Manuscript accepted on :21-04-2022
Published online on: 30-06-2022
Plagiarism Check: Yes
Reviewed by: Dr. Guy-Armel Bounda
Second Review by: Dr. Mario Vincenzo Russo
Final Approval by: Dr. Ian James Martin
Miti Gandhi* , Harshit Zaveri
, Harshit Zaveri , Ipseeta Ray
, Ipseeta Ray and Rakesh Gildhiyal
and Rakesh Gildhiyal
Department of Pharmacology, Mahatma Gandhi Missions Medical College, Navi Mumbai, Maharashtra, India.
Corresponding Author E-mail: mitigandhi26@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2448
Abstract
Background: Psychotropic medications, used at normal therapeutic or maintenance doses, have been associated with Adverse drug reactions (ADRs). Multiple studies have noted the high burden of ADRs caused by psychiatric medications. Hence, reporting of likely ADRs is warranted as pharmacovigilance data will help health care professionals to provide reliable, balanced information for the effective assessment of the risk/benefit profile of medicines. Methodology: Necessary approval from the Institutional Ethics Committee will be obtained before initiating the study. It is a prospective, observational, single centre pilot study. 30 patients attending the Psychiatry OPD in MGM Hospital, Kamothe, and experiencing ADRs were enrolled in the study. Results: People who experienced an ADR had a median age of 43±16.23 yrs. A total of 35 ADRs were noted in 30 patients on psychiatric medications. Therapeutic Failure (57%) was observed to be the most common ADR followed by gastric reflux (22%). Benzodiazepines (19%) and SSRIs (17%) seems to be the most common class of drugs, while Trihexyphenydyl (14%) was noted to be the most common drug, causing ADRs. Conclusion: Therapeutic Failure (57%) was observed to be the most common ADR and benzodiazepines (19%) seems to be the most common drug class causing ADR. Under reporting of ADRs is an issue that arises from the lack of awareness amongst patients as well as healthcare professionals. Hence, pharmacovigilance in psychiatry units alerts the physicians and protects the patients from ADRs that can be avoided.
Keywords
Adverse Drug Reaction; Observational; Psychiatry; Pilot; Pharmacovigilance
Download this article as:| Copy the following to cite this article: Gandhi M, Zaveri H, Ray I, Gildhiyal R. Adverse Drug Monitoring in the Psychiatry Outpatient Department in a Tertiary Care Hospital-A Pilot Study. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Gandhi M, Zaveri H, Ray I, Gildhiyal R. Adverse Drug Monitoring in the Psychiatry Outpatient Department in a Tertiary Care Hospital-A Pilot Study. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3OObsvx |
Introduction
Adverse drug reactions (ADRs) lead to a substantial increase in morbidity and mortality, invariable causing a rise in the overall cost of healthcare. Psychotropic medications prescribed even at therapeutic and maintenance doses in psychiatry, have been commonly linked to various ADRs. Hence, the role of pharmacovigilance is an important one, especially to alert the healthcare professionals of likely ADRs and to safeguard patients being treated with psychotropic medications.1
The commonest clinical diagnosis observed in the psychiatry OPD seem to schizophrenia and related psychoses (schizotypal and other delusional disorders), followed by depression.2 The commonest ADRs that have been observed are tremors, somnolence, dyspepsia, weight gain and constipation. Atypical antipsychotics and SSRIs are suspected be the most common class of drugs leading to ADRs. 2,3
The burden of ADRs in psychiatry is high and it has been noted as such by multiple studies.4 Lack of awareness, both at the level of healthcare professionals and patients, has been seen to lead to under reporting.5 Setting up a robust pharmacovigilance program in psychiatry units can be extremely beneficial in preventing under reporting and preventing avoidable harm to the user population.2
Hence, a pilot study was conducted to monitor and report the adverse drug reactions that were encountered in the psychiatry OPD department and undertook causality assessment.
Material and Methods
The pilot study was conducted as prospective, observational study design at a single centre i.e the Psychiatry OPD, MGM Medical College and Hospital, Kamothe, Navi Mumbai, over a period of 2 months. All necessary approvals from the Institutional Ethics Committee were obtained before initiating the study (Approval no.- NEC/2020/08/58). All patients attending the psychiatry OPD over the age of 18 years, experiencing an ADR and willing to give consent, were enrolled in the study. Patients fulfilling the inclusion and exclusion criteria were considered. Informed consent of the participants was taken by explaining the purpose of the study. Data from the CDSCO ADR reporting form fed into MS Excel and analysed. Causality assessment was done using the WHO UMC scale. Following data was collected from the prescribed prescriptions and recorded in the CDSCO Suspected ADR reporting form:
Patient information
Suspected Adverse Reaction
Suspected Medication
Reporter Details
Results
Demographic profile of patients
A total of 30 patients were enrolled, with an almost equal division between male and female (Male=48% and Female=52%). The participants were divided into 3 age groups and the no. of patients and ADRs/ age group is shown in Table 1. The median age of all the groups was calculated to be 42±14.21 years.
Table 1: Distribution of patients based on their age groups.
| Age of the patients | No. of patients (n=30) | No. of ADRs/age group (n=35) |
| 18-30 years | 7(23%) | 9(25%) |
| 31-60 years | 21(7%) | 23(65%) |
| >60 years | 2(6%) | 2(5.7%) |
Incidence of ADRs
A total of 35 adverse drug reactions (ADRs) were seen amongst the 30 patients. The incidence rate was found to be 6.8%. Out of all the prescriptions, majority (83%) had only 1 ADR/ prescription while 16% of the prescriptions had 2 ADRs/prescription.
The most common diagnoses observed were depression disorder followed by adjustment disorder. The variety of diagnoses seen in the psychiatry OPD are reflected in Graph
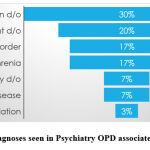 |
Graph 1: Diagnoses seen in Psychiatry OPD associated with ADRs. |
Common ADR’s Encountered
There were several ADRs that presented to the psychiatry OPD and their distribution is shown in Graph 2, with the most frequent (63%) being drug therapeutic failure. The other ADRs noted were dyspepsia (14%), followed by weight gain (6%), fine tremors (6%) and sedation (5%) at an almost equal frequency, while merely 3 % of patients experienced sleep disturbance and abdominal pain each.
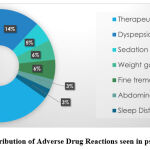 |
Graph 2: Distribution of Adverse Drug Reactions seen in psychiatry OPD. |
Drug classes responsible for ADR
The total number of drugs that caused the ADRs were 67, with 49 being single drugs while 18 of them were fixed dose combinations (FDCs). The average number of drugs taken by a patient who experienced atleast 1 ADR is 2.6. The class of drugs of drugs that caused the ADRs were noted to be atypical antipsychotics (22%), benzodiazepines(22%), selective serotonin reuptake inhibitors(SSRIs) (16%), aliphatic carboxylic acid antiepileptic(5%), central anticholinergic (5%), iminostilbene antiepileptic (5%), mood stabiliser (5%), newer non benzodiazepine hypnotic (5%), phenyltriazine antiepileptic (5%) and tricyclic antidepressant (TCA) (5%). Many drugs were involved in causing the ADRs as shown in Graph 3. Out of these the most common were olanzapine, trihexyphenidyl, lorazepam and escitalopram. The FDCs involved in causing the ADRs, from the most common to the least, were trihexyphenidyl +risperidone (28%), escitalopram+ etizolam (22%), escitalopram+ clonazepam (22%), sodium valproate + valproic acid (11%), donepezil +memantine (11%) and amitriptyline+ chlordiazepoxide (6%).
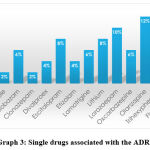 |
Graph 3: Single drugs associated with the ADRs. |
Causality assessment
The causality assessment conducted by WHO-UMC scale revealed that 100% of the ADRs could be categorized as “Possible” because no dechallenge and rechallenge information was available.
Discussion
An adverse drug reaction (ADR) can be defined as ‘A response to a drug which is noxious and unintended, and which occurs at doses normally used in man for the prophylaxis, diagnosis, or therapy of disease, or for the modifications of physiological function’. (6) Some of the important reasons for increasing ADRs are a variety of new drugs introduced into the market, rising polypharmacy and the increase in the age of the population. (7) Properly conducted pharmacovigilance is an extremely essential step in recognizing as well as controlling adverse drug reactions. Castburg et al discovered that almost half of the physicians had never reported an ADR. (8) Under reporting seems to be the main challenge when it comes to pharmacovigilance, which seems to be due to lack of time and a greater importance given to diagnoses than drugs by practicing physicians.9
The need for pharmacovigilance in psychiatry due to several reasons. Most patients need polypharmacy and long-term medications to treat psychiatric disorders.10 Many clinical trials of psychiatric medications are conducted under controlled conditions where comorbidities and other factors are not taken under consideration and a publication bias has been noted in many of the trials.11 Pharmacovigilance units for psychiatric medication can be extremely useful to predict expected ADRs, prevent them by early detection and provide a continuity in providing healthcare.12
In our study we found that majority of the ADRs occurred in the 31-60 years age group, which was also noted by Gawali et al.2 We also found that the vast majority of the patients experienced minimum 1 ADR/ prescription just like the study conducted by Sridhar et al.1
The commonly seen diagnoses that were associated with ADRs were similar to that found in the study by Sengupta et al, i.e depression disorder, bipolar affective disorder and schizophrenia.3
The most common ADR noted in our study was drug therapeutic failure which was not observed by most other studies. Drug therapeutic failure(DTF) is defined as ‘an adverse drug reaction in which the expected drug effects do not occur following a prescribed pharmacological treatment, including any clinical event that could be related to a low prescribed dose or lack of compliance’. 13 DTF was followed by dyspepsia, weight gain, fine tremors, sedation, abdominal pain and sleep disturbance as the prominent ADRs seen in our study which are comparable to those noticed in studies like those conducted by Baryaliya et al and Sengupta et al. 14, 3
The class of drugs found to be most associated with the ADRs in our study were the same as those found in the study done by Lucca et al, which were psycholeptics like atypical antipsychotics, benzodiazepines and psychoanaleptics like SSRIs.15 Olanzapine was found to be the most common single drug to be linked to the ADRs by our study as well as studies carried out by Sengupta et al and Chakravarty et al. (16, 3)
The causality assessment by WHO UMC scale revealed that all the ADRs in our study were under the “Possible” category which was different than the study conducted by Scandashree et al, where the majority ADRs were under the “Probable” category.17
It has been noted by multiple studies that pharmacovigilance needs to become an integral part of healthcare. Awareness amongst physicians, patients and their guardians need to be raised regarding the possible ADRs. In our study, we have been able to emphasize the need to recognize and report ADRs, especially drug therapeutic failure in the psychiatry OPD. An in depth look at the common ADRs and their awareness, needs to be carried out to understand the current obstacles that lead to under reporting of ADRs.
There were several limitations to our study such as the small sample size, short duration and single site of the study.
Conclusion
Adverse drug reactions lead to numerous issues for the healthcare system by lengthening hospital stay, burdening the patient with finances and most importantly, affecting the safety of the patient. Majority of the patients with ADRs experienced atleast 1 ADR/ prescription and the incidence rate was noted to be 6.8% which reflects the fact that ADRs are common place with psychiatric medications. Therapeutic Failure (57%) was observed to be the most common ADR and benzodiazepines (19%) seems to be the most common drug class causing ADR. Under reporting of ADRs is an issue that arises from the lack of awareness amongst patients as well as healthcare professionals. Hence, pharmacovigilance in psychiatry units alerts the physicians and protects the patients from ADRs that can be avoided.
Conflict of Interest
There are no conflict of interest.
Funding Source
There is no funding source.
References
- Sridhar SB, Al-Thamer SS, Jabbar R. Monitoring of adverse drug reactions in psychiatry outpatient department of a Secondary Care Hospital of Ras Al Khaimah, UAE. Journal of basic and clinical pharmacy. 2016 Jun;7(3):80.
CrossRef - Gawali UP, Kesari HV, Gawand KS. Adverse drug reaction profile at psychiatry outpatient department of a tertiary care centre. Int J Basic Clin Pharm. 2017;6:2428-33.
CrossRef - Sengupta G, Bhowmick S, Hazra A, Datta A, Rahaman M. Adverse drug reaction monitoring in psychiatry out-patient department of an Indian teaching hospital. Indian journal of pharmacology. 2011 Feb;43(1):36.
CrossRef - Szabo CP. Common adverse drug reactions with psychiatric medications and an approach to their management: Adverse drug reactions are as important in psychiatric practice as they are in any other branch of medicine. Continuing Medical Education. 2011;29(6).
- Singh H, Mamatha K, Rani S, Magdalene S, Sabu J, Jeny R. A study of adverse drug reactions associated with antipsychotic drugs among female patients attending outpatient psychiatry department. Archives of Mental Health. 2017 Jan 1;18(1):4.
- Safety of Medicines A guide to detecting and reporting adverse drug reactions. Geneva: World Health Organization; 2002 [cited 4 June 2021]. Available from: http://apps.who.int
- Schatz S, Weber RJ. Adverse drug reactions. Pharmacy Practice. 2015 Aug 24;1(1).
- Castberg I, Reimers A, Sandvik P, Aamo TO, Spigset O. Adverse drug reactions of antidepressants and antipsychotics: Experience, knowledge and attitudes among Norwegian psychiatrists. Nordic journal of psychiatry. 2006 Jan 1;60(3):227-33
CrossRef - Dikshit RK. Challenges in pharmacovigilance. Indian journal of pharmacology. 2010 Dec;42(6):333
CrossRef - Rajkumar RP, Melvin G. Pharmacovigilance for psychiatrists: An introduction. Indian journal of psychiatry. 2014 Apr;56(2):176.
CrossRef - Dharman D, Krishnan P, Ravikumar KG, Dharan SS, Rajan S. The era of pharmacovigilance and the need of pharmacovigilance in psychiatry: A review. Journal of Drug Delivery and Therapeutics. 2019 Feb 15;9(1-s):449-52.
CrossRef - Nalini R. Pharmacovigilance study in psychiatry out-patient department of a tertiary care teaching hospital. Int J Basic Clin Pharmacol 2017;6:2375-9
CrossRef - Franceschi A, Tuccori M, Bocci G, Vannozzi F, Di Paolo A, Barbara C, Lastella M, Blandizzi C, Del Tacca M. Drug therapeutic failures in emergency department patients: a university hospital experience. Pharmacological research. 2004 Jan 1;49(1):85-91
CrossRef - Barvaliya S, Panchal JR, Desai MK, Parikh M. Pattern of adverse drug reactions into psychiatric patients. International Journal of Basic & Clinical Pharmacology. 2019 May;8(5):1059
CrossRef - Lucca JM, Ramesh M, Parthasarathi G, Ram D. A Prospective Surveillance of Pharmacovigilance of Psychotropic Medicines in a Developing Country. Psychopharmacology bulletin. 2016 Mar 1;46(1):54
- Chakravarty P, Neog P, Dewan B. Study of adverse drug reactions of atypical antipsychotic drugs in the department of psychiatry in a tertiary care hospital of Assam. Open Journal of Psychiatry & Allied Sciences. 2017;8(1):24-8
CrossRef - Udaykumar P. A cross-sectional study to assess the adverse effect profile of second-generation antipsychotics: risperidone, olanzapine and quetiapine. International Journal of Basic & Clinical Pharmacology. 2016 Sep;5(5):1785.
CrossRef







