L, Sun P, Zhang S.Q and Zhao Y.P. Asterosaponins from the starfish Culcita novaeguineae and their bioactivities. Fitoterapia., 2006;77: 28 – 34.
CrossRef - Tran H.Q, Lee D, Han S, Kim C and Yim J.H. Steroids from the cold water starfish Ctenodiscus crispatus with cytotoxic and apoptotic effects on human hepatocellular carcinoma and glioblastoma. Bull. Korean. Chem. Soc., 2014; 35: 2335 – 2341.
CrossRef - Kicha A.A, Ivanchina N.V, Huong T.T, Kalinovsky A.I and Dmitrenok P.S. Two new asterosaponins, archasterosides A and B, from the Vietnamese starfish Archaster typicus and their anticancer properties. Bio. Med. Chem. Lett., 2013; 20: 3826 – 3830.
CrossRef - Das S, Lyla P.S and Khan S.A. Marine microbial diversity and ecology: Importance and future perspectives. Curr. Sci., 2006; 90: 1325 – 1335.
- Andersson L, Bohlin L, Iorizzi M, Riccio R, Minale L and Moreno-López W. Biological activity of saponins and saponin-like compounds from starfish and brittle-stars. Toxicon., 1989; 27: 179 – 188.
CrossRef - Chamundeeswari K, Saranya S and Rajagopal S. Exploration of potential antimicrobial activity of sea star Astropecten indicus. J. App. Phar. Sci., 2012; 2: 125 -128.
CrossRef - Prabha D, Solimabi W, Tonima K and Lisette D.S. Screening of marine organism for antimicrobial activity against clinical pathogen. Indian. J. Geo. Mar. sci., 2011; 40: 338 – 346.
- Mayer AMS, Rodriguez AD, Berlinck RGS, and Hamann MT. Marine pharmacology in 2005–2006: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, antiinflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochem. Et. Bio. Acta., 2009; 5: 283 – 308.
CrossRef







