Manuscript accepted on :25-03-2022
Published online on: 09-06-2022
Plagiarism Check: Yes
Reviewed by: Dr. Shashi Rashmi R. Acharya
Second Review by: Dr. Ankur Singh Bist
Final Approval by: Dr. Ian James martin
Kiran Singbal1* , Michelle Kuah Wei Shan1, Sulagna Dutta2
, Michelle Kuah Wei Shan1, Sulagna Dutta2 and Kranthi Raja Kacharaju1
and Kranthi Raja Kacharaju1
1Department of Conservative Dentistry and Endodontics, Faculty of Dentistry, MAHSA University, Malaysia.
2Department of Oral Biology and Biomedical Sciences, Faculty of Dentistry, MAHSA University, Malaysia.
Corresponding Author E-mail: kiransingbal@mahsa.edu.my
DOI : https://dx.doi.org/10.13005/bpj/2406
Abstract
Background: The Dental Public Health Services of the Ministry of Health, Malaysia have introduced Cention N as a primary restorative material in the health care centers all over the country. Thus, in this scenario, a comprehensive evaluation of Cention N as compared to other restorative materials, in terms of fluoride release, assumes to be of utmost relevance. Fluoride release plays a pivotal role in the prevention of secondary dental caries, and dental restorations facilitate direct fluoride delivery to the susceptible tooth surface. Aim: To evaluate and compare the fluoride releasing property of Cention N with other restorative materials, EQUIA Forte, Beautifil II and Estelite Quick. Methods and Material: A total of 20 disk-shaped brass-mold samples (6±0.1mm in diameter and 2±0.1mm thickness) for the dental restorative materials were prepared, with five samples for each of Cention N, EQUIA Forte, Beautifil II and Estelite Quick. Following the immersion of samples in deionizing water, the released fluoride ions were measured over 28 days using ion chromatography. Data were obtained at day 1, 7 and 28 post-immersion. Statistical analysis: Data obtained from this investigation was analyzed using the MedCalc statistical software (v. 19.05). Kolmogorov-Smirnove test followed by Repeated Measures ANOVA and ‘Comparison of multiple method’ was applied to statistically compare the fluoride release efficacy of Cention N individually with each of the other three materials. Any difference found was considered significant at P<0.05. Results: Cention N showed significantly higher fluoride release than Beautifil II and Estelite Quick when observed on Day 1, 7 and 28. ‘Comparison of multiple method’ confirmed the results obtained via ANOVA and showed Cention N is a significantly better (P<0.05) material in terms of fluoride release as compared to Beautifil II and Estelite Quick on Day 1, 7 and 28. Conclusions: Cention N displays consistent fluoride release while weighing against other contemporary and more expensive restorative materials.
Keywords
Cention N; Composite Resin; Fluoride Ion; Glass Ionomer; Ion Chromatography
Download this article as:| Copy the following to cite this article: Singbal K Shan M. K. W, Dutta S, Kacharaju K. R. Cention N Compared to Other Contemporary Tooth-Colored Restorative Materials in Terms of Fluoride Ion Releasing Efficacy: Validation of a Novel Caries-Prevention-Initiative by the Ministry of Health, Malaysia. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Singbal K Shan M. K. W, Dutta S, Kacharaju K. R. Cention N Compared to Other Contemporary Tooth-Colored Restorative Materials in Terms of Fluoride Ion Releasing Efficacy: Validation of a Novel Caries-Prevention-Initiative by the Ministry of Health, Malaysia. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3my49fc |
Introduction
Dental caries is one of the most prevalent chronic diseases affecting the global population 1. Even with the current technology and advances, it remained as a major issue of concern to most of the adults and almost 60 to 70% of school-going children 1. Secondary or recurrent caries has been ascertained to be one of the prime causes of restoration failures. It is defined by the Federation Dentaire International (FDI) as a ‘positively diagnosed carious lesion which occurs at margin of existing restoration’ 2. Replacement of restorations has been reported to occupy approximately half of all restorative dentistry work, while about 60% of these replacements are attributed to recurrent caries 3.
Fluoride incorporation into the restorative material composition is an effective strategy for preventing secondary decay in restorations. Free fluoride ions and hydroxyapatite crystals in the inorganic portion of the tooth combine to form fluoroapatite crystals. This caries resistant complex aids in the reduction of enamel acid solubility 4 and prevents initiation and progression of caries 5. The mechanism of action of fluoride is to facilitate remineralization and preventing demineralization of inorganic components of tooth 6. Numerous dental restorative materials containing fluoride have been promoted 6 that leach fluoride ions into the adjoining enamel margins and hence inhibit recurrent caries around restorations 7.
In this regard, it is noteworthy that composite materials like Cention N, developed recently, are gaining popularity for its cost-effectiveness. Cention N which is an ‘alkasite’, utilizes an alkaline glass filler that is capable of releasing acid-neutralizing ions 8. This material is self-curing with additional light curing option. As a dual-cured restorative material, it can be used as a full volume replacement material. It is dispensed as a powder containing various glass fillers, initiators and pigments as well as liquid comprising of dimethacrylates and initiator 9.
Resin modified glass ionomer (RMGIC) and conventional GICs both possess similar level of efficacy, while RMGICs have been reported to be more effective in terms of fluoride release 10. EQUIA, a RMGIC, is a hybrid of conventional glass ionomer and resin components. Ion-releasing fluoro-aluminosilicate glass particles are the powder components in the material with an initiator for the dual cure reaction. Fluoride is also incorporated into the glass particles for optimizing the setting characteristics and used as a flux in order to decrease the fusion temperature11. The liquid component contains water and polyacrylic acid or polyacrylic acid modified with methacrylate or hydroxyethyl methacrylate (HEMA) monomers. This material initially sets by slow acid-base reaction which contributes to the unique maturing process and the final strength of the restorative material [27, 28]. Silica gel surrounding the unreacted glass is bound together by hydrated calcium and aluminium poly-salts in the cured material. The unique feature of EQUIA is that it enables fluoride to be released into the oral environment without compromising the structural integrity of the restoration because fluoride is not a part of the matrix formation.
Beautifil II also finds relevance as one of the contemporary materials, which is a giomer and is similar to the acid-modified composites provided to the dentists as single paste material. The aluminosilicate glass reacts with the polyacid to form a pre-reacted glass-polyalkenoate complex. The reaction products are set through light-activated free radical addition polymerization 12. After the setting reaction, the final set cement has a long-term fluoride release and the ability to be recharged with topically applied fluoride. Whereas, the Estelite Quick is a dental composite which uses a blend of aromatic and aliphatic dimethacrylates monomers such as bis-GMA, triethylene glycol dimethacrylate (TEGDMA), and urethane dimethacrylate (UDMA) to form a highly cross-linked polymeric material reinforced by the dispersion of glass, crystalline or resin filler particles and short fibers bound to the matrix by silane coupling agents 4. The justification for including Estelite Quick as a part of the study is to act as a control since the manufacturers do not claim to have included fluoride.
The Ministry of Health Malaysia (KKM) has focused on Cention N as the primary restorative material to replace amalgam mainly on the basis of its cost-effectiveness. Hence, Cention N, due to its fluoride releasing anti-cariogenic activity, is the focus of the present study. Its widespread use will have far-reaching implications influencing the long-term oral health status of the Malaysian populace.
Several techniques have been identified to measure the quantum of fluoride ions release in various media, such as artificial saliva, deionized water and lactic acids. The techniques include ion chromatography (IC), ion-selective electrodes (ISE) and capillary electrophoresis (CE) 13. Thus, the aim of the present study is to evaluate the fluoride releasing efficacy of the recently extensively used restorative material, Cention N using ion chromatography (IC) in deionized water which is a neutral pH solutions7, 14 and compare the same with other three contemporary tooth colored restorative materials such as, EQUIA, Beautifil II and Estelite Quick.
Materials and Methods
A total of 20 disk shaped samples, 5 for each restorative material, Cention N (A), EQUIA Forte (B), Beautifil II (C) and Estelite Quick (D), were prepared using a teflon mould of 10.0mm x 10.0mm specification. The restorative materials were categorized as per their composition that included a resin modified glass ionomer cement (RMGIC), giomer, alkasite and composite resin. Each of these dental restorative materials was purchased from the market with its own expiry date and batch number listed on their packaging.
Sample Preparation
Samples were prepared from 4 restorative materials. Five pellets were made from material A, B, C and D resulting in a total of 20 pellets (Table 1). The pellets prepared from these dental restorative materials were manipulated based on the manufacturer’s instructions and were packed into a teflon mould of 10.0mm x 10.0mm specification placed on a glass slab and subsequently covered with glass slide, to ensure standardization of shape and size of each pellet (Figure 1). The pellets were light cured for 40 seconds or left to set at room temperature for 10 minutes. Finishing procedures were not incorporated as the cured surface already had a satisfactory finish. All samples were premeasured with digital weighing scale for weight-standardization. The samples were then immersed and stored in individual plastic containers with uniform volume of 5ml deionized water at 37º± 0.5°C. Deionized (DI) water is devoid of any ions and thereby its use in the present study was important to create an environment to measure even the miniscule quantities of fluoride ion release by the materials with utmost precision 15, 16. A tweezer was used to remove the samples from their containers at the pre-determined time intervals of 1, 7- and 28-days post sample preparation and the storage solution was then analyzed for its fluoride ion concentration using ion chromatography (Figure 1). Each of the samples was then transferred into different containers consisting of fresh uncontaminated deionized water, and storage was resumed.
 |
Figure 1: Sample Preparation and experimental procedures. |
Determination of Fluoride Release
The present study used an ion chromatograph (DX 100; Dionex, Camberley, UK) with suppressed conductivity to determine the release of free fluoride ions (Figure 1). The instrument was fixed to an ION PAC AS14 analytical column (Dionex) and ION PAC AG14 Guard column (Dionex). For analysis, a 20 µl loop was fixed to the column. 0.5ml of each sample solution was injected into the injection loop of Ion Chromatograph with a flow rate of 1.0 ml/min. Chromatogram determined the peak fluoride released as the free fluoride ions released can retain in the solution for a longer period of time. The peak area represents free fluoride concentrations by linear interpolation between standard solutions of concentration slightly higher and lower than the test solution. The measurement of fluoride ions released was repeated thrice to an accuracy of 0.001 ppm. The free fluoride ion released from each sample solution was measured in Parts per Million (PPM) and all the collected data were subjected to statistical analysis.
Statistical analysis
Data obtained from this investigation was analyzed using the MedCalc statistical software (v.19.05). In brief, summary statistics of the concentration of fluoride ion released were initially done to evaluate the distribution, normality and homogeneity of the data. Continuous variables were reported as Mean ± Standard Deviation (SD). Based on Kolmogorov-Smirnov test, the normality distribution assumption for majority of the variables between the groups were met (p>0.05), thus parametric tests were performed. The Repeated Measures ANOVA was applied to determine the significant difference of changes of fluoride ions concentration from Day 1, Day 7 and Day 28 between groups of tooth colored restorative materials. ‘The Bland-Altman plot 17, or difference plot, was plotted to compare Cention N with each of the other dental restorative materials. The differences in terms of fluoride ions release between the two restorative materials are plotted against the averages of the two materials. Any difference found was considered to be significant if the p-value showed less than 0.05.
Results
A total of 20 pellets were used in this study, five samples for each of the four restorative materials (Table 1). The average of fluoride concentration for each group based on each day is presented in Table 2.
Table 1: Categorization of dental restorative materials
| Group | Restorative Material | Type | Manufacturer (Batch No.) | P/L Ratio | Type of Curing |
| A | EQUIA | Resin modified glass ionomer cement | GC (1609051) | 1:3 | Light cure |
| B | Beautifil II | Fluoride releasing composite | Shofu (011506) | – | Light cure |
| C | Cention N | Alkasite | Ivolar Vivadent (W07377) | 1:2 | Light cure/ Self cure |
| D | Estelite Quick | Composite | Tokuyama Dental (111E66) | – | Light cure |
Table 2: Distribution of average fluoride ions released by four tooth colored restorative materials (n = 20).
| Restorative material | Day | Mean | Std. Deviation |
| Cention N | DAY1 | 0.10 | 0.008 |
| DAY7 | 0.43 | 0.050 | |
| DAY28 | 0.33 | 0.015 | |
| EQUIA | DAY1 | 0.85 | 0.068 |
| DAY7 | 0.35 | 0.015 | |
| DAY28 | 0.32 | 0.010 | |
| Beautifil II | DAY1 | 0.01 | 0.006 |
| DAY7 | 0.03 | 0.044 | |
| DAY28 | 0.01 | 0.001 | |
| Estelite Quick | DAY1 | 0.02 | 0.004 |
| DAY7 | 0.01 | 0.001 | |
| DAY28 | 0.01 | 0.002 |
Pairwise comparison along with Bonferroni method was done within each tooth colored restorative material (Table 3). Cention N shows significance difference of all paired days; Day 1 vs Day 7 (p<0.001), Day 1 vs Day 28 (p<0.001) and Day 7 vs Day 28 (p=0.022). EQUIA, Day 1 vs Day 7 (p<0.001) and Day 1 vs Day 28 (p<0.001) have significant difference of mean fluoride concentration. Estelite Quick also shows significant difference of all paired days; Day 1 vs Day 7 (p=0.012), Day 1 vs Day 28 (p=0.003) and Day 7 vs Day 28 (p=0.002).
Table 3: Comparison of fluoride concentration based on time within each type of tooth colored restorative material (n=20).
| Restorative materials | Mean Difference | 95% Confidence Interval for Difference | p-value | ||
| Cention N | Day 1 | Day 7 | -0.33* | -0.414, -0.241 | <0.001 |
| Day 28 | -0.23* | -0.262, -0.193 | <0.001 | ||
| Day 7 | Day 28 | 0.10* | 0.022, 0.179 | 0.022 | |
| EQUIA | Day 1 | Day 7 | 0.50* | 0.395, 0.599 | <0.001 |
| Day 28 | 0.53* | 0.395, 0.665 | <0.001 | ||
| Day 7 | Day 28 | 0.03 | -0.005, 0.071 | 0.080 | |
| Beautifil II
|
Day 1
|
Day 7 | -0.02 | -0.103, 0.056 | 0.915 |
| Day 28 | 0.01 | -0.007, 0.016 | 0.522 | ||
| Day 7 | Day 28 | 0.03 | -0.050, 0.107 | 0.675 | |
| Estelite Quick | Day 1 | Day 7 | 0.01* | 0.003, 0.014 | 0.012 |
| Day 28 | 0.02* | 0.009, 0.026 | 0.003 | ||
| Day 7 | Day 28 | 0.01* | 0.005, 0.013 | 0.002 | |
(*significance level, P≤0.5)
In order to evaluate the fluoride releasing ability from four tooth coloured restorative materials, Cention N, EQUIA, Beautifil II and Estelite Quick, Repeated Measures ANOVA were used. The amount of fluoride concentration on Day 1, Day 7 and Day 28 post treatment, were compared within each group (Figure 2). Result showed that fluoride ions released by Beautifil II and Estelite Quick were significantly lower as compared to that by the Cention N at Day 1, 7 and 28. Fluoride ions released by EQUIA was significantly higher than that by Cention N at Day 1, while at Days 7 and 28 post treatment, EQUIA released less fluoride but not significantly lower than that by the Cention N.
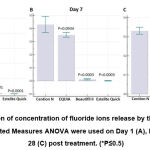 |
Figure 2: Comparison of concentration of fluoride ions release by the dental restorative materials: Repeated Measures ANOVA were used on Day 1 (A), Day 7 (B) and Day 28 (C) post treatment. (*P≤0.5). |
Discussion
Fluoride plays an essential role in prevention of dental caries and promoting oral health. Among the fluoride releasing restorative materials, Cention N belongs to a new category that is reported to release substantial levels of fluoride ions compared to traditional glass ionomers. It has been considered as a substitute to amalgam since it is more cost effective and thus ideally suited for use in government dental clinics. Hence, its properties such as anti-cariogenicity due to fluoride release is highly relevant and rationalizes its inclusion in the present study.
This study has used Ion chromatography (IC) to measure the concentration of fluoride release precisely up to 0.001ppm, and can differentiate between fluoride ions and fluoride complexes released from materials, as compared to the commonly used Ion-selective electrode (ISE) connected to an ion meter species 18. Free flowing fluoride ions are reported to be the only substances that amplify the defense of tooth structure against recurrent caries around restorations. This is because of the ability of tooth structure to absorb free fluoride ions and transform the hydroxyapatite into fluoroapatite 19. Another advantage of IC is that the time taken to analyze a sample is typically only around ten minutes 12, 20. As per convention, based on several similar studies, the time intervals for the analysis of fluoride ions concentration has been adopted as 1, 7 and 28 days post-setting 9, 21, 22.
After day 1, all restorative materials except Estelite Quick showed more release of fluoride ions followed by a declining trend in fluoride ions release on the 7th and 28th days. This may be explained by the phenomenon of the ‘wash-off effect’23, which resulted in peak fluoride ion release after day 1. The dental restorative materials, namely Beautifil II, EQUA and Cention N have shown to possess two phases of fluoride ion release, the initial ‘burst release’ phase followed by a sustained release 23. A huge amount of fluoride ions is integrated into the product matrix during the initial acid dissolution of powder particles surfaces. The “burst effect” causes fluoride to be released quickly from the matrix exposed on the surface of the material. It is then slowly replaced by fluoride ions from the matrix below the surface contributing to the sustained release of fluoride ions after 24 hours 24, 25. This creates a favorable environment to decrease the viability of bacteria in the inner layer of carious dentin and it also serves to prevent caries as well as to enhance the remineralization of the susceptible tooth surfaces. The ability of a sustained release of fluoride ions enhances the defense against new carious lesion in enamel and dentin 23.
The present study demonstrated the following order of concentration of fluoride ions released from the dental restorative materials: EQUIA (RMGIC) > Cention N (Alkasite) > Beautifil II (Giomer). Results showed a rapid decrease of fluoride release after day 7 and a more gradual decline till the 28th day. Whereas, Estelite Quick showed only a trace amount of fluoride ion release 26.
Results showed that Cention N had significantly higher (P<0.05) fluoride release than Beautifil II and Estelite Quick when observed on Day 1, 7 and 28. This observation may be explained by the fact that alkaline glass in Cention N accounts for 24.6% in weight of the final material and this releases substantial levels of fluoride ions as compare to those released by Beautifil II 8. Moreover, the amount of fluoride ion released by Beautifil II were lesser as the origin of the fluoride ions is limited only to the surface of this material upon setting, the surface pre-reacted glass ionomer (S-PRG) reacting with the poly acids 27, 28.
When the mean fluoride ion released by Cention N was compared to that by EQUIA, a higher fluoride release was observed on day 7 and 28, but the values were not statistically significant. EQUIA showed progressive decrease in fluoride ion released due to the ‘wash off’ effect. Cention N had a spike of fluoride release on the 7th day which may be originating from unreacted particles of calcium barium aluminum fluorosilicate glass and calcium fluorosilicate glass within the self-cured polymerized material8. While in Day 1, EQUIA has significantly higher (p<0.05) fluoride ion release than Cention N, the reason being EQUIA a dual cure material undergoing acid-base reaction as well as polymerization reaction. Hence, the acid-base reaction in EQUA contributes to the initial surface burst effect 24, 29. Cention N only undergoes polymerization reaction, the reaction product is formed immediately at the end of setting period which rendered the fluoride ion release by Cention N lesser than EQUIA. Besides, Cention N releases a significantly larger amount of ions when the pH is low (acidic)8, while in the present study use of deionized water (neutral pH) as the medium for restorative material, the fluoride ions released by Cention N was found lower in day 1, as compared to EQUIA.
Conclusion
All the tested restorative materials demonstrated fluoride ion release over the observed time frame. Cention N has shown promising results owing to its consistently high fluoride release which may contribute to its anti-cariogenic property. The findings of the present study, along with the cost-effectiveness and convenient manipulation of Cention N, validate the novel initiative of the Ministry of Health, Malaysia in introducing Cention N as the primary restorative material in dental clinics in Malaysia.
Conflict of interest
Authors declare no conflict of interest.
Funding Sources
There is no funding source.
References
- Petersen PE, Bourgeois D, Ogawa H, Estupinan-Day S, Ndiaye C. The global burden of oral diseases and risks to oral health. Bulletin of the World Health Organization. 2005;83:661-9.
- Quinn JB, Quinn GD. A practical and systematic review of Weibull statistics for reporting strengths of dental materials. Dent. Mater. 2010;26(2):135-47.
CrossRef - Babar MG, Lin SL. Cariostatic effect of fluoride-containing restorative materials: a review. Malaysian Dent. J. 2009;30(2):130-6.
- Anusavice KJ, Shen C, Rawls HR. Phillips’ science of dental materials: Elsevier Health Sciences; 2012.
- Nigam AG, Jaiswal J, Murthy R, Pandey R. Estimation of Fluoride Release from Various Dental Materials in Different Media—An In Vitro Study. Int. J. Clin. Pediatr. Dent. 2009;2(1):1.
CrossRef - Ten Cate J. Remineralization of caries lesions extending into dentin. J Dent. Res. 2001;80(5):1407-11.
CrossRef - Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials—fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent. Mater. 2007;23(3):343-62.
CrossRef - Sharma S, Maurya S. Cention N: A Review. J Curr Res. 2018;10(05):69111-2.
- Lecture BLBM. Dentin caries: progression and clinical management. Oper dent. 2002;27(3):211-7.
- Mitra S. In vitro fluoride release from a light-cured glass-ionomer liner/base. J dent res. 1991;70(1):75-8.
CrossRef - Singh TM, Suresh P, Sandhyarani J, Sravanthi J. Glass ionomer cements (GIC) in dentistry: a review. Int J Plant, Anim & Environ Sci. 2011;1(1):26-30.
- McCabe JF, Walls AW. Appl. dent. mat: John Wiley & Sons; 2013
- Itota T, Carrick TE, Rusby S, Al-Naimi OT, Yoshiyama M, McCabe JF. Determination of fluoride ions released from resin-based dental materials using ion-selective electrode and ion chromatograph. J dent. 2004;32(2):117-22.
CrossRef - Frost PM. An audit on the placement and replacement of restorations in a general dental practice. Prim Dent Care. 2002;9(1):31-6.
CrossRef - Mousavinasab SM, Meyers I. Fluoride release by glass ionomer cements, compomer and giomer. Den res j. 2009;6(2):75.
- Virmani S, Hegde M, Shetty S, Sadananda V. Comparative Evaluation of Fluoride Release from Three Glass Ionomer Cements–An in vitro Study. Bri J App Sci Tec. 2016;18:1-6.
CrossRef - Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat meth med res. 1999;8(2):135-60.
CrossRef - Shahid S, Duminis T. Glass-ionomer cement: chemistry and its applications in dentistry. Adv Dent Biomat: Elsevier; 2019. p. 175-95.
CrossRef - Moslemi M, Fekrazad R, Tadayon N, Ghorbani M, Torabzadeh H, Shadkar M. Effects of ER, Cr: YSGG laser irradiation and fluoride treatment on acid resistance of the enamel. Pediatr. Dent.. 2009;31(5):409-13.
- McCABE JF, Yan Z, Al Naimi OT, Mahmoud G, Rolland SL. Smart materials in dentistry—future prospects. Dent mat j. 2009;28(1):37-43.
CrossRef - Yip H-k, Smales RJ. Fluoride release from a polyacid-modified resin composite and 3 resin-modified glass-lonomer materials. Quintessence Int. 2000;31(4).
- Cury J, Conceicao E. Fluoride release from fluoride-containing materials. Ope Dent. 1996;21:185-90.
- Zafar MS. Effects of surface pre-reacted glass particles on fluoride release of dental restorative materials. World Appl Sci J. 2013;28(4):457-62.
- Danelon M, Camara D, Miyasaki M, Scarpa J, Delbem A, Sassaki K. Effect of fluoride gels supplemented with hexametaphosphate in inhibiting demineralization. J Dent Res. 2012;91:162761.
- Delbem A, Pedrini D, Franca J, Machado T. Fluoride release/recharge from restorative materials-effect of fluoride gels and time. Operative Dentistry-University Of Washington-. 2005;30(6):690.
- Mathew B, Thomas AM. Comparitive Evaluation of the Fluoride Release and Rechargability of Chitosan Modified Glass Ionomer Cement and a Glass Ionomer Cement–An in Vitro Study. Indian J Public Health Res Dev. 2019;10(1).
CrossRef - Dionysopoulos D, Koliniotou-Koumpia E, Helvatzoglou-Antoniades M, Kotsanos N. Fluoride release and recharge abilities of contemporary fluoride-containing restorative materials and dental adhesives. Dent mat j. 2013;32(2):296-304.
CrossRef - Jingarwar MM, Pathak A, Bajwa NK, Sidhu HS. Quantitative assessment of fluoride release and recharge ability of different restorative materials in different media: An in vitro study. J Clin Diagn Res. 2014;8(12):ZC31.
CrossRef - Conceição JMd, Delbem ACB, Danelon M, da Camara D, Wiegand A, Pessan JP. Fluoride gel supplemented with sodium hexametaphosphate reduces enamel erosive wear in situ. J dent. 2015;43(10):1255-60.
CrossRef







