Manuscript accepted on :12-01-2022
Published online on: 21-01-2022
Plagiarism Check: Yes
Reviewed by: Dr. Kulvinder Kaur
Second Review by: Dr. Bhumika Brahmbhatt
Final Approval by: Dr. Ayush Dogra
M. Vani , M.Uma
, M.Uma , V. K. Nirmala
, V. K. Nirmala and P. Uma Maheswari Devi*
and P. Uma Maheswari Devi*
Department of Applied Microbiology and Biochemistry, Sri Padmavati Mahila Visvavidyalayam, Tirupati, Andhra Pradesh, 517502, India
Corresponding Author E-mail: umadevi66@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2380
Abstract
Liver, an important organ of the body, plays a key role in the anabolism of biomolecules along with detoxification of several xenobiotics. Thus, the protection of liver from alcohol toxicity and its causative effects such as steatosis, necrosis, fibrosis, cirrhosis and steato-hepatitis is of global concern.The consumption of alcohol results in the production of ROS and RNS in the liver thereby causing oxidative stress and free radical injuries. Therefore, reliable hepato-protective agents are at demand to combat against alcohol induced toxicity. At this point, we investigated the synergistic efficacy of aqueous extracts of Green tea and seagrassin the regression of liver damage induced by alcohol intoxication. Alcohol –induced rat models were adopted to perform biochemical and histo-pathological studies. The results demonstrated tremendous decline in alcohol induced hepatotoxicity with mixed extract which indicates the synergistic and supra additive activity of phytochemicals of both green tea and seagrass.
Keywords
Alcohol; Antioxidants; Camellia Sinensis; Hepato-protection; Oxidative Stress; Seagrass Halophila Beccarii
Download this article as:| Copy the following to cite this article: Vani M, Uma M, Nirmala V. K, Devi P. U. M. Synergistic Activity of Green Tea and Seagrass Extract in the Regression of Alcohol Induced Liver Toxicity in Rats. Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Vani M, Uma M, Nirmala V. K, Devi P. U. M. Synergistic Activity of Green Tea and Seagrass Extract in the Regression of Alcohol Induced Liver Toxicity in Rats. Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3AkXXgP |
Introduction
Liver is a vital organ involved in the metabolic functions of the body (Price and Wilson, 2005). Besides, its chief role in the metabolism of biomolecules like proteins, carbohydrates and lipids and it plays a crucial role in the storage of vitamins, iron and copper along with the detoxification of several exogenous and endogenous substances. Alcohol use disorder is the foundation for considerable diseases, and the liver is the most adversely affected organ due to the effect of alcohol on diverse cellular and molecular process of liver (Wang et al., 2015). The excessive consumption of alcohol is the root cause and imperative lead factor for the progression of liver damage (Gao and Bataller, 2015). Globally, alcohol liver diseases are one of the major health problems due to its high morbidity and mortality (Saalu et al., 2012).
Liver is the only organ able to metabolize the alcohol into acetaldehyde through the catalytic function of alcohol dehydrogenase (ADH) and catalase (CAT) in microsomal ethanol oxidizing system (MEOS). Eighty percent of consumed ethanol is oxidized by the catalytic action of ADH and MOES and the remaining 20% of ethanol by catalase. During the oxidation process, the molecular oxygen accepts the released electrons and converts H2O2 into water through the action of catalase. The acetaldehyde formed by mitochondrial enzymes then enters into citric acid cycle. However, high consumption of alcohol leads to over production of acetaldehyde, a major toxic metabolite, which stimulates the oxidative stress. Further, the diminution in the antioxidant mechanism facilitates oxidative burst due to imbalance between pro oxidants and antioxidants (Linawati et al., 2007). Hence, there is a need for natural antioxidants as dietary supplement to inhibit the free radical generation.
Literature is replete with beneficial health results of poly phenols in several pathological symptoms and diseases associated with oxidative stress such as alcohol liver diseases, cancer, neurodegenerative and cardiovascular disorders and chronic diabetes (Kim et al., 2014).In addition to terrestrial plants, the marine plants like seagrasses are gaining popularity due to their adaption to the marine environment as well as possession of high levels of antioxidants, Vitamin C&E and other phytochemical compounds (Vani et al., 2019). As per the literature, Green tea assumed a prominent status as herbal drink based on the abundance of poly phenols, flavonoids and phenolic compound (Paper et al., 2012, Ramah et al., 2014). Thus, in the present work, we aimed to evaluate the synergistic activity of seagrass Halophila beccarii and green tea Camellia sinensis extracts against alcohol induced hepatotoxicity.
Methods
Seagrass and Camellia sinensis aqueous extracts
The seagrass Halophila beccarii plants collected from the Pulicat Lake and green tea Camellia sinensis leaves brought from local market were shade dried and extracted by boiling with water for one hour. The seagrass aqueous extract (SGE), green tea aqueous extract (GTE) and mixed extract (ME) of seagrass & green tea aqueous extract (50:50) were filtered ,evaporated and stored at 4 ºC for further use.
Qualitative Phytochemical analysis of Halophila beccarii and Camellia sinensis
The SGE, GTE and ME were used for qualitative detection of phenols, saponins, tannins, flavonoids, terpenoids, steroids, carboxylic acids and coumarins. Standard methods were adopted to analyze the phytochemical constituents of all extracts (Singh et al., 2013).
Quantitative analysis for secondary metabolites
Quantitative analysis was carried out to measure the prevalence of secondary metabolites in the SGE, GTE and ME.The content of flavonoids was quantitatively detected at 510 nm by using spectrophotometer with quercetin as standard as per the method described by Sahu and Saxena (Sahu and Saxena, 2013). The total phenol content of extracts was estimated by the considering gallic acid as a standard at 750 nm (McDonald et al., 2001). The quantitative estimation of saponins was carried out at 544 nm with Diosgenin as a standard (Makkar et al., 2007).
Measurement of phytonutrients (Vitamin C and Vitamin E)
SGE, GTE and ME were used to quantify the levels vitamin C by adding the extract (100-500µg) with 3.0 ml of acidified reaction mixture containing sodium phosphate and ammonium molybdate and the levels were recorded at 695 nm after boiling the mixture 2h. The amount of vitamin E present in SGE, GTE and ME was measured at 540 nm by the addition of a thiourea (10 %) and 0.25 ml 2 % Dinitro phenyl hydrazine to the extracts (Prieto et al., 1999).
In vitro antioxidant activity of seagrass and green tea
The standard method was adopted for measuring the total antioxidant activity of SGE, GTE and ME [100-500 µg/ml] (Prieto et al., 1999, Oyaizu 1986). The reducing power of SGE, GTE and ME was measured at 700nm by comparing with standard ascorbic acid (Oyaizu, 1986). The Free Radical Scavenging activity of the SGE, GTE and ME was measured as per the method optimized in our laboratory (Vani et al., 2019). The hydrogen peroxide radical scavenging activity of the extracts was calculated by measuring the absorbance at 510 nm (Ruch et al., 1989).
Effect of seagrass, green tea and mixed extracts on alcohol induced hepato-toxic animals
Experimental Animals
All animals (150-200 grams) were maintained and experiments were performed as per the ethical standards (Institutional Animal Ethical Committee of Sri Padmavati Mahila visvavidyalyam, Tirupati, A.P. India (1677/Po/Re/S/2012/CPCSEA/30). The animals were categorized as different groups with six albino rats in each. Group 1 was the normal control group (NC group). Group-II: Normal Control supplemented with mixed extract 250 mg/kg (NCME) and Group 3 was the negative control group (ALC) treated with 20% w/v alcohol (l0ml/kg b.w.) 16 days. Groups 4, 5, and 6 were the intervention groups treated with alcohol (10 mL/kg. b.w) and Green tea extract, seagrass extract and mixed extract (GTE+ALC, SGE+ALC, and ME+ALC group) at a concentration of 250mg/kgb.w. respectively for 16 days. After treatment, blood was collected from retro- orbital plexus and serum was separated for the analysis of alanine amino transaminase , Aspartate amino transaminase and Alkaline phosphatase (Rashmi et al., 2016). Then the animals were dissected to collect liver tissue to estimate the lipid peroxides, MDA content (Fraga et al., 1988), levels of antioxidants such as superoxide dismutase (Kakkar et al., 1984; Crouch et al., 1981), catalase (Sinha, 1972) and histopathological studies.
Histopathological observations of lung tissue
For histopathological examination, small sections (0.5 cm) of hepatic tissues sliced from lobe edges were processed for paraffin embedding, fixed with 10% formaldehyde and staining was carried out using hematoxylin and eosin (Bancroft and Cook HC, 1984).
Statistical analysis
Three independent data sets were generated for all the experiments and n=6 unless otherwise noted. The mean ± SEM of the experimental data was calculated using Graph pad prism (7.0). One-Way ANOVA followed by Dunnet’s test was followed to compare with control and Statistical significance is denoted by an asterisk (*) when p values are *p<0.03 to 0.05, *p<0.001, ***p<0.0001 and ****p<0.00001.
Results
Phytochemical profile of Green tea and Seagrass
The extracts of Green tea and Seagrass exhibited the abundance of alkaloids, phenols, saponins, flavonoids, tannins, coumarins, glycosides, steroids, terpenoids (Table 1.).
Table 1: Phytochemical profile of SGE, GTE and ME
| S. No | Phytochemical Constituents |
GTE | SGE | ME |
| 1 | Alkaloids | – | + | ++ |
| 2 | Cardiac glycosides | + | – | + |
| 3 | Coumarins | – | + | + |
| 4 | Flavonoids | + | + | ++ |
| 5 | Glycosides | + | + | ++ |
| 6 | Phenols | + | + | ++ |
| 7 | Saponins | – | + | ++ |
| 8 | Steroids | – | + | ++ |
| 9 | Tannins | – | + | ++ |
| 10 | Terepenoids | + | + | ++ |
Compared with green tea, seagrass extract showed larger amount of coumarins, saponins and tannins. Both the extracts are enriched with vitamin C and vitamin E. All the secondary metabolites and vitamins seem to be abundant in the mixed extract compared to seagrass and green tea extract (Table 2).
Table 2: Abundance of phytochemical constituents in SGE, GTE and ME
| Compounds | GTE mg/g | SGE mg/g | ME mg/g |
| Phenols | 6.44 ± 0.035 | 8.15±0.025 | 19.44 ± 0.032 |
| Flavonoids | 4.25 ± 0.048 | 6.05±0.025 | 11.59 ± 0.035 |
| Saponins | 2.02 ± 0.025 | 5.04±0.082 | 13.02 ± 0.062 |
| Tannins | 0.36 ± 0.025 | 3.15±0.025 | 5.75 ± 0.045 |
| Vitamin C | 5.84 ± 0.047 | 7.15±0.055 | 10.96 ± 0.110 |
| Vitamin E | 4.95 ± 0.108 | 18.50±0.080 | 23.86 ± 1.202 |
Values are expressed as mean ± SEM of independent experiments, statistical significance 1% level (p ≤ 0.05).
In vitro anti-oxidant potential of SGE, GTE and ME
The mixed extract of seagrass and green tea demonstrated significant antioxidant activity compared parent extract and levels were highly considerable on comparison with standard ascorbic acid (Fig.1).
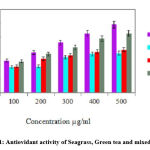 |
Figure 1: Antiovidant activity of Seagrass, Green tea and mixed evtracts |
Values are expressed as mean ± SEM of independent experiments, statistical significance 1% level (p ≤ 0.05).
The DPPH and H2O2 scavenging assay are simple, fast and widely used methods for measuring the free radical scavenging ability. All the three extracts exhibited dose-dependent free radical scavenging activity and 50% scavenging of DPPH and H2O2 was noticed with 300 µg/ml of Mixed extract (Table 3).
Table 3: Free radical scavenging by SGE, GTE and ME
| Conc µg/ml | Scavenging activity (%) on DPPH | Scavenging activity (%) on H2O2 | ||||||
| GTE | SGE | ME | Ascorbic acid | GTE | SGE | ME | Ascorbic acid | |
| 100 | 23 | 25 | 37 | 40 | 21 | 26 | 49 | 40 |
| 200 | 25 | 35 | 42 | 47 | 25 | 28 | 56 | 47 |
| 300 | 37 | 42 | 51 | 55 | 38 | 40 | 57 | 55 |
| 400 | 45 | 53 | 57 | 67 | 42 | 45 | 63 | 67 |
| 500 | 52 | 60 | 65 | 75 | 50 | 55 | 70 | 75 |
Values are expressed as mean ± SEM of independent experiments, statistical significance 1% level (p ≤ 0.05).
Reducing power of Mixed aqueous extract
The antioxidant potential of secondary metabolites is calculated based on the reducing power which reduces ferric ions to ferrous ions by their electron donating activity. The mixed extract demonstrated equivalent reducing power on comparison with ascorbic acid standard (Fig. 2).
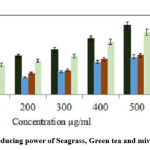 |
Figure 2: Reducing power of Seagrass, Green tea and mived extract. |
Values are expressed as mean ± SEM of independent experiments, statistical significance 1% level (p ≤ 0.05).
Protective effect of SGE,GTE and ME on liver bio markers in Alcohol induced Rats
The markedly increased activities of hepatic biomarker enzymes confirm the liver damage caused by alcohol toxicity. Alcohol induced rats were supplemented with SGE,GTE and mixed extract independently at a dose level of 250 mg/kg bwt for about 16 days. When compared with SGE and GTE, mixed extract represented significant decrease in AST, ALT, ALP levels of alcohol induced rats (Table 4).
Table 4: Effect of SGE, GTAE and ME on the Liver Function Tests as a marker of liver damage in alcohol induced hepatotoxicity in rats
| Groups | AST(IU/L) | ALT(IU/L) | ALP(IU/L) |
| Group-I: Control | 40.2 ± 1.01 | 30.5 ± 0.09 | 94.7 ± 0.05 |
| Group- II: Control + ME | 30.7 ± 1.09* | 21.8 ± 0.5* | 90.5 ± 1.04** |
| Group- III: Alcohol Control(ALC) | 70 .4± 1.08** | 80.1 ± 0.1* | 210.5± 0.07* |
| Group-IV: ALC + GTE | 50.7 ± 1.02* | 39.2 ± 0.8* | 170.4± 1.09* |
| Group-V: ALC + SGE | 45.7 ± 1.04* | 32.8 ± 0.9* | 152.8 ± 1.3* |
| Group-VI: ALC +ME | 42.9 ± 1.07** | 31.6± 1.01** | 145.4± 1.5** |
Values are expressed as mean ± SEM of independent experiments, statistical significance 1% level (p ≤ 0.05).
Protective effect of SGE,GTE &ME on liver Antioxidant enzymes and TBARS
The range of liver antioxidant enzymes such as SOD, catalase and TBARS levels was measured to detect the protective effect of SGE, GTE&ME on hepatotoxicity and oxidative damage induced by alcohol. The alcohol control group (ALC) showed 50 % reduction in the expression level of antioxidant enzymes on comparison with normal control group. Administration of SGE, GTE and ME significantly improved the level of antioxidants enzymes as compared to alcohol group. Lipid peroxidation levels were increased in ALC group due to alcohol toxicity. The augmented level of TBARS is an indicative of oxidative damage caused by peroxidation of lipids on oxidative stress. The TBARS levels were brought to the normal by the administration of mixed extract when compared with seagrass and green tea extracts which confirms the synergistic effect of seagrass and green tea in the alleviation of hepatotoxicity induced by alcohol (Table 5).
Table 5: Effect of SGE, GTE and ME on liver antioxidant enzymes in Alcohol induced rats
| Groups | SOD | Catalase | TBARS |
| Group-I: Control | 30.36 ± 0.37 | 56.4 ± 0.33 | 0.19 ± 0.002 |
| Group- II: Control + ME | 24.34 ± 0.33* | 52.13±0.31* | 0.20 ± 0.05* |
| Group- III: Alcohol Control(ALC) | 15.28± 0.05** | 23.4 ± 0.07* | 43.4 ± 0.07* |
| Group-IV: Alcohol + GTE | 18.41 ± 0.07* | 46.9 ± 0.42* | 0.30 ± 0.02* |
| Group-V: Alcohol + SGE | 22.54 ± 0.27* | 47.7 ±0.065* | 0.26 ± 0.04* |
| Group-VI: Alcohol +ME | 29.02 ± 0.31* | 52.8 ± 0.20* | 0.25 ± 0.01* |
Values are expressed as mean ± SEM of statistical significance 1% level (p ≤ 0.05)
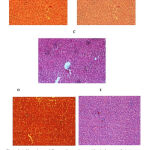
Histopathological examination
the hepatocyte cells are found to be normal with clearly visible nuclei, central vein, hepatic stroma, parenchyma and portal triad in control group(Fig.3A). Normal control rats treated with mixed extract showed normal appearence of hepatocytes (Fig.3B). The animal groups treated with alcohol showed disarranged hepotocytes with cytomegaly along with vacuolated degenerating cells with fatty changes called steatosis and necrosis of hepatic cells (Fig.3C). Appearance of steatosis with small and large droplets of fat is classical hallmark of alcoholic hepatitis in alcohol induced rats(Fig.3D). The administration of mixed extract stand for several changes like drastic reduction in the vacuolar changes associated with cytomegaly, disappearance of cloudy swelling, fatty changes and areas of regeneration (Fig.3E).
Discussion
In this work, seagrass and green tea were selected based on the presence of phytochemical
constituents such as phenols, flavonoids, tannins and saponins.The antioxidant activity of aqueous extracts of seagrass and green tea represents positive relationship between regulation of oxidative stress and secondary metabolites such as phenols and flavonoids (Zahari et al., 2016). Due to correlation between phytochemicals and the antioxidant activity of seagrass and green tea, the hepato protective potential of the mixed extract was determined using the alcohol-induced hepato toxic rats as animal models.
The elevated levels of serum bio markers, a hallmark for hepatic damage, confirmed the seriously affected Liver functions in alcohol-treated animals (Vidhya and Indira, 2009). The oxidative stress, as a result of generation of ROS, is elevated due to alcohol intoxication which facilitates the diffusion of ions and leakage of ALT and AST from cytoplasm into blood after hepatic cellular damage (Vermaulen et al., 1992). However, treatment with mixed extract has decreased the levels of hepatic markers as compared to the alcohol induced control group.Our results are in agreement with the extract of Silybum marianum plant which possess antioxidant activity due to presence of flavonoids (Soufy, 2012, Ighodaro and Omole, 2012). Similar trend in the decreased levels of hepatic enzymes by quercetin was reported in ethanol induced toxicity (Pari and Karthikesan, 2007). The protective effect of mixed extract, in the alcohol mediated hepatotoxicity, is mainly due to the stabilization of cell membrane which prevents the leakage of hepatic enzymes and their translocation into the serum.
Similarly, total antioxidant activity is one of the primary indicator of oxidative stress that indicates the collective cause of all antioxidants found in a cellular system (Remmer et al., 1989). Thus, the quantitative estimation of total antioxidant activity presents the status of exact endogenous antioxidant status. In alcohol induced hepatotoxicity, alcohol causes the hepatic damage by cytokines induced inflammatory mediators mainly TNF –α from hepatic Kupffer cells. Chronic alcohol administration results in hepatotoxicity, due to auto oxidation of hepatic cells by lipid peroxidation which generate prooxidants and reduces antioxidant levels (Eswar Kumar et al., 2013).The oxidative stress of liver was augmented by alcohol intoxication, as represented by 50% reduction in the expression level of antioxidant enzymes and increased MDA content of liver tissues relative to the normal control group. The lipid peroxidation generates higher levels of TBARS levels cause significant deformations in hepatic and extra hepatic cellular metabolism and further results in whole cell deformity and death (Maruthappan and Sakthi Shree, 2009, Arun and Balasubramanian, 2011). Superoxide dismutase (SOD) expression is the representative index for damage in liver cells as it plays a key role in alleviating the toxicity generated by free radicals (Kharpate et al., 2007). Administration of rats with the mixed extract and extracts of seagrass and green tea has decreased the elevated liver MDA and increased the expression of antioxidant enzymes such as SOD and catalase with varied degrees, and the maximum effect was found with the mixed extract of seagrass and green tea.
The biochemical investigations were also correspondingly correlated with the histopathological observations. The animal group treated with alcohol showed disarranged hepotocytes with cytomegaly along with vacuolated degenerating cells with fatty changes called steatosis and necrosis of hepatic cells. Appearance of Steatosis with small and large droplets of fat is classical hallmark of alcoholic hepatitis in alcohol induced rats. The administration of mixed extract stand for several changes like drastic reduction in the vacuolar changes associated with cytomegaly and disappearance of steatosis.
Conclusion
The conspicuous decrease of Alcohol-elevated ALT,AST and ALP levels due to the administration of mixed extract represents the stabilization of plasma membranes, repair of hepatocellular damage along with the improvement in biliary dysfunction. With the support from biochemical and histopathological observations, the current paper demonstrated the synergistic activity of seagrass and green tea extracts in the regression of hepatotoxicity induced by alcohol intoxication.
Acknowledgement
The authors are thankful to CTR (Centre for translational research) Sri padmavati Mahila Visvavidyalayam, Tirupati for supporting to do analysis.
Authors’ Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding Sources
None
References
- Price, S. A., Wilson, L.M., 2005. Pathophysiology:Clinical Concepts of Disease Processes, sixth ed. EGC Medical Book Publishers, Jakarta
- Wang, Z., Su, B., Fan, S., Fei, H., Zhao, W. Protective effect of oligomeric proanthocyanidins against alcohol induced liver steatosis and injury in mice. Biochem Biophys Res Commun. 458: 757-762 (2015.)
CrossRef - Gao, B. and Bataller, R.. Alcoholic liver disease: pathogenesis and new therapeutic Gastroenterology. 141: 1572–1585 (2011)
CrossRef - Saalu, L.C., Ogunlade, B., Ajayi, G.O., Oyewopo, A.O., Akunna, G.G. Ogummodede, O.S. The hepato-protective potentials of Moringa oleifera leaf extract on alcohol– induced hepato-toxicity in wistar rats. Am J Biotechnol Mol Sci. 2: 6-14 (2012)
CrossRef - Linawati, Y., Apriyanto, A., Susanti, E., Wijayanti, I., Donatus, A. Hepatoprotective effect of herbal decoction of putrimalu (Mimmosa pigra L.) in rats induced paracetamol. J Farmasi Indones. 10: 2007-2017 (2007).
- Kim, H.S., Quon, M.J., Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol 10: 187-195 (2014).
CrossRef - Vani, M., Murthy, S.D.S., Uma, P. Phytochemicals and in-vitro antioxidant activity of Halophila beccarii. Int J of Pha Sci and Res. 10: 1000-1007 (2019).
- Paper, O., Santoso, J., Anwariyah, S. Phenol content, antioxidant activity and fibers Profile of four tropical seagrasses from Indonesia. J Coast Dev. 15: 189-196 (2012).
- Ramah, S., Etwarysing, L., Auckloo, N. Prophylactic antioxidants and phenolics of seagrass and seaweed species: A seasonal variation study in a Southern Indian Ocean Island, Mauritius. Internet J Med Update. 9: 27-37 (2014)
- Singh, A.R., Bajaj, V.K., Sekhawat, P.S., Singh, K.V. Phytochemical estimation and antimicrobial activity of aqueous and methanolic extract of Ocimum sanctum. J Nat Prod Plant Resour. 3: 51-58 (2013).
- Sahu, R. and Saxena, J. Screening of total phenolic and flavonoid content in conventional and nonconventional species of Curcuma. Pharmacogn Phytochem. 2: 76-179 (2013).
- McDonald, S., Prenzler, P.D., Antolovich, M. and Robards, K. Phenolic contentand antioxidant activity of olive extracts. Food Chem. 73: 73-84 (2001).
CrossRef - Makkar, H.P.S., Sidhuraju, P., Becker, K. Plant Secondary Metabolites. Methods Mol Biol. 393: 1-122 (2007).
CrossRef - Prieto P., Pineda M., Aguilar M. Spectorophometric quantification of antioxidant, capacity through the formation of phosphomolybdenum complex: Specific application to the determination of vitamin E. Biochem. 269(2): 337-341 (1999).
CrossRef - Oyaizu, M. Studies on product of browning reaction prepared from glucose amine. Eiyo Hyoka To Chiryo. 44: 307-315 (1986).
CrossRef - Ruch, R.J., Cheng, S.J., Klaunig, J.E. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 10: 1003-1008 ( 1989).
CrossRef - Ramshi, H.K.,Vani, M., Uma maheswari devi, P. Therapeutic activity of conjugated linoleic acids synthesized by lactobacillus plantarum. Int J Pharm Bio Sc 7: 215-223 (2016).
CrossRef - Fraga, C.G., Leibovitz, B.E., Toppel, A.L. Lipid peroxidation measured as TBARS in tissues. Free Radic Biol Med. 4: 155-161 (1988).
CrossRef - Kakkar, P., Das, B., Viswanathan, P.N. A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophys. 21: 130-132 (1984).
- Crouch, R.K., Gandy, S.E., Kimsey, G., Galbraith, R.A., Galbraith, G.M., Buse, M.G. The inhibition of islet superoxide dismutase by diabetogenic drugs. Diabetes. 30: 235-241 (1981).
CrossRef - Sinha, K.A. Colorimetric assay of Catalase. Anal Biochem. 47: 389-394 (1972)
CrossRef - Bancroft, J.D. and Cook, H.C. Manual of Histopathological Techniques, Routine morphological staining (1st edn), Churchill Livingstone, Singapore, pp: 18-25 (1984)
- Zahari, A., Ablat, A., Sivasothy, Y., Mohamad, J., Choudhary, M.I., Awang, K. In-vitro antiplasmodialand antioxidant activities of bisbenzylisoquinoline alkaloids from Alseodaphne corneri. Asian Pac J Trop Med. 9: 328-332 (2016).
CrossRef - Vidhya, A. and Indira, M. Protective effect of Quercetin in the regression of ethanol–induced hepatotoxicity. Indian J. Pharm. Sci. 71: 527-532 ( 2009).
CrossRef - Vermaulen, N.P.E. and Bessems, J.G.M. Van de straat. Molecular aspects of paracetamol – induced hepatotoxicity and its mechanism –based prevention. Drug Metals Dev. 24: 367-407 (1992).
CrossRef - Soufy, I.N. Hepatoprotective and antioxidant effects of Silybum marianum planta gainst hepatotoxicity induced by carbon tetrachloride in rats. J of Am Sci. 8: 479-86 (2012)
- Ighodaro, O.M. and Omole, J.O. Ethanol–induced hepatotoxicity in male wistar rats: effects of aqueous leaf extract of Ocimum gratissimum. J of Med and Med Sci 3: 499-505 (2012).
- Pari, L. and Karthikesan, K. Protective role of caffeic acid against alcohol-induced biochemical changes in rats. Fund Clin Pharmacol. 21(4): 355-361 (2007).
CrossRef - Remmer, H., Kessler, W., Einsele, H., Hintze, T., Diaz de Toranzo, G., Gharaibeh AM. Ethanol promotes oxygen-radical attack on proteins but not on lipids. Drug Metab Rev. 20: 219-232 (1989).
CrossRef - Eswar Kumar, K., Harsha, K.N., Shabana, S., Neelkanta Rao, N., Giri Babu, N. Evaluation of in –vitro antioxidant activity and in vivo hepatoprotective activity of Moringa oleifera seeds extract against ethanol induced liver damage in wistar rats. IOSR-PHR. 3: 10-15 (2013)
CrossRef - Maruthappan, V., Sakthi, S.,Hepatoprotective effect of Azadirachta indica (Neem) leaves against alcohol induced liver injury in albino rats. J Pharm Res. 2: 655-59 (2009).
- Arun, K. and Balasubramanian, U. Comparative study on Hepatoprotective activity of Phyllanthus amarus and Eclipta prostrata against alcohol induced in albino rats. Int J EnvironSci. 2: 361-379 (2011).
- Kharpate, S., Vadnerkar, G., Jain, D. and Jain, S. Hepatoprotective activity of the ethanol extract of the leaf of Ptrospermum acerifolium. Indian J Pharm Sci. 69: 850-852 (2007).
CrossRef