Manuscript accepted on :06-04-2022
Published online on: 09-05-2022
Plagiarism Check: Yes
Reviewed by: Dr. Yerbolat Iztleuov
Second Review by: Dr. Bhumika Brahmbhatt
Final Approval by: Dr. Ian James Martin
Shanthi P* , Sownthariya C
, Sownthariya C and Thiripura Sundari U
and Thiripura Sundari U
Department of Botany, Holy Cross College (Autonomous), Tiruchirappall, India.
Corresponding Author E-mail: dr.p.shanthiraj@gmail .com
DOI : https://dx.doi.org/10.13005/bpj/2437
Abstract
Medicinal plants are potential source of antimicrobial agents, used traditionally to treat various human microbial infections worldwide. The present study was aimed to determine the antibacterial efficacy of Melia azedarach Linn. leaf extracts against secondary bacterial pathogens of dermatophytosis such as Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus and to investigate the presence of phytocompounds through High Performance Thin Layer Chromatographic (HPTLC) method of the potential extract. The leaf extracts of the selected plants were tested for antibacterial potentiality using disc diffusion method and gentamicin (10µg/disc) was used as positive control. The results revealed that the positive control had more inhibitory effect than the solvent extracts used. Comparatively, acetone extract of M. azedarach leaves was more effective against two test pathogens, S. aureus (12.93±0.65 mm) at 150 µl/disc and P. aeruginosa (11.5±0.10 mm) at 100 µl/disc concentration with significant difference of p≤0.05 using one-way analysis of variance (ANOVA). The varying degree of extract concentrations has a greater influence in the inhibitory effect against test pathogens. The different Rf values, maximum percentage concentration, area percentage of polyvalent chemical constituents was recorded in HPTLC profiling of acetone leaf extract, where the maximum percentage concentration was found to be 14.07% at 0.09 Rf. The HPTLC studies has confirmed that the compounds present in the acetone extract might be responsible for the inhibitory effect against the bacterial pathogens and are more soluble in semi-polar solvent. Therefore, the present investigation forms the basis as preliminary study of antibacterial efficacy of M. azedarach leaf extracts and phytocompound HPTLC profiling of potential extract, which could be used for quality evaluation of compound and standardisation of drug in future work.
Keywords
Antibacterial; Dermatophytosis; High Performance Thin Layer Chromatography; Inhibitory effect; Melia azedarach; Phytocompounds;.
Download this article as:| Copy the following to cite this article: Shanthi P, Sownthariya C, Sundari U. T. HPTLC Profiling and Antibacterial Efficacy of Melia Azedarach Linn. Leaf Extracts Against Secondary Bacterial Pathogens of Dermatophytosis. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Shanthi P, Sownthariya C, Sundari U. T. HPTLC Profiling and Antibacterial Efficacy of Melia Azedarach Linn. Leaf Extracts Against Secondary Bacterial Pathogens of Dermatophytosis. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3vX7OZM |
Introduction
Dermatophytic infections are considered as global health problem. Dermatophytosis is an infectious condition caused by keratinophilic pathogenic fungi which belong to three major genera Trichophyton, Microsporum and Epidermophyton sps. It has a tendency of reoccurrence due to several reasons such as poor hygiene, over population, humid environmental conditions and invasion of opportunistic microbes on the infected part. Skin infection occurs when pathogenic micro-organisms (bacterial, fungal, viral and parasitic) penetrate the skin, spread and cause swelling, colour change, pain and discomfort. A rash is an area of swollen or irritated skin, primarily remain as symptom and then paves way for opportunistic microbes causing secondary bacterial infections1.
The major cause of skin infections is the occurrence of secondary bacterial skin infections which are common complications of primary dermatoses or dermatophtosis, primary non-bacterial skin infections, traumatic lesions, ulcers, cutaneous infestations. Aerobic and anaerobic, gram-negative and gram-positive organisms present in such secondary infections, include Staphylococcus aureus, S. epidermidis, Streptococcus sps., Escherichia coli, Enterobacter sps., Pseudomonas aeruginosa, Proteus sps, Peptostreptococcus sps., Clostridium sps., Eubacterium sps., Bacteroides sps., Porphyromonas sps., Fusobacterium sps., Candida sps. Local application of antibacterial agents remains as an important component of treatment whereas, infection may also persist as a result of resistance to antibiotic drugs. Thus, treatment of serious skin infections should include systemic antimicrobial therapy2.
Medicinal herbs with high therapeutic value are used to treat multitude of ailments and diseases. Plants synthesize abundant chemical compounds (phytochemicals) that possess pharmacological actions with medicinal properties widely used in traditional medicine, since pre-historic times. Phytochemicals are rich in secondary metabolites such as alkaloids, flavonoids, tannins and terpenoids, which are known to possess antimicrobial properties3 in phyto-research field. Several innovative therapeutic approaches revealed that phtyochemicals exhibit potent activity against bacterial resistance3,4,5,6,7.
The complex mixtures of phytocompounds commonly known an ‘active constituents’ are standardised, analysed and purified for its therapeutic potentiality against various diseases using several chromatographic techniques. High Performance Thin Layer Chromatography (HPTLC) has been well known for its advanced technology that provide qualitative and quantitative data of an active ingredient or phytoconstituent8. It is an effective analytical technique extensively used to identify phytocompounds, standardize and provide quality control of herbal formulations in the development of potential drug9. The significant degradation of active compounds due to exposure of heat, light and air can be corrected and minimized by increasing the volume of analyte in HPTLC plate, which serves as a major advantage when compared to other analytical methods10.
In south Asia, Melia azedarach, Linn. is well known for its tremendous medicinal properties. It is a tree belonging to the family meliaceae. In traditional system of medicine, the plant was recognized to possess significant therapeutic properties such as blood detoxifier, anti-inflammatory, antipyretic, anthelmintic and antimicrobial agent especially used in the treatment of skin diseases11. With the knowledge of traditional medicinal system and the medicinal properties of plants, the present investigation was aimed to analyze the antibacterial efficacy of traditional medicinal plant Melia azedarach Linn. leaf extracts against three bacteria Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, which are responsible for secondary bacterial infections of dermatophytosis, also to study High Performance Thin Layer Chromatographical (HPTLC)profiling of the plant extract.
Materials and Methods
Collection and Preparation of Plant Extracts
The fresh leaves of Melia azedarach Linn. was collected from Tiruchirappalli district, Tamil Nadu, washed several times in tap water and shade dried at room temperature for 10 – 15 days. The dried leaves were powdered using an electric grinder.
Soxhlet extraction (hot continuous extraction) procedure was undertaken to extract the phytocompounds of the plant sample. 15g of coarsely ground leaf powder was mixed with 100ml solvents such as petroleum ether, chloroform, ethanol, acetone, and aqueous in the Soxhlet apparatus. The extracts were sequentially collected in separate containers and was evaporated at low pressure using Buchi Rotavapor at 10ᵒC. The crude extracts were stored at 4ᵒC for further use.
Antimicrobial Assay
Selection of Microbes
The microorganisms selected for the present study were obtained from the Department of Microbiology, K.A.P Viswanathan Govt. Medical College, Tiruchirappalli, Tamil Nadu. The three bacterial strains employed as test organisms were listed in appendix-1.
Appendix 1
| S.No. | Bacteria | Strain |
| 1. | Staphylococcus aureus | Gram – positive |
| 2. | Escherichia coli | Gram – negative |
| 3. | Pseudomonas aeruginosa | Gram – negative |
Preparation of microbial inoculums
Nutrient broth selected for the growth of the bacteria
The peptone broth (nutrient broth) was procured from Himedia laboratory Pvt. Ltd., Bombay, India. Microbial inoculums were prepared by sub culturing the commercially available strains procured from clinics. A loop full of organisms were taken and inoculated into 5ml of nutrient broth and incubated at 37ºC for 24 hours till a moderate turbidity was developed. This was used as a source of bacterial inoculum.
Preparation nutrient medium
Nutrient medium preparation for the growth of the bacteria
The Muller – Hinton agar medium (nutrient media) was procured from Himedia laboratory Pvt. Ltd., Bombay, India. 28g of nutrient agar medium was taken in a conical flask and dissolved in 1000ml of distilled water. The contents were mixed thoroughly. Then, the conical flask with the medium was tightly plugged with cotton and subjected to sterilization.
Sterilization of nutrient medium
The steam sterilization process was carried out using an autoclave. Along with the nutrient medium, necessary glass wares such as petridishes, forceps and inoculation needle were also sterilized at 15lb psi pressure at 121ºC for 15 minutes.
Antibacterial Sensitivity test – Disc Diffusion method
Nutrient agar plates were prepared for each bacterium in sterilized petriplates. 20ml of the sterile Muller- Hinton agar medium was poured carefully under aseptic conditions and allowed to remain undisturbed for the medium to solidify. Each petriplate was labeled according to the bacterial strains to be used for streaking. Each bacterial pure culture was swabbed on the surface of the nutrient medium. On the petriplates, antibiotic discs with plant extracts along with the positive control antibiotic disc (gentamicin, 10µg/disc) were placed at equidistant on the surface.The inhibition zone formed against each bacterial strain by the plant extracts was compared with the standard positive control antibiotic disc. The diameter of the inhibition zone was denoted in millimeters(mm) using a measuring scale12.
Statistical analysis
The results of the antibacterial activity were expressed as mean ± SD of three experiments. All the data were analysed statistically using one-way analysis of variance
(ANOVA).
High Performance Thin Layer Chromatography
The acetone leaf extract of M. azedarach was analyzed for their qualitative phytoconstituent fingerprinting by HPTLC method13,14.
Sample Preparation
The acetone leaf extract was evaporated under reduced pressure using rota-evaporator and the extract residue was re-dissolved in 1ml of chromatographic grade solvent methanol, which was used as sample.
Developing Solvent System
A number of solvent systems were tried and a satisfactory resolution was obtained in the solvent system of Toluene:Ethylacetate:Methanol:Formicacid (6:2:1.5:0.5) for the extract used.
Sample Application
Application of bands of each extract was carried out (6mm in length and 100µl in concentration for leaf) using spray technique. The sample was applied in duplicate on pre-coated silica gel 60F254 aluminium sheets (4 x 10 cm) with the help of Linomat 5 applicator attached to CAMAG TLC Scanner system, which was programmed through winCATS Planar chromatography manager software.
Development of Chromatogram
After the application of sample, the chromatogram was developed in Twin trough glass chamber 10 x 10 cm saturated with solvent Toluene:Ethylacetate:Methanol:Formicacid (6:2:1.5:0.5) with the total volume of 10ml for 20 minutes between 60 to 120°C.
Detection of Spots
The air-dried plates were viewed in ultra-violet radiation to mid-day light. The chromatograms were scanned by densitometer at 420nm after spraying with specific reagent. The plates were kept in photo-documentation chamber and images were captured at white light, UV- 254nm and UV- 366nm wavelengths. After derivatization, the plates were scanned for peak table, display and densitogram were recorded. The Rf values and % area were calculated using win CATS software.
Results
The antibacterial efficacy of Melia azedarach leaf extract was determined against Staphylococcus aureus, Escherichia coli and Pseudomonas aeruginosa. The different solvent extracts used were ethanol, methanol, acetone, at 100, 150, 200 and 250µl/disc concentrations whereas, chloroform and petroleum ether at 200, 300, 400 and 500µl/disc concentration. The values were expressed in mean ± standard deviation of three replicates indicating a significant difference of p≤0.05, according to One-Way Analysis of Variance (ANOVA). The positive control gentamicin antibiotic disc (10µg/disc), revealed maximum inhibitory effect against all the test organisms when compared to all the leaf extracts used. The results were tabulated in Table – 1. Acetone leaf extract was more effective against two test pathogens, S. aureus and P. aeruginosa, whereas, the maximum zone of inhibition was registered to be 12.93±0.65 mm at 150 µl/disc and 11.5±0.10 mm at 100 µl/disc concentration respectively (Fig.3). The maximum zone of inhibition (12.70±0.20 mm) by ethanolic leaf extract against P. aeruginosa was recorded at 100 µl/disc concentration (Fig.1). Methanolic leaf extract exhibited inhibition (11.56±0.35mm) against S. aureus at 250 µl/disc concentration (Fig.2). Chloroform leaf extract inhibited Escherichia coli (11.45±0.63) at 500 µl/disc concentration (Fig.4). Also, petroleum ether extract registered inhibitory effect (10.26±0.24) against S. aureus at 200 µl/disc concentration (Fig.5). Correspondingly, S. aureus was inhibited by methanol, acetone and petroleum ether leaf extracts. There was no inhibition by ethanol and chloroform extracts. Inversely, E. coli was inhibited only in chloroform extract and did not show any inhibition against all other leaf extracts. Whereas, P aeruginosa was inhibited in ethanol and acetone leaf extracts. Also, methanol, chloroform and petroleum ether did not exhibit any inhibition against the organism. Therefore, difference in the concentrations of leaf extracts might be responsible for the wide range of variations in the inhibitory effect.
Table 1: Antibacterial efficacy of different solvent extracts of M. azedarach leaf.
|
Name of the Solvent
|
Concentration (µl) |
Diameter of inhibition zones (mm)* | ||
| Staphylococcus aureus | Escherichia coli | Pseudomonas aeruginosa | ||
|
Ethanol |
100 | – | – | 12.70±0.20 |
| 150 | – | – | 10.16±0.40 | |
| 200 | – | – | 9.20±0.30 | |
| 250 | – | – | 11.20±0.30 | |
|
Methanol |
100 | 9.43±0.50 | – | – |
| 150 | 4.26±0.35 | – | – | |
| 200 | 8.46±0.51 | – | – | |
| 250 | 11.56±0.35 | – | – | |
|
Acetone |
100 | 11.53±0.35 | – | 11.5±0.10 |
| 150 | 12.93±0.65 | – | 10.96±0.47 | |
| 200 | 10.93±0.25 | – | 11.40±0.25 | |
| 250 | 9.10±0.70 | – | 9.11±0.20 | |
|
Chloroform |
200 | – | 8.53±0.32 | – |
| 300 | – | 10.31±0.42 | – | |
| 400 | – | 9.77±0.44 | – | |
| 500 | – | 11.45±0.63 | – | |
|
Petroleum ether |
200 | 10.26±0.24 | – | – |
| 300 | 9.57±0.37 | – | – | |
| 400 | 8.66±0.52 | – | – | |
| 500 | 5.39±0.22 | – | – | |
|
Standard antibiotic disc (Gentamicin)
|
16.16±0.25
|
15.60±0.36
|
16.86±0.90
|
|
* Values are expressed as Mean±Standard deviation of three replicates indicate significant difference (p≤0.05) according to One-Way Analysis of Variance (ANOVA).
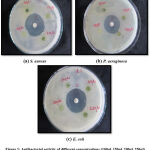 |
Figure 1: Antibacterial activity of different concentrations (100µl, 150µl, 200µl, 250µl) of ethanolic leaf extract against bacterial pathogens. |
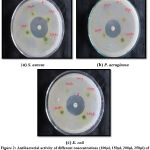 |
Figure 2: Antibacterial activity of different concentrations (100µl, 150µl, 200µl, 250µl) of methanolic leaf extract against bacterial pathogens. |
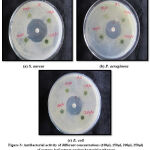 |
Figure 3: Antibacterial activity of different concentrations (100µl, 150µl, 200µl, 250µl) of acetone leaf extract against bacterial pathogens. |
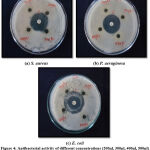 |
Figure 4: Antibacterial activity of different concentrations (200µl, 300µl, 400µl, 500µl) of chloroform leaf extract against bacterial pathogens. |
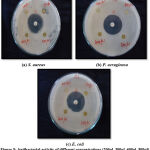 |
Figure 5: Antibacterial activity of different concentrations (200µl, 300µl, 400µl, 500µl) of petroleum ether leaf extract against bacterial pathogens |
Based on the preliminary invitro examination on antibacterial efficacy, acetone and ethanol extract was considered to be more effective and was selected for further investigation, to examine the phytochemical compound profile, through HPTLC method. The results obtained from HPTLC fingerprint scanned at 420nm wavelength, confirmed the presence of twelve different phytoconstituents with different Rf values. The corresponding Rf values recorded in the ascending order of 0.04 to 0.94, with maximum percentage of concentration 14.07% at 0.09 Rf. The polyvalent chemical constituents with different Rf values, maximum percentage, area percentage, present in the acetone leaf extract were tabulated in Table 2 and shown in Fig. 6. Also, the 3-dimensional chromatogram and spectra of the extract was depicted in Fig. 7 and 8 respectively. Thus, it has been found that acetone extract of the M. azedarach leaf contains mixture of compounds and the pharmacological activity revealed by them are due to the cumulative effect of all the composite compounds.
Table 2: HPTLC profile of track 1.
| Peak | Start Rf | Start Height | Max Rf | Max height | Max % | End Rf | End Height | Area | Area % |
| 1 | -0.03 | 2.0 | 0.02 | 292.4 | 10.38 | 0.04 | 190.5 | 6029.7 | 8.18 |
| 2 | 0.04 | 192.7 | 0.07 | 432.6 | 15.35 | 0.09 | 318.8 | 10367.1 | 14.07 |
| 3 | 0.09 | 321.1 | 0.10 | 332.6 | 11.80 | 0.16 | 58.0 | 7210.8 | 9.78 |
| 4 | 0.16 | 58.6 | 0.18 | 73.8 | 2.62 | 0.21 | 50.8 | 1954.0 | 2.65 |
| 5 | 0.24 | 58.9 | 0.30 | 196.3 | 6.96 | 0.31 | 114.9 | 4815.5 | 6.53 |
| 6 | 0.31 | 115.2 | 0.33 | 147.4 | 5.23 | 0.36 | 104.2 | 3971.0 | 5.39 |
| 7 | 0.36 | 104.9 | 0.40 | 287.2 | 10.19 | 0.42 | 163.4 | 6088.5 | 8.26 |
| 8 | 0.42 | 165.5 | 0.43 | 254.0 | 9.01 | 0.46 | 88.6 | 4331.9 | 5.88 |
| 9 | 0.46 | 89.3 | 0.49 | 278.1 | 9.87 | 0.53 | 77.0 | 7666.6 | 10.40 |
| 10 | 0.54 | 77.3 | 0.58 | 203.1 | 7.21 | 0.68 | 51.6 | 8815.5 | 11.96 |
| 11 | 0.74 | 52.3 | 0.81 | 120.3 | 4.27 | 0.82 | 119.6 | 4007.8 | 5.44 |
| 12 | 0.82 | 118.8 | 0.86 | 200.6 | 7.12 | 0.94 | 2.1 | 8439.6 | 11.45 |
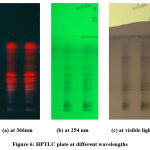 |
Figure 6: HPTLC plate at different wavelengths. |
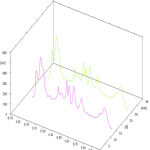 |
Figure 7: HPTLC 3-dimensional chromatogram of M.azedarach acetone leaf extract. |
 |
Figure 8: HPTLC spectra of acetone extract of M. azedarach leaf. |
Discussions
The present study finds supportive evidence of several scientific research reports. The influence of ethanolic and methanolic extract on inhibition against selected micro-organisms were found to be some extent, depending upon the varying solvent concentrations. The ethanolic extract of Limonia acidissima leaves15 and methanolic leaf extract of Vitellaria paradoxa 16 were reported to possess maximum inhibitory effect against some bacterial pathogens. The antimicrobial activity of methanol, ethanol and aqueous extracts of seven medicinal plants were found to have good inhibitory effect against medicinally important bacteria such as Staphylococcus sp., Escherichia coli, Klebsiella sp., Pseudomonas sp17. Similar results were obtained in aqueous and ethanolic extracts of six different medicinal plants against some bacterial pathogens18. The methanol, ethanol and chloroform extracts of Argemone mexicana, reported 80% antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Klebsiella pneumonia, Vibrio cholera and Enterobacter aerogenes19 . Antimicrobial potential of Amaranthus viridis ethanolic extracts was studied against two Gram positive bacterial strains, Staphylococcus aureus and Bacillus subtilis, and four Gram negative bacterial strains via; Proteus vulgaris, Pseudomonas picketii, Klebsiella pneumonia and Escherichia coli 20. Varying degree of inhibition zone was recorded in different solvent extracts of F. limonia fruit against the selected bacterial organisms21. Our results are in line with the report on six medicinal plants, where acetone extracts was reported to have good inhibitory effect against some pathogenic bacteria22. Regarding the results of chloroform and petroleum ether extracts, our findings were inversely proportional with the report on the antimicrobial activity of Murraya koenigii root extracts against Staphylococcus aureus, Micrococcus luteus, Bacillus subtilis, E. coli, Pseudomonas aeruginosa and Aspergillus niger. They reported that the chloroform extract showed good inhibitory properties against all pathogens and even at very low concentrations23. Moringa oleifera leaf extracts of different solvents showed varying degree of inhibition according to the type of solvent extract and its concentration against Bacillus subtilis and Klebsiella pneumoniae which revealed susceptibility as well resistant to all the extracts24. A significant antibacterial activity has been reported in M. azedarach leaves25,26,27,28 and fruit extracts29 against certain gram- positive and gram- negative strains. Therefore, it can be concluded that M. azedarach may contains certain antimicrobial components that could be very useful in the treatment for various infectious diseases, especially against secondary bacterial pathogens of skin infection.
The quality of plant extracts depends on the presence of active phytoconstituents which can be identified and determined by an analytical technique High Performance Thin Layer Chromatography (HPTLC). Densitometry provides data with peak area, peak height for the quantitative determination of bioactive constituents30. The HPTLC finger printing of Pisonea aculeata chloroform leaf extract revealed 14 peaks with Rf values in the range of 0.03 to 0.95, ethyl acetate extract of leaf showed 6 peaks with Rf values in the range of 0.04 to 0.94 and 90% ethanolic extract of leaf revealed 11 peaks with Rf values in the range of 0.03 to 0.9331. The methanol and aqueous extract of Sterculia lychnophora seeds confirmed the presence of different secondary metabolites with different concentrations, from HPTLC fingerprint scanned at different wavelengths32. Similar studies on the crude extract of different parts of Vernonia cinerea L indicated the chemical profile of potential compounds that possess biological activity33. A HPTLC densitogram reported major phytoconstiutents with several peaks scanned at 254 nm and 366nm from the methanolic extract of Fumaria parviflora (whole plant)34. The methanolic extracts of Verbesina sphaerocephala leaves and flowers were reported to possess high phenolic and flavonoid compounds through HPTLC analysis with relevant antibacterial and antioxidant activity35. Two phytocompounds rutin and kaempferol-3-O-rutinoside were identified in Bauhinia rufescens by HPTLC with antioxidant and antidiabetic potential36. The methanolic leaf and root extracts of Hypochaeris radicata has confirmed the existence of major phytocompounds through HPTLC method responsible for bioactivity against pathogenic organisms of communicable and non-communicable aliments37. Thus, HPTLC fingerprinting helps to determine the major active biocompounds especially secondary metabolites, present in medicinal plants with reliable scanning profiling of qualitative and quantitative measurements.
Conclusion
According to World Health Organisation (WHO), every year, millions of fatalities occur due to microbial infections all around the world. This remains a biggest challenge in health society. In this regard, researchers focus on natural products as an alternative to existing less effective antibacterial drugs. The present research findings may provide an authentic conclusion that, the phytochemical compounds present in the leaves of M. azedarach may have a promising role in the antibacterial activity against the tested microbes. These phytocompounds may serve as selective agents for the maintenance of human health and a potent remedy for secondary bacterial pathogens of dermatophytosis. Also, the HPTLC profiling has proved the presence of major phytocompounds in the acetone leaf extract. Based on the separation of bands along with obtained Rf values, percent area of the potent extract and its correlation with literature study, it can be stated that the chemical constituents present in the extract may include phenols, flavonoids, alkaloids, terpenoids and other secondary metabolites which can be authenticated and purified as maker compounds for drug delivery in future work.
Acknowledgement
We are grateful to thank DST-FIST for providing the infrastructural facilities. We are extremely thankful to Department of Microbiology, K.A.P Viswanathan Govt. Medical College, Tiruchirappalli, for providing the microbial strains to undergo antibacterial study. Also, we extend our sincere gratitude to SRM university, Chennai, for providing facilities to undertake HPTLC analysis.
Conflict of Interest
The authors declare no conflict of interest.
Funding Sources
There is no funding sources.
References
- medicalnewstoday.com/articles/324654
- Itzhak Brook. Secondary bacterial infections complicating skin lesions. Journal of medical microbiology. 2002. 51:808-812.
CrossRef - Cowan MM. 1999. Plant products as antimicrobial agents. Clin Microbiol Rev.12(4):564–82.
CrossRef - Baym M, Stone LK, Kishony R. 2016. Multidrug evolutionary strategies to reverse antibiotic resistance. Science. 351(6268):3292.
CrossRef - Khameneh B, Diab R, Ghazvini K, Fazly Bazzaz BS. 2016. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb Pathog.95: (32–42).
CrossRef - Shakeri A, Sharifi MJ, Fazly Bazzaz BS, Emami A, Soheili V, Sahebkar A. 2018. Bioautography detection of antimicrobial compounds from the essential oil of salvia Pachystachys. Curr Bioact Compd.14(1):80–85.
CrossRef - Fazly Bazzaz BS, Khameneh B, Zahedian Ostad MR, Hosseinzadeh H. 2018. In vitro evaluation of antibacterial activity of verbascoside, lemon verbena extract and caffeine in combination with gentamicin against drug-resistant Staphylococcus aureus and Escherichia coli clinical isolates. Avicenna J Phytomed. 8(3):246–53.
- Houghton P. Mukherjee PK. Evaluation of herbal medicinal products:perspectives on quality, safety and efficacy. Pharmaceutical Press London. UK. 2009.
CrossRef - Tistaert C, Dejaegher B, Vander Heyden Y. Chromatographic separation techniques and data handling methods for herbal fingerprints: A Review. Anal Chim. Acta. 2011. 148 -161.
CrossRef - Paixao N, Perestrelo R, Marques JC, Camara JS. Relationship between antioxidant capacity and total phenolic content of red, rose and white wines. Food Chemistry. 2007. 105:204-214.
- Satyavati GV, Raina MK, Sharma M. Medicinal plants of India. Indian council of medical research, New Delhi. 1976. 201-206.
CrossRef - Bauer A.W. Kirby W.M.M. Sherries S.C. and Tunk M 1966. Antibiotic susceptibility of testing by a standard single disc method. J. Clinical Pathology. 36: 492 – 496.
CrossRef - Harbone JB. Phytochemical methods. 3rd edition, London: Champman and Hall. 1998.
- Wanger H, Baldt S. Plant Drug Analysis: A thin layer chromatographic Atlas. Berlin:Springer;1996.
- Neelamadhab Panda, V. Jagannath Patro, Basanta Kumar Jena, P.K. Panda. 2013 1imonia acidissima Leaves. International Journal of Herbal Medicine. 1(1): 22-27
- Olaleye , O.O., Adetunji C. O. , Kolawole O .M. 2015. Identification of Phytochemical Constituents of the Methanolic extract of Vitellaria paradoxa responsible for Antimicrobial Activity against Selected Pathogenic Organisms .SMU Medical Journal vol.2.
- Selvamohan , and Ramadas, Shibila Selva Kishore. 2012. Antimicrobial activity of selected medicinal plants against some selected human pathogenic bacteria. Advances in Applied Science Research. 3 (5):3374-3381.
- Ada Zwetlana, Nandini, M., Kusuma Dorcas , 2014. Antimicrobial activity of medicinal plant extracts on gram negative bacteria. Journal of Medicinal Plants Studies 2(5): 51-54.
- Andleeb S, Alsalme A, Al-Zaqri N, Warad I, Alkahtani J and Bukhari SM. In-vitro antibacterial and antifungal properties of the organic solvent extract of Argemone mexicana L. Journal of King Saud University – Science. 2020.32:2053–2058
CrossRef - Sumaira Sarwar , Maimoona Sabir , S., Sajid Raza , and Malik , S.A., 2016. Analysis of antimicrobial activity of medicinal plant Amaranthus viridis. International Journal of Innovation and Scientific Research. 20(2):494-499.
- Jayashree VH and Ramesh Londankar. Comparative phytochemical studies and antimicrobial potential of fruit extracts of Feronia limonia Linn. International Journal of Pharmacy and Pharmaceutical Sciences.2014. 6(1): 731-734.
- de Boer, H. J., A. Kool, A. Broberg, W. R. Mziray, I. Hedberg, and J. J. Levenfors. 2005.Antifungal and antibacterial activity of some herbal remedies from Tanzania. J Ethnopharmacol. 96:461-469.
CrossRef - Manisha Vats, Harneet Singh and Satish Sardana. 2011. Phytochemical Screening and antimicrobial activity of roots of Murraya koenigii (Linn.) Spreng. Rutaceae. Brazilian Journal of microbiology. 42:1569-1573.
CrossRef - Ashok ,V. Gomashe, Pranita A. Gulhane Megha P. Junghare and Neeta A. Dhakate , 2014. Antimicrobial Activity of Indian Medicinal Plants: Moringa oleifera and Saraca indica. International Journal of Current Microbiology and Applied Sciences. vol. 3: 161-169 .
- Ramya S, Jepachanderamohan PJ, Alaguchamy N, Kalayanasundaram M, Jayakumararaj R. 2009. In Vitro Antibacterial Prospective of Crude Leaf Extracts of Melia azedarach Linn. against Selected Bacterial Strains. Ethnobotanical Leaflets. 13: 254-58.
- Ratna China, Sayani Mukherjee, Sauradip Sen, Sreedipa Bose, Sanjukta Datta, Hemanta Koley, Santinath Ghosh, Pubali Dhar. Antimicrobial activity of Sesbania grandiflora flower polyphenol extracts on some pathogenic bacteria and growth stimulatory effect on the probiotic organism Lactobacillus acidophilus. Microbiol. Res. 2012.167(8):500-6.
CrossRef - Abdul Viqar Khan, Qamar Uddin Ahmed, M Ramzan Mir, Indu Shukla and Athar Ali Khan. 2011. Antibacterial efficacy of the seed extracts of Melia azedarach against some hospital isolated human pathogenic bacterial strains. Asian Pacific Journal of Tropical Biomedicine.1(6): 452-455.
CrossRef - Hussein J. Hussein , Ali H. Al-Marzoqi. 2020. The antibacterial efficacy of the secondary metabolites extracted from (Melia azedarach L.) leaves against pathogenic microorganisms isolated from burns and gingivitis infections. Eurasian Journal of Biosciences. 14 (1): 561-565.
- Nazar Jabbar Al-Khafaji, Raad Mahmood Al-Zubaedi, Shaimaah Jabbar Al-Azawi. 2016. Evaluation of antibacterial effects of Melia azedarach fruit extracts against some isolated pathogenic bacteria. Veterinary Science Development. 6:6080.
CrossRef - Moulishankar A, Ganesan P, Elumalai M and Lakshmanan K. Significance of TLC and HPTLC in Phytochemical Screening of Herbal Drugs. Journal of Global Pharma Technology. 2020. 13(01):30-45.
- Mamoon Hussain Syed, Ayesha Yasmeen, Mohammad Suleman Hussain, N. Siva Subramanian,M.Ramadevi. Preliminary Phytochemical Screening and HPTLC Fingerprinting of Leaf Extracts of Pisonea aculeata. Journal of Pharmacognosy and Phytochemistry. 2013. 2 (1):36-42.
- Anuradha Palve, Pooja Shetty, Mukesh Pimpliskar and R. N. Jadhav. HPTLC method for qualitative determination of phytochemical compounds in extract of Sterculia lychnophora. Int. J. Res. Ayurveda Pharm. 2015;6(3):358-365 http://dx.doi.org/10.7897/2277-4343.06370.
CrossRef - Giby Abraham. Pharmacognostical and phytochemical studies of the plant Sahadevi [Vernonia cinerea(L.)Less.]. Int. J. Res. Ayurveda Pharm. 2015; 6(1):47-54. http://dx.doi.org/10.7897/2277- 4343.06112
CrossRef - Bhargava A, Shrivastava P and Tilwari A. HPTLC analysis of Fumaria parviflora (Lam.) methanolic extract of whole plant. Future Journal of Pharmaceutical Sciences 2021.7:1. https://doi.org/10.1186/s43094-020-00150-x
CrossRef - Rodríguez-Valdovinos KY, Salgado-Garciglia R, Vázquez-Sánchez M, Álvarez-Bernal D, Oregel-Zamudio E, Ceja-Torres LF and Medina-Medrano JR. Quantitative Analysis of Rutin by HPTLC and In Vitro Antioxidant and Antibacterial Activities of Phenolic-Rich Extracts from Verbesina sphaerocephala. Plants. 2021;10:475. https://doi.org/10.3390/plants10030475
CrossRef - Osman W, Mohammed MS, Khalid HS, Muddathir A, Shantier SW, Osman B, and Abdoon I. HPTLC Fingerprint Profile and Identification of Antidiabetic and Antioxidant Leads from Bauhinia rufescens L. Advances in Pharmacological and Pharmaceutical Sciences. 2020. 3201463:18. https://doi.org/10.1155/2020/3201463
CrossRef - Jamuna S and Paulsamy S. HPTLC Fingerprints of Various Secondary Metabolites in the Traditional Medicinal Herb Hypochaeris radicata L. Jounal of Botany. 2016. Article ID 5429625. https://doi.org/10.1155/2016/5429625
CrossRef







