Manuscript accepted on :20-01-2022
Published online on: 10-02-2022
Plagiarism Check: Yes
Reviewed by: Dr. Swastika Maity
Second Review by: Dr. Mohammed Najim Abed
Final Approval by: Dr. Alessandro Leite Cavalcanti
Rosamarlina1,2 , Mochammad Hatta2,3,
, Mochammad Hatta2,3, , Irawaty Djaharuddin4
, Irawaty Djaharuddin4 , Ilhamjaya Patellongi5
, Ilhamjaya Patellongi5 , Agus Dwi Susanto6
, Agus Dwi Susanto6 , Andi Asadul Islam7
, Andi Asadul Islam7 , Muhammad Nasrum Massi3
, Muhammad Nasrum Massi3 , Agussalim Bukhari8
, Agussalim Bukhari8 , Arif Santoso4
, Arif Santoso4 , Nur Ahmad Tabri4
, Nur Ahmad Tabri4 , Farida Murtiani1
, Farida Murtiani1 , Ade Rifka Junita2,3
, Ade Rifka Junita2,3 , Ahmad Syukri Saleh2,3, Ressy Dwiyanti9
, Ahmad Syukri Saleh2,3, Ressy Dwiyanti9  and Sesilia Rante Pakadang10
and Sesilia Rante Pakadang10
1Sulianti Saroso Infectious Diseases Hospital, Jakarta, Indonesia
2Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
3Molecular Biology and Immunology Laboratory, Faculty of Medicine Hasanuddin University, Makassar, Indonesia.
4Department of Pulmonology and Respiratory Medicine, Faculty of Medicine Hasanuddin University, Makassar, Indonesia.
5Department of Physiology, Faculty of Medicine Hasanuddin University, Makassar, Indonesia.
6Department of Pulmonology and Respiratory Medicine, Persahabatan Hospital, Jakarta, Indonesia.
7Department of Neurosurgery, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
8Department of Nutrition, Faculty of Medicine, Hasanuddin University, Makassar, Indonesia
9Department of Microbiology, Faculty of Medicine, Tadulako University, Palu, Indonesia
10Department of Pharmacy, Health Polytechnic of Makassar, Indonesia
Corresponding Author E-mail: hattaram@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2344
Abstract
Background: Increasing resistance to TB drugs raises the challenge of TB eradication. Miana leaves is Indonesian traditional herbal medicine, have antimicrobial, anti-inflammatory, and immunoregulatory action. Not much is known about the effect of Miana on HIF-1α and ICAM-1, the immunoregulators of infection and inflammation. This study aims to elucidate the effect of Miana on HIF-1α and ICAM-1 in M. tuberculosis (Mtb) infected mice. Materials and Methods: This experimental study used Mtb infected Balb/c mice were divided into 4 groups; group 1 is placebo, group 2 is treated with Rifampicin as Anti TB drug, group 3 is treated with Miana, and group 4 is treated with Miana + Anti TB drug. HIF-1α and ICAM-1 serum levels were analyzed using ELISA. Results: There is a significant difference of mean HIF-1α (p= 0.00, F = 114.21) and ICAM-1 (p= 0.00, F = 113.11) between the four groups after treatment. HIF-1α level is significantly lower in anti TB treatment, Miana, and Miana + anti TB treatment compared to placebo (mean difference (MD) 35,764.67, p=0.00; 29,230.98, p=0.000; 38,489.62, p=0.00, respectively). Furthermore, ICAM-1 level is significantly lower in anti TB treatment, Miana, and Miana + anti TB treatment compared to placebo (MD 95,449.68, p=0.00; 79,509.69, p=0.00; 108,672.83, p=0.00, respectively). Conclusion: HIF-1α and ICAM-1 expression was reduced after Miana administration. Miana can be a potential complement to anti-TB treatment but cannot replace rifampicin as anti-TB drugs.
Keywords
Coleus scutellariodes; HIF-1α; ICAM-1; Miana leaf; M. tuberculosis; Rifampicin
Download this article as:| Copy the following to cite this article: Rosamarlina R, Hatta M, Djaharuddin I, Patellongi I, Susanto A. D, Islam A. A, Massi M. N, Bukhari A, Santoso A, Tabri N. A, Murtiani F, Junita A. R, Saleh A. S, Dwiyanti R, Pakadang S. R. The Changes of HIF-1α and ICAM-1 Expression after Miana (Coleus Scutellariodes [L]) Treatment in Balb/C Mice with Mycobacterium Tuberculosis Infection. Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Rosamarlina R, Hatta M, Djaharuddin I, Patellongi I, Susanto A. D, Islam A. A, Massi M. N, Bukhari A, Santoso A, Tabri N. A, Murtiani F, Junita A. R, Saleh A. S, Dwiyanti R, Pakadang S. R. The Changes of HIF-1α and ICAM-1 Expression after Miana (Coleus Scutellariodes [L]) Treatment in Balb/C Mice with Mycobacterium Tuberculosis Infection. Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3syDJMY |
Introduction
Tuberculosis (TB) is one of the major global health problem. It is estimated that two thirds of TB burden lies in eight countries including Indonesia which held 8% of the burden. WHO 2018 report estimated there were more than 1 million new TB cases in indonesia.1,2 Tuberculosis has been increasingly harder to treat because of the growing problem of multi-drug-resistant (MDR) TB. Therefore, there is a need for additional means to antituberculosis drugs to treat TB.3,4,5,6
The use of traditional herbal medicine has been a staple alternative and complement to conventional medicine for Indonesian. Miana (Coleus scutellariodes [L] Benth) is known as one of the traditional herbal medicine that is often used. It contains flavonoid, tanin, triterpenoid, steroid and atsiri oil that have antibacterial, antiinflammatory and antioxidant properties.7,8,9,10 In one in vitro study, Miana extract can suppress the growth of Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, and M. tuberculosis.6,11,12,13,14Administration of Miana extract also increases the number of CD4 T cells, IFN-γ levels, and TNF-α and also reduces the M. tuberculosis colonies in Wistar mice’s lungs. Miana extract can affect proliferation of T cells in mice that was given 510mg/kgBW of Miana extract.15,16,17,18,19
Miana has an effect on host immune response. HIF-1α and ICAM-1 played an important role in immune response to bacterial infection. M.tuberculosis infection triggers the host’s immune response and causes inflammation. Local hipoxia caused by the inflammation increases cellular level of HIF-1α.10,11 HIF-1α induces phagocytotic activities, nitric oxide synthase (NOS) and antimicrobial peptides which have a direct antimicrobial activities. ICAM-1 is also stimulated by inflammation and facilitates the transmigration of leukocytes.20
Not much is known about the effect of Miana leaves extract on HIF-1α and ICAM-1 in M.tuberculosis infection. This can be useful to find out how the molecular mechanism of Miana against M. tuberculosis infection and is a modality for prevention, supplementation and treatment in TB patients.
This study aims to elucidate the effect of Miana on HIF-1α and ICAM-1 in Balb/c mice infected with M. tuberculosis.
Material and methods
Ethical Statement
This study was conducted in accordance with standard guidelines for the use and care of experimental animals. The use of these animals will be reviewed and approved by the ethics committee of the Faculty of Medicine, Hasanuddin University, Makassar, Indonesia, No: 177/UN4.6.4.5.31/ PP36/ 2021, date: 17 March 2021.
Research design
This experimental study used 20 Balbc/c mice and was conducted in July 2020 in Molecular Biology and Immunology Laboratory, Faculty of Medicine, Hasanuddin University (UNHAS), Makassar, Indonesia. The mice were divided into four groups which Group 1 is a negative control group treated with placebo; Group 2 is a positive control group treated with Rifampicin as anti TB drug); Group 3 is an intervention group treated with Miana extract; and Group 4 is an intervention group treated with Miana extract and Rifampicin as anti TB drug.
Balb/c mice
12 weeks old pathogen free Balb/c mice weighing 30-40 grams were used in this experiment. The animals were acclimatized for 7 days before the start of the study, all mice were kept in a room temperature, a 12-hour light and dark cycle, and were given standard natural pellet food and adequate drink3,4,5,8,9
Miana Extract
Miana leaves were obtained from Toraja, South Sulawesi, Indonesia. The Miana extract was made using 10 grams of Miana Leaves that were washed and dried in 50°C oven. The dried leaves were grinded and sieved using size 100 mesh sieve to achieve fine powder form. A total of 30 grams of Miana powder was diluted with ethanol with 1:10 ratio and mixed using a shaker for 24 hours in room temperature. The mixture was filtered using Whatman filter with 20 µm of pore size . The filtrate was evaporated using a rotary evaporator in 50°C temperature until concentrated and then dried using a freeze dryer. The concentrated extract was made into a pellet. Keep in black light glass bottle and the pellets were stored in a refrigerator until use. The pellets will be diluted with distilled water (Otsu-WI) and given to the mice using a nasogastric tube for 7 days.3,4,5,7,9,14,21
Research Protocol
Shown on Figure 1, all 20 Balb/c mice were put into 4 groups randomly. On day 0, the first blood draw of 0.2 ml of venous blood in tail was drawn (before infection and before treatment blood sample) from all mice in all groups and all mice were infected by M. tuberculosis. On day 14 after infection, the second blood draw (after infection before treatment blood sample) was taken. Group 1 was given 10 mg/kgBW/day of distilled water as placebo, group 2 was given 78 mg/kgBW of Rifampicin as anti TB drug, group 3 was given Miana extract, group 4 was given anti TB drug and Miana extract. On day 14 after treatment and day 28 after infection, the final blood draw in tail vena was done3,7,9,11,13.
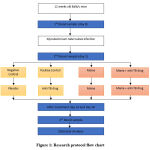 |
Figure 1: Research protocol flow chart |
Venous blood sample was centrifugated to obtain the blood serum. The serum was stored in -20o C until analysis. HIF-1α and ICAM-1 protein level was measured in pg/ml. HIF-1α and ICAM-1 protein level before induction, before treatment, and after treatment of all groups were done. All sample analysis was done in duplicate to ensure validity of ELISA analysis results.
Statistical Analysis
Normality test was done using Shapiro-Wilk test and Levene’s test was done for homogeneity test. Protein levels of each group were presented in means and standard deviation. One-way ANOVA test was used to determine the differences between the means of HIF-1α and ICAM-1 protein levels of the experimental groups. Post-hoc analysis was done to further elucidate the differences of means. P value below 0.05 is determined as statistically significant. All statistical analyses were done using SPSS 20.0 software for Windows.
Results
Table 1 showed HIF-1α and ICAM-1 protein levels in each group. The highest HIF-1α protein levels before infection were found in group 1 (mean ± SD: 16,275.15 ± 3,660.47; 15,287.939 ± 4,346.87; 15,060.39 ± 4,482.69; 14,537.32 ± 3,030.49, respectively), followed by group 2, 3 and 4. HIF-1α protein levels after M. tuberculosis infection before treatment were highest in group 3, followed by group 4, 1, and 2 (mean ± SD: 48,996.60 ± 1,880.71; 46,719.28 ± 3,258.97; 45,914.73 ± 3,636.34; 45,388.40 ± 4,205.40, respectively). After treatment, the lowest levels of HIF-1α were found in group 4, followed by group 2 and 3 and the highest levels in group 1 (mean ± SD: 16,918.79 ± 1,967.44; 19,643.73 ± 4,229.83; 26,177.42 ± 2,647.28; 55,408.41 ± 5,095.27, respectively).
Before infection, ICAM-1 levels were highest in group 1 (mean ± SD: 70,476.65 ± 10,567.54), then group 3 (mean ± SD: 65,033,86 ± 10309.90), group 2 (mean ± SD: 61,600.63 ± 1,1104.79), Group 4 (mean ± SD: 54,353.08 ± 4,494.03). After infection, ICAM-1 levels increased and were highest in group 3 (mean ± SD: 148.821.02 ± 10,819.83), followed by group 4 (mean ± SD: 142,790.84 ± 8,440.84), group 2 (mean ± SD: 141,055.38 ± 10,881.41), and group 1 (mean ± SD: 138,759.95 ± 7,690.45). After treatment, ICAM-1 levels decreased with the lowest ICAM-1 levels found in group 4 (mean ± SD: 60,802.51 ± 9,207.74), then group 2 (mean ± SD: 74,025.65 ± 13,324.45), Group 3 (mean ± SD: 89,965.65 ± 6,229.42), and the highest in group 1 (mean ± SD: 169,475.34 ± 10,914.71).
Table 1: HIF-1α and ICAM-1 levels in before infection, after M.tb infection and after treatment of each group
| Group | Before infection | After Mtb Infection | After Treatment | F | p value | |
| HIF-1α | Anti-TB drug* | 15,287.93 ± 4,346.87 | 45,388.40 ± 4,205.40 | 19,643.73 ± 4,229.83 | ||
| Miana | 15,060.39 ± 4,482.69 | 48,996.60 ± 1,880.71 | 26,177.42 ± 2,647.28 | |||
| Anti-TB drug + Miana | 14,537.32 ± 3,030.49 | 46,719.28 ± 3,258.97 | 16,918.79 ± 1,967.44 | 114.21 | 0.00 | |
| Placebo | 16,275.15 ± 3,660.47 | 45,914.73 ± 3,636.34 | 55,408.41 ± 5,095.27 | |||
| ICAM-1 | Anti-TB drug | 61,600.63 ± 1,1104.79 | 141,055.38 ± 10,881.41 | 74,025.65 ± 13,324.45 | ||
| Miana | 65,033.86 ± 10,309.90 | 148,821.02 ± 10,819.83 | 89,965.65 ± 6,229.42 | 113.11 | 0.00 | |
| Anti-TB drug + Miana | 54,353.08 ± 4,494.03 | 142,790.84 ± 8,440.84 | 60,802.51 ± 9,207.74 | |||
| Placebo | 70,476.65 ± 10,567.54 | 138,759.95 ± 7,690.45 | 169,475.34 ± 10,914.71 |
*Anti -TB drug : Anti Tuberculosis (Rifampicin)
One-Way ANOVA test was performed to determine whether there was a significant difference between the mean protein levels in each group after being given treatment (table 1). Results showed a significant difference in HIF-1α and ICAM-1 protein levels between four groups (HIF-1α (P value = 0.00, F count = 114.21), ICAM-1 (P value 0.00, F count = 113.11)).
A post-hoc test (table 2) was conducted to further analysed the differences in the levels of HIF-1α and ICAM-1 between groups. HIF-1α level is significantly lower after rifampicin, Miana, and Miana + rifampicin administration compared to placebo (mean difference 35,764.67, p=0.00; 29,230.98, p=0.00). ICAM-1 level is significantly lower after rifampicin, Miana, and Miana + rifampicin administration compared to placebo (mean difference 95,449.68, p=0.00; 79,509.69, p=0.00; 108,672.83, p=0.00, respectively). There was no difference in protein levels of HIF-1α and ICAM-1 levels between the group given anti TB drug + Miana compared to the group that were given anti TB drug alone.
Table 2: Mean comparison of HIF-1α and ICAM-1 levels in each group after intervention
| Group | HIF-1 α | ICAM-1 | ||||
| Mean Difference | p value | Mean Difference | p value | |||
| Anti-TB drug | Miana | -6,533.69* | .01 | -15,939.99* | .02 | |
| Anti-TB drug* Miana | 2,724.94 | .26 | 13,223.14 | .05 | ||
| Placebo | -3,5764.67* | .00 | -95,449.68* | .00 | ||
| Miana | Anti-TB drug | 6,533.69* | .01 | 15,939.99* | .02 | |
| Anti-TB drug Miana | 9,258.63* | .00 | 29,163.14* | .00 | ||
| Placebo | -29,230.98* | .00 | -79,509.69* | .00 | ||
| Anti-TB drug + Miana | Anti-TB drug | -2,724.94 | .26 | -13,223.14 | .05 | |
| Miana | -9,258.63* | .00 | -29,163.14* | .00 | ||
| Placebo | -38,489.62* | .00 | -108,672.83* | .00 | ||
| Placebo | Anti-TB drug | 35,764.67* | .00 | 95,449.68* | .00 | |
| Miana | 29,230.98* | .00 | 79,509.69* | .00 | ||
| Anti-TB drug + Miana | 38,489.62* | .00 | 108,672.83* | .00 | ||
*Anti -TB drug : Anti Tuberculosis (Rifampicin)
Observation of bacterial load between mice in each group showed a decrease in bacterial load up to no bacteria seen in microscope’s field of view after treatment in the group that was given Anti TB drug alone and the group that was given Anti TB drug combined with Miana. In the group that was given Miana alone, the bacterial load also decreased dramatically even though there were still few bacteria seen in observation (table 3).
Table 3: Observation of changes in the M.tuberculosis count in mice
| Group | Mice | Result (AFB/100 field) | ||
| Before Infection | Post Infection | After treatment | ||
| Anti-TB drug | 1 | 0 | 178 | 0 |
| 2 | 0 | 182 | 0 | |
| 3 | 0 | 197 | 0 | |
| 4 | 0 | 201 | 0 | |
| 5 | 0 | 173 | 0 | |
| Miana | 1 | 0 | 191 | 95 |
| 2 | 0 | 186 | 55 | |
| 3 | 0 | 180 | 70 | |
| 4 | 0 | 175 | 62 | |
| 5 | 0 | 195 | 67 | |
| Anti-TB drug + Miana | 1 | 0 | 180 | 0 |
| 2 | 0 | 187 | 0 | |
| 3 | 0 | 196 | 0 | |
| 4 | 0 | 202 | 0 | |
| 5 | 0 | 193 | 0 | |
| Placebo | 1 | 0 | 176 | 1321 |
| 2 | 0 | 188 | 1542 | |
| 3 | 0 | 185 | 1500 | |
| 4 | 0 | 200 | 1278 | |
| 5 | 0 | 190 | 1652 | |
Discussion
The results of this study showed that the pattern of mean protein levels of HIF-1α and ICAM-1 in mice with tuberculosis infection were similar after administration of Rifampicin as anti-TB drug, Miana extract, anti-TB drug and Miana, and distilled water as placebo. The levels of HIF-1α and ICAM-1 in mice with tuberculosis infection were significantly lower after administration of Miana extract than mice given placebo. The same thing happened to infected mice that were given Anti TB drug and Anti TB drug + Miana. However, the levels of HIF-1α and ICAM-1 were still lower in mice given Anti TB drug than in Miana extract alone. There was no significant difference between the levels of HIF-1α and ICAM-1 between mice given Anti TB drug and Miana compared to mice given Anti TB drug.
Various studies have shown HIF-1α levels are increased in M.tuberculosis infection in response to local tissue hypoxia.22,23 Hypoxia-inducible factor 1 alpha (HIF-1α) is induced by pro-inflammatory cytokines, growth factors, and various infections. Its induction is a common component of the body/host response to infection. HIF-1α is required for pro-inflammatory Th17 cell differentiation, activation and regulation of pro-inflammatory cytokine release.22,23,24,25 HIF-1α levels decreased after administration of antituberculosis drugs and the same thing happened after administration of Miana extract. However, there was no significant decrease in HIF-1 levels between the administration of anti-TB drug alone compared with the administration of anti-TB with Miana extract.12,14,26
ICAM-1 levels are known to be elevated in M.tuberculosis infection. In this study, ICAM-1 levels were also increased in the serum of Balc/c mice infected with M.tuberculosis.27 ICAM-1 levels decreased after the intervention. Giving Miana extract or anti TB drug alone to mice infected with M.tuberculosis caused a decrease in ICAM-1 levels. However, there was no significant decrease in ICAM-1 in mice that were given Anti TB drug with the addition of Miana extract compared to those given Anti TB drug alone. This shows that Miana extract has a similar effect to Anti TB drug on Mtb. However, the mechanism by which Miana reduces ICAM-1 levels in M.tuberculosis infection has not been elucidated in this study.28,29,30
Based on the results of this study, Miana extract had the same effect as Anti TB drug in mice infected with tuberculosis. However, the mechanism by which Miana can reduce HIF-1α levels has not been elucidated in this study. One theory that can explain this phenomenon is the content of flavonoids, tannins, saponins, and terpenoids in Miana leaves which have antimicrobial, anti-inflammatory and antioxidant properties.11,31,32These effects may play a role in reducing inflammation due to infection and leading to improvement of cellular hypoxic conditions, suppressing bacteria, inflammation and overcome ROS and hypoxia results in a decrease in the induction of HIF-1α production.4,33,34,35,36,37 While ICAM-1 is increased due to proinflammatory cytokine response due to M.tuberculosis infection. Miana’s effect can decreased inflammation. Thus, the inducer of ICAM-1 activation decreases so that ICAM-1 levels decrease.21,38,39
Although the role of Miana extract on HIF-1α and ICAM-1 levels is known by this study, this study cannot explain the exact mechanism of how Miana extract affects HIF-1α and ICAM-1 in M.tuberculosis infection. However, Miana extract which contains flavonoids which acts as a type of antibacterial as well as immunoregulatory to enhance host immune response to fight bacteria and anti-inflammatory action. In this study may explain the possible molecular mechanism of Miana on TB infection via HIF-1α and ICAM-1 pathway. Further research is needed to elucidate more specific of the pharmacodynamics of Miana leaf extract on immunoregulation of host.
Conclusion
Miana leaf extract can reduce the HIF-1α and ICAM-1 protein levels. There was no significant difference in HIF-1α and ICAM-1 levels between mice given anti TB drug alone and mice given anti TB drug with additional Miana leaf extract. The levels of HIF-1α and ICAM-1 were significantly lower in mice given Rifampicin as anti TB drug and anti TB drug with the addition of Miana leaves. This suggests that the administration of Miana leaf extract has the same effect as anti TB drug but cannot replace the function of anti TB drug. Miana extract can be a complement or adjuvant to anti TB drug although the effect of anti TB drug with Miana complement on HIF-1α and ICAM-1 is not significant.
Acknowledgement
The author would like to thank Mr. Romi Usman, Mr. Marwani, Mr. Wilhelmus Jebaru and all staff of the Molecular Biology and Immunology Laboratory, Makassar, Indonesia and consultants for their continuous help and support for the completion of this study.
Conflict of interest
There is no conflict of interest.
Funding source
This study does not receive any funding from any type of institution.
References
- Organization. WH. Global Tuberculosis Report 2020. 2020;Geneva: World Health Organization.
- Scheelbeek PFD, Wirix AJG, Hatta M, Usman R, Bakker MI. . Risk factors for poor tuberculosis treatment outcomes in Makassar, Indonesia. Southeast Asian J Trop Med Public Health. 2014;45(4).
- Rosamarlina, Hatta M, Sridiana E, Djaharuddin I, Patellongi I, Murtian F. The effect of Miana (Coleus scutellariodes [L]) on Vascular Endothelial Growth Factor expression in Balb/c mice infected with Mycobacterium tuberculosis. Biomedical and Pharmacology Journal 2021;14(2):525-32. https://dx.doi.org/10.13005/bpj/2154.
CrossRef - Wahyuni TD, Hatta M, Bukhari A, Santoso A, Massi MN. Increasing Natural Resistance Associated Macrophage Protein 1 serum level after Miana treatment in BALB/c induced Klebsiella pneumoniae experimental research. Annals of Medicine and Surgery 2021;65. https://doi.org/10.1016/j.amsu. 2021.102262
CrossRef - Amsyah UK, Hatta M, Tahir H, Alam G, Asmawati A. Expression of IL-10 in A.actinomycetemcomitans Induced Rat Treated by Purple Miana Leaves Biomedical and Pharmacology Journal. 2019;12(4):2099-104. . doi : http://dx.doi.org/10.13005/bpj/1845.
CrossRef - Hatta M, Rofia AS, Tandirogang N, Masjudi M , Yadi Y. Detection and identification of mycobacterium in sputum from suspected tuberculosis patients. BMC Research Notes. 2010;3:72. doi: 10.1186/1756-0500-3-72.
CrossRef - Syamsuri F, Hatta M, Natzir R, Alam A, Massi MN, Bahar B, et al. Expression of TLR-4 in Salmonella typhi-Induced Balb/c Mice Treated by Miana Leaves (Coleus scutellaroides (L) Indian Journal of Public Health Res Dev. 2018;9(12):1449-54.
CrossRef - Karo MBr, Tambaip T, Hatta M, Simanjuntak T, Irmawaty L, Rina T, Kamelia E, Rahmawati F, Bintang M. A mini Review of Indonesian Medical Plants for vulvovaginal candidiasis. Rasayan J. Chem. 2017; 10(4):1280-1288. http://dx.doi.org/10.7324/ RJC.2017.1041887.
- Karo MBr, Hatta M, Patellongi I, Natzir R, Tambaip. T. IgM antibody and colony fungal load impacts of orally administered ethanol extract of Plectranthus scutellarioides on mice with systemic candidiasis [Impacto de la administración oral del extracto etanólico de Plectranthus scutellarioides sobre anticuerpos IgM y la carga fúngica en ratones con candidiasis sistémica]. Journal of Pharmacy & Pharmacognosy Research. 2018;6(1):27-34. http://jppres.com/jppres
- Ramakrishnan S, Anand V, S R. Vascular Endothelial Growth Factor Signaling in Hypoxia and Inflammation. Journal of Neuroimmune Pharmacology. 2014;9(2):42-60.
CrossRef - Yanto TA, Hatta M, Bukhari A, Natzir R. Molecular and Immunological Mechanisms of Miana Leaf (Coleus Scutellariodes [L] Benth) in Infectious Diseases. Biomedical and Pharmacology Journal. 2020;13(4):1607-18. https://dx.doi.org/10.13005/bpj/2036
CrossRef - Tambaip T, Karo M, Hatta M, Dwiyanti R, Natzir R, Massi MN, Islam AA, Djawad K. Immunomodulatory effect of orally red fruit (Pandanus conoideus) extract on the expression of CC chemokine receptor 5 mRNA in HIV patients with antiretroviral therapy. Res J Immunol. 2018;11:15-21. DOI: 10.3923/rji.2018.15.21
CrossRef - Simanjuntak TP, Hatta M, Tahir M, Sirait RH, Karo M, Tambaib T, Dwiyanti R, Noviyanthi RA, Junita AR. Analysis of Anti-toxoplasma Immunoglobulin G and Immunoglobulin M Antibody Levels after Intervention with Curcuma Longa Extract on Early Pregnant Mice with Acute Toxoplasmosis. J Global Infect Dis. 2019;11:25-9. DOI: 10.4103/jgid.jgid_28_18
CrossRef - Simanjuntak TP, Hatta M, Rauf S, Prabandari SA, Siagian, Dwiyanti R. Tumor Necrosis Factor-alpha Levels and Histopathology Finding after Intervention with Curcuma longa Extract. J Med Sci. 2018;18(2):56-62. DOI: 10.3923/jms.2018.56.62.
CrossRef - Neufeld G, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9-22.
CrossRef - Suryanto AA, van den Broek J, Hatta M, de Soldenhoff R, van der Werf MJ. Recurrence of tuberculosis in patients treated with single-dose drugs in Combi-packs and fixed-dose combination (4FDC) drugs in South Sulawesi, Indonesia. Int J Tub Lung Dis. 2008;12(2):174-9
CrossRef - Umar F, Hatta M, Husain DR, Dwiyanti R, Natzir R, Sjahril S, Junita AR, Primaguna MR. Molecular characterization of mutation associated with resistances to first- and second-line tuberculosis drug among tuberculosis patients in Makassar, Indonesia. J Taibah Univ Med Sci. 2020;15(1):54-8. https://doi.org/10.1016/j.jtumed.2019.12.003
CrossRef - Umar F, Hatta M, Husain DR, Bahar B, Bukhari A, Dwiyanti R, Junita AR, Primaguna MR. Verapamil as an Efflux Inhibitor Against Drug Resistant Mycobacterium Tuberculosis: A Review. Sys Rev Pharm. 2019;10(1): Suppl s43-s48. DOI:10.5530/srp.2019.1s.22.
CrossRef - Umar F, Hatta M, Husain DR, Natzir R, Dwiyanti R, Junita AR, Primaguna MR. The effect of anti-tuberculosis drugs therapy towards mRNA efflux pump gene expression of Rv1250 in Mycobacterium tuberculosis collected from tuberculosis patients. New Microb New Infect. 2019;32(C):1-7. https://doi.org/10.1016/j.nmni.2019.100609
CrossRef - Bhalla K, Chugh M, Mehrotra S, Rathore S, Tousif S. Host ICAMs play a role in cell invasion by Mycobacterium tuberculosis and Plasmodium falciparum. Nature Communication. 2015;6:6049.
CrossRef - Karo M, Hatta M, Salma W, Patellongi I, Natzir R. Effects of Miana (Coleus scutellaroides [L] Benth) to expression of mRNA il-37 in Balb/C mice infected Candida Albicans. Pharmacognosy Journal. 2018;10(1):16-9. DOI : 10.5530/pj.2018.1.3.
CrossRef - Braverman J, Sogi, K., Benjamin, D., Nomura, D. and Stanley, S. HIF-1α is an essential mediator of IFN-γ dependent immunity to Mycobacterium tuberculosis J Immunol. 2016;197(4).
CrossRef - Shi L, Eugenin, E. and Subbian, S. Immunometabolism in Tuberculosis. : Front Immunol; 2016 [Available from: https://www.frontiersin.org/articles/10.3389/fimmu.2016.00150/full.
CrossRef - Kasim VN, Hatta M, Natzir M, Hadju V, Hala Y, Budu, Alam G, As’ad S, Febriza A, Idrus HH. Antibacterial and anti-inflammatory effects of lime (Citrus aurantifolia) peel extract in Balb/c mice infected by Salmonella typhi. J Biol Res – Bollettino della Società Italiana di Biologia Sperimentale. 2020;93(2):81-4. DOI: 10.4081/jbr.0.8951.
CrossRef - Idrus HH, Hatta M, Febriza A, Kasim VN. Antibacterial activities of Sapodilla fruit extract inhibiting Salmonella typhi in mice Balb/c. Int J Applied Pharmaceutics. 2019;11(5):121-6. DOI: http://dx.doi.org/10.22159/ijap.2019.v11s5.T0095
CrossRef - Sirait LI, Massi MN, Hatta M, Prihantono. The Effects of Extract Andaliman Fruit (Zanthoxylum acanthopodium Dc) to CAMP mRNA expression and Bacterial Load in Mice Balb-C after Gardnerella vaginal Infection. Indian J Pub Health Res Dev. 2019;9(11):607-611. DOI: 10.5958/0976-5506.2018.01525.5
CrossRef - Hamzaoui A, Hamzaoui, K., Kahan, A. and Chabbou, A. Levels of soluble VCAM-1, soluble ICAM-1, and soluble E-selectin in patients with tuberculous pleuritis. Mediators of Inflammation. 1996;5(4):276-9.
CrossRef - Kamelia E, Islam AA, Hatta M, Miko H, Karo M. Evaluation of the Activity of F2-isoprostane in Alzheimer’s Disease Rats Given Banana Extract. Pakistan J Med Health Sci. 2020;14(2):1459-564.
- Febriza A, Natzir R, Hatta M, Uiterwaal CSPM, As’ad S, Budu B, Alam G, Kasim VN, Idrus HH. Curcumin Effects in Inducing mRNA Gene Cathelidicin Antimicrobial Peptide (CAMP) in Balb/c Mice Infected with Salmonella Typhi. J Biol Res – Bollettino della Società Italiana di Biologia Sperimentale. 2020;93(2):76-80. https://doi.org/10.4081/jbr.0.8942
CrossRef - Irawaty DA Hasanuddin T, Hatta M, Harun A, Ainul W. Differences of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Moringa Leaf Extract (Moringa Oliefera L.) on Bacteria Aggregatibacter Actinomycetemcomitans and Porphyromonas Gingivalis. Indian J Pub Health Res Dev. 2019;10(8):896- 900. DOI : 10.5958/0976-5506.2019.02007.2
CrossRef - Aritonang R Natzir R, Sinrang W, Massi MN, Hatta M, M.Kamelia E. The Effect of Administration of Extract from Areca Nut Seeds (Areca Catechu L) on the Estradiol and Estrus Cycle Balb/C Female Rats. J Physics: Conference Series. 2020;1477(6):1-6. doi:10.1088/1742-6596/1477/6/062026
CrossRef - Djais AI, Hasanuddin T, Hatta M, Achmad H, Sari M. Effect of Moringa Leaf Extract (Moringa Oleifera) on Increasing the Number of Osteoblast as a Marker of Bone Remodeling. Indian J Pub Health Res Dev. 2019;10(9):1394-8.
CrossRef - Rosyidi RM, Januarman, Priyanto B, Islam AA, Hatta M, Bukhari A. The Effect of Snakehead Fish (Channa striata) Extract Capsule to the Albumin Serum Level of Post-operative Neurosurgery Patients. Biomed Pharmacol J. 2019;12(2):893-9. http://dx.doi.org/10.13005/bpj/1714
CrossRef - Mulyawan E, Ahman MR, Islam AA, Massi MN, Hatta M, Arif SK. Effect of Valerian Extract on GABRB3 Gene Expression and Sedation in BALB/C Mice. Current Bioactive Compounds. 2020;16(8):1249-57. DOI: 10.2174/1573407216999200620185627
CrossRef - Royani I Asaad S, Mappaware NA, Hatta M, Rabia. Effect of Ajwa Dates Consumption to Inhibit the Progression of Preeclampsia Threats on Mean Arterial Pressure and Roll-Over Test. BioMed Research J. 2019;2019. https://doi.org/10.1155/2019/2917895
CrossRef - Farsida, Hatta M, Patellongi I, Prihantono, Shabariyah R, Larasati RA, Islam AA, Natzir R, Nasrum M, Hamid F, Dwi Bahagia A. The Correlation of Foxp3+ Gene and Regulatory T Cells with Scar BCG Formation among Children with Tuberculosis. J Clin Tub Other Mycobact Dis. 2020;100202:1-7. https://doi.org/10.1016/j.jctube.2020.100202
CrossRef - Farsida, Shabariah R, Hatta M, Patellongi I, Prihantono, Massi MN, Islam AA, Natzir R, Dwi Bahagia A, Hamid F, Fatimah, Akaputra R, Savitri PA. Relationship between expression mRNA gene Treg, Treg, CD4+, and CD8+ protein levels with TST in tuberculosis children: A nested case-control. Ann Med Surgery. 2021;61:44-7. https://doi.org/10.1016/j.amsu.2020.12.011
CrossRef - Syarif L, Junita AR, Hatta M, Dwiyanti R, Kaelan K, Sabir M, Primaguna MR, Purnamasari NI. A Mini Review: Medical Plants for Typhoid Fever in Indonesia. Systematic Reviews in Pharmacy. 2020;11(6):1171-80. doi:10.31838/srp.2020.6.170
- Syamsuri F, Hatta M, Natzir R, Alam G, Massi MN, Dwiyanti R, Bahar B. . A Review: Worldwide Medicinal Plants for Typhoid Fever. Indian J Pub Health Res Dev. 2018;9(8):1461-5. DOI: 10.5958/0976-5506.2018.00938.5
CrossRef







