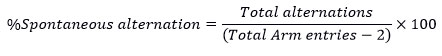Manuscript accepted on :10-01-2022
Published online on: 13-01-2022
Plagiarism Check: Yes
Reviewed by: Dr. Hanefi Ozbek
Second Review by: Dr. Jagdish Joshi
Final Approval by: Dr. H Fai Poon
Hariom Kumar 1 , Vishal Diwan 2 and Bhupesh Sharma 1, 3*
, Vishal Diwan 2 and Bhupesh Sharma 1, 3*
1Department of Pharmacology, Amity Institute of Pharmacy, Amity University Uttar Pradesh, India.
2UQ Diamantina Institute, Translational Research Institute, The University of Queensland, Australia.
3CNS Pharmacology, Conscience Research, India.
Corresponding Author E-mail: bhupeshsharmaresearch@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2343
Abstract
Autism spectrum disorder (ASD) mainly diagnosed with social behavioral problems, lack of communication, social interaction, and repetitive behavior along with cognitive dysfunction. Ryanodine receptors are involved in various neurological and behavioral impairments in different conditions. The role of Ryanodine receptors has not been explored in experimental ASD. The present study explicates the role of ryanodine receptor antagonist; ruthenium red (RR) in prenatal valproic acid (Pre-VPA) administered experimental ASD phenotypes. Three chamber social behavior, Y-Maze were utilized to assess social interaction, spontaneous alteration, respectively. Hippocampus and Prefrontal cortex (PFC) were utilized for various biochemical assessments, whereas cerebellum was used for assessments of blood brain barrier (BBB) permeability. Pre-VPA rats showed reduction in spontaneous alteration, social interaction. Pre-VPA administration were decreased PFC levels of IL-10, and GSH along with hippocampus cAMP response element-binding protein (CREB) and brain-derived neurotrophic factor (BDNF). Also, the animals have shown increase in PFC levels of IL-6, TNF-α, TBARS, Evans blue leakage and water content. Daily administration of R Red considerably diminished Pre-VPA administered reduction in spontaneous alteration, social interaction, CREB, BDNF and increase in inflammation, oxidative stress, BBB permeability. Conclusively, Pre-VPA has induced autistic phenotype, which were attenuated by ryanodine receptor antagonist. Ryanodine receptor antagonists may further test for their pharmacological effects in ASD phenotypes.
Keywords
Blood Brain Barrier; CREB; IL-6, BDNF; Ryanodine; Social Interaction
Download this article as:| Copy the following to cite this article: Kumar H, Diwan V, Sharma B. Remediate Effect of Ryanodine Receptor Antagonist in Valproic-Acid Induced Autism . Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Kumar H, Diwan V, Sharma B. Remediate Effect of Ryanodine Receptor Antagonist in Valproic-Acid Induced Autism . Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3zVCUBq |
Introduction
A neurodevelopmental disorder, autism spectrum disorder (ASD) is currently being diagnosed with communication problems, repetitive behavior along with social behavior dysfunction. Shockingly, there is no affirmed treatment accessible till presently. This is following an upward example and overall budgetary weight. Males are increasingly inclined to ASD when contrasted with females. More than one percent of the populace overall is experiencing ASD 1. Several environmental and genetic factors seem to be responsible but the exact cause still unknown. Autism has diverse genetic relations along with different environmental causes including infection, pesticides and drugs 2,3. Valproic acid is a typically used antiepileptic drug and it is used to treat depressive disorders, migraine, anxiety, and neuropathic pain. Prenatal valproic acid (Pre-VPA) treatment is one of the best characterized models of autism rats with solid choice, face and predictive validity 4. Our lab and several researchers have reported that Pre-VPA exposure leads to neuro-inflammation, oxidative stress and mitochondrial dysfunction 5–9. The autistic individuals have some genetic defects in calcium channel genes, which regulates the neurodevelopment 10.
Elevation of the concentration of cytosolic calcium induces the release of neurotransmitters at synaptic junctions, leads to dendritic function, regulates behavior-dependent changes in synaptic plasticity and gene expression 11. Calcium homeostasis is generally regulated by various kind of calcium channels and their receptors. ryanodine receptor is one of the calcium Ruthenium red is being known as selective intracellular ryanodine receptor (RyR) antagonist. Antagonism of the RyR results into constructive effect in different brain conditions such as Alzheimer’s disease and Huntington’s disease12–14. It has been documented that antagonism of ryanodine receptor exert neuro-protective effect along with decreases infarct volume and decrease levels of intracellular calcium in brain 15.
Some previous studies also reported that ruthenium red gives useful impacts by declining brain inflammation 16 and oxidative stress 17. The antagonistic role of ryanodine receptors in ASD still not explored. This manuscript explored the novel job of ryanodine receptors antagonist; ruthenium red in Pre-VPA administered autistic phenotypes in rodents.
Materials and Methods
Animals
Adult albino wistar rats were kept in polypropylene cages in Amity University (Reg No. 1327/PO/ReBi/S/10/CPCSEA) at a temperature of 25 ± 2°C with relative humidity of 50 ± 5%. The animals had free access to water and standard laboratory pellet chow diet (Ashirwad Industries, Punjab, India). Animals were exposed to the natural light and dark cycle with 12 hours of light (starting at 07:00 hrs. and ending at 19:00 hrs.) followed by 12 hours of dark (starting at 19:00 hrs. and ending at 07:00 hrs.). The Institutional Animal Ethics Committee of Amity University, Uttar Pradesh, India, has approved all experiments.
Drugs chemicals and reagents
Analytical and laboratory grade chemicals and reagents were used in the present study. Sodium salt of valproic acid was taken from Sun Pharma Pvt. Ltd. Evans blue, ethylene glycol tetra acetic acid (EGTA) was purchased from SISCO Research Laboratory Pvt. Limited, Mumbai, India. Lowry’s reagent, N-naphthylethylenediamine and 5, 5`-dithiobis (2-nitrobenzoic acid) (DTNB) were obtained from Sigma-Aldrich, India. Hydrogen peroxide and pyridine was obtained from Rankem Laboratories Pvt. India, Ltd.
Administration of drugs
Drugs and their solutions were prepared before the procedure. For autism induction, sodium valproate was dissolved in 0.9 % saline (3.3 ml/kg) and given single dose (500 mg/kg; i.p; on12.5th day of embryonic phase) to the pregnant female rats 7. Ruthenium Red was dissolved in 0.9 % saline (3.3 ml/kg; i.p.) and given orally (1 or 3 mg/kg; from postnatal day 21st to 50th) between 9.00 and 18.00 h by oral gavage to the rats 18.
ASD induced by Pre-VPA
Pregnancy was identified by the existence of a vaginal plug on the embryonic day 1st. A single dose of sodium valproate with concentration of 500 mg/kg injected through intra-peritoneal route to pregnant females on embryonic 12.5th day received sodium valproate. These sodium valproate treated pregnant females were accommodated weaning of offspring (post-natal day 20) The only male offspring born were recruited for multiple tests for the analysis 6,19.
Protocol design
In total five groups of animals, with each group containing eight (n=8) animals were used for the protocol of the study. Choice of animals was based on research already published, effectively using albino Wistar rats to model experimental ASD like condition 7.
The timeline, groups, and parameters assessed for present study are seen in Figure 1.
Fertilization was ascertained using a vaginal examination for the presence of sperm cells, this day was considered as gestational day 1. Pregnant dams were housed individually till the day of parturition and weaning of the pups. The pups were weaned and then distributed randomly into their respective groups on post-natal day 20 7,19.
Group I—Control group– Pregnant dams were injected with single dose of 0.9 % saline (3.3 ml/kg) on gestational 12.5th day. The received male pups were taken as control group. Group II and III — Ruthenium Red (R Red) D1 and D2 per se: Male pups born to untreated pregnant dams were randomly selected for these group, received R Red D1 and R Red D2 (3 mg/kg and 6 mg/kg, i.p.) respectively from post-natal (PND) 21st to PND 50th ; Group III—Prenatal valproic acid (Pre- VPA) group: Pregnant dams were injected with single dose of sodium valproate (500 mg/kg, i.p;) on gestational 12.5th day; Group IV and V —Pre-VPA +R Red D1 and D2 group: Males exposed to Pre-VPA were randomly selected and received R Red D1 (3 mg/kg, i.p.) and R Red D2 (6 mg/kg, i.p.) from PND 21st to PND 50th (Figure 1).
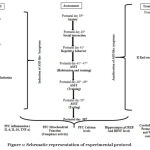 |
Figure 1: Schematic representation of experimental protocol |
E- Embryonic day; PFC- Prefrontal cortex; P- Postnatal day; Pre-VPA – Prenatal valproic acid; R Red – Ruthenium Red; D1- dose 1 (3 mg/kg); D2- dose 2 (6mg/kg).
Social interaction assessment
On postnatal day 48th – 49th, social interaction was evaluated by Three-Chamber Sociability and Social Novelty Test apparatus (30 cm long and 70 cm wide which divided into three identical chambers) for the rat. We have used this mehod in our previous research adopted from Kim et al,. 2011 with slightly modification 20. Concisely, the test arena measured 76 cm x 30 cm x 35 cm and was divided into three equal chambers with an access point between each chamber. Animals were given free access to all the chambers and Each trial started with the animal placed in the central chamber. To encourage exploration of the side chambers, all animals were accustomed to the apparatus for 5 min prior to starting of the test trial. After ending of the habituation period, the rats were tested in the sociability phase lasting for 10 min. Animals to be placed under a wire cage were spent 30 minutes at the wire cage, prior to initiation of the sociability phase. In the sociability phase, a stranger animal was placed under the wired cage in either (left or right) side chamber, while in the other chamber called as stranger chamber and empty chamber, respectively. Upon conclusion of the sociability phase the social preference phase was initiated 2 hrs after the last animal trial. In the social preference phase each animal was allotted 10 min to look at the complete arena. During this phase, the animal earlier considered as stranger, was now rendered familiar and introducing another novel animal into the paradigm, along with the familiar animal. The two chambers now would be called as familiar and novel chamber. The time spent by test animals in both the side chambers was measured. Sociability index (SI) and social preference index (SPI) were calculated according to the following formula 5,6,21.
Repetitive behavior assessment
Repetitive behavior measured through Y-Maze by observing the decrease in percentage spontaneous alternations (5). The rats were exposed to the starting arm during an 8-minute trial with free exploration in the three arms of the maze. The series of Arm entries were noted down to calculate spontaneous alternations numbers made. The alternation was noted as the rat came successively into the three different arms. The following formula was used to calculate
Preparation for biochemical assessments
On postnatal day 50th, a high dose of thiopental sodium (90 mg / kg; i.p) was used to isolate the PFC tissue by anaesthetization. The homogenate supernatant was collected for various biochemical estimates as defined in the procedures below. Absorption was taken with a spectrophotometer (PerkinElmer). Prefrontal cortex, hippocampus and cerebellum are the main brain regions implicated in ASD pathogenesis and neurochemical disorders 7,22 So, for this study, we selected prefrontal cortex and cerebellum brain regions.
Oxidative stress assessments
Glutathione (GSH) and lipid peroxidation (TBAR) are known as oxidative stress markers, so in this study, we evaluated these markers in prefrontal cortex 5,6
Glutathione (GSH)
The quantitative estimation of reduced form of glutathione (GSH) was assessed using spectrophotometer (PerkinElmer) at 412 nm (7). In a test tube, PFC supernatant and trichloroacetic acid (10 per cent w / v) were combined at 1:1 ratio and the test tubes were centrifuged (1000 g at 4 ° C for 10 min). 0.3 M disodium hydrogen phosphate (2 mL) and supernatant (0.5 mL) is mixed. The spectrophotometric absorbance was observed at 412 nm following the addition of .25 mL of freshly prepared DTNB (DTNB dissolved in 1 percent w / v sodium citrate). 10-100 μM of the reduced form of glutathione was used for plotting of standard curve. The effects of reduced glutathione consistent with mg of protein were expressed as micromoles.
Lipid peroxidation
Lipid peroxidation was assessed as close to our previous investigations 7. The PFC supernatant sample (0.2 mL) was taken in test tube. In addition, 8.1 per cent sodium dodecyl sulphate (0.2 mL), 30 per cent acetic acid pH 3.5 (1.5 mL) and 0.8 per cent thiobarbituric acid (1.5 mL) were added, followed by distilled water up to 4 mL.. The test tubes were put in the incubator at 950C for 1 hour. The incubated mixture was then cooled by adding distilled water (1 mL) followed by adding n-butanol-pyridine mixture (15:1 v / v) to 5 mL. The centrifuge tubes were handled at 4000 g for 10 min. The spectrophotometric absorbance was taken on developing pink color. 1, 1, 3, 3-tetra methoxy propane (1-10 nM) was used for plotting standard calibration curve. Nano moles per mg of protein were used for expression of result.
Elisa Method for Iterleukin-6 (IL-6), interleukin-10 (IL-10) and tissue necrosis factor-α (TNF-α) , Brain-derived neurotrophic factor (BDNF) and cAMP response element-binding protein (CREB)
These were assessed by enzyme-linked immune-sorbent assay- sandwich method in PFC and hippocampus area of brain. The IL-6, IL-10, TNF-α, BDNF and CREB were estimated by using RayBio® Rat ELISA kits. The procedure mentioned in the product information leaflet was completely followed and samples were run in triplicate for optical density measurement, average optical density was considered for final calculation of concentration. Briefly, IL-6, IL-10, TNF-α, BDNF and CREB quantitation was made in PFC supernatant at 450 nm on 96-well percolated to specific antibodies plates. Supernatant and standard were taken out in to well. The immobilized antibody was bound with IL-6, IL-10, TNF-α, BDNF and CREB present in sample, respectively. Washing was given to the tubes, and the biotinylated anti-Rat IL-6, IL-10, TNF-α, BDNF and CREB antibodies were applied to the plates. Unbound biotinylated antibody was removed by washing the well. The HRP conjugated streptavidin was added to the wells. After washing a TMB substratum solution was applied to the wells. A blue color formation showed the IL-6, IL-10, TNF, BDNF and CREB have linked in sample. The stop solution was pipette to the wells which changed the color to yellow. Results were expressed in pg/ml and ng/ml 23.
Blood-brain barrier (BBB) permeability and water content
BBB permeability and water content performed by the concentration of Evans blue dye in the cerebellum as previously reported methods 6,24. Concisely, 4% of dye /Evans blue (intra-peritoneal; 4 ml/kg) was injected to subject and was permitted to circulate for 2 hours. Before collecting the samples, the subjects (anesthetized) were trans-cordially perfused with saline to flush out the remaining dye in the vessels. Weighed cerebellum for quantitative spectroscopic calculation. In short, the cerebellum precisely measured. The cerebellum processed at pH 7.4 dye extraction in 3.5 mL of 0.1 mol / L phosphate buffer saline by a homogenizer. Then, for protein precipitation, 6 ml of 60 per cent trichloroacetic acid was added. This processed cerebellum was vortexed after 30 minutes of cooling for 2 minutes. The cerebellum was processed for 40 minutes through a centrifuge at 4000 rpm to obtain pellets. The dye levels were measured at 610 nm via a spectrophotometer. The findings were expressed using a standardized curve of Evans blue as μg of Evans blue / g of cerebellum tissue. For 48 hours wet-weighted cerebellum tissue was put in the oven at 105 ° C. Weighted out after 48 hours of dry cerebellum. Wet-dry method was used to calculate the volume of water in the cerebellum. The percentage water content was calculated as: (wet weight – dry weight)/wet weight × 100%. and results shown in percentage of water content 24.
Statistical analysis
Sigma Stat (v3.5) was used for statistical analysis. The findings were demonstrated in the form of mean ± standard deviation. Statistics for all variables were analyzed using two-way ANOVA followed by a post-test by Bonferroni. This at p<0.05 was considered statistically important.
Results
Social behavior
Sociability, sociability index, social preference, and social preference index
Pre-VPA has decreased time went through in stranger chamber and expanded time went through in empty chamber, with contrast to control rats, which shows lower sociability in Pre-VPA treated rats. Administration of R Red has significantly mitigated Pre-VPA associated reduction of time went through in stranger chamber followed by increased time went through in the empty chamber. Pre-VPA treated rats, has shown lower sociability index when compared to control rats, which was markedly attenuated by R Red (Figure 2).
Pre-VPA administration has decrease time went through in novel chamber and expand time went through in the familiar chamber, with contrast to control rats, which shows lower social preference in Pre-VPA treated rats. Administration of R Red has significantly mitigated Pre-VPA associated reduction of time went through in novel chamber and increased time went through in the familiar chamber (Figure 2). Pre-VPA treated rats have shown lower the social preference index, contrast to control rats, which was significantly mitigated by R Red.
 |
Figure 2: Sociability, sociability index social preference and social preference index on Three-Chamber Sociability and Social Novelty Test apparatus |
Results are mean ± standard deviation, two-way ANOVA followed by Bonferroni’s post-test. a p<0.05 vs control rats, b p<0.05 vs Pre-VPA treated rats
A-Stranger: a F1, 42 = 106.277; b F2, 42 = 31.543; Empty: a F1, 42 = 228.979; b F2, 42 = 68.632;
B-Sociability Index: a F1, 42 = 502.727; b F2, 42 = 131.026; C-Familiar: a F1, 42 = 502.086; b F2, 42 = 60.493; Novel: a F1, 42 = 1477.037; b F2, 42 = 421.444; D-Social preference Index: a F1, 42 = 2585.419; b F2, 42 = 444.524.
Pre-VPA – Prenatal valproic acid; R Red – Ruthenium Red; D1- dose 1 (3 mg/kg); D2- dose 2 (6mg/kg).
Repetitive behavior
Pre-VPA treatment has considerably reduced the % spontaneous alteration. This shows repetitive behavior of Pre-VPA exposed rats. Treatment with R Red suggestively mitigate Pre-VPA associated decreased % spontaneous alteration (Figure 3), which suggests a decrease of Pre-VPA induced repetitive behavior.
 |
Figure 3: Repetitive behavior measured as % spontaneous alteration on Y maze. |
The results are a mean ± standard deviation, two-way ANOVA with Bonferroni’s post-test.
a p<0.05 vs control rats, b p<0.05 vs Pre-VPA treated rats
a F1, 42 = 623.665; b F2, 42 = 274.682
Pre-VPA – Prenatal valproic acid; R Red – Ruthenium Red; D1- dose 1 (3 mg/kg); D2- dose 2 (6mg/kg).
PFC neuro-inflammation, oxidative stress and, and hippocampus CREB and BDNF
Pre-VPA has increased PFC inflammation (IL-6 levels, TNF-α levels and decreased IL-10 levels), oxidative stress (decrease GSH levels and increase TBARS levels) along with decreased hippocampus CREB and BDNF. R Red treatment has significantly mitigated Pre- VPA induced increased PFC inflammation (IL-6 levels, TNF-α levels and decreased IL-10 levels), oxidative stress (decrease GSH levels and increase TBARS levels) along with decreased hippocampus CREB and BDNF (Figure 4, Figure 5 and Figure 6).
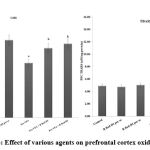 |
Figure 4: Effect of various agents on prefrontal cortex oxidative stress |
Results are mean ± standard deviation; two-way ANOVA followed by Bonferroni’s post-test.
a p<0.05 Vs control rats, b p<0.05 Vs Pre-VPA treated rats
GSH- a F1, 18 = 163.265; b F2, 18 = 685.007; TBARS: a F1, 18 = 1257.099; b F2, 18 = 175.039.
Pre-VPA – Prenatal valproic acid; R Red – Ruthenium Red; D1- dose 1 (3 mg/kg); D2- dose 2 (6mg/kg).
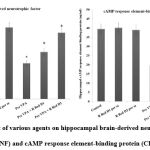 |
Figure 5: Effect of various agents on hippocampal brain-derived neurotrophic factor (BDNF) and cAMP response element-binding protein (CREB) |
Results are mean ± standard deviation; two-way ANOVA followed by Bonferroni’s post-test.
a p<0.05 vs control rats, b p<0.05 vs Pre-VPA treated rats
BDNF a F1, 18 = 476.678; b F2, 18 = 78.896: CREB F1, 18 = 199.643; b F2, 18 = 29.509
Pre-VPA – Prenatal valproic acid; R Red – Ruthenium Red; D1- dose 1 (3 mg/kg); D2- dose 2 (6mg/kg).
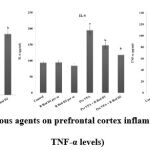 |
Figure 6: Effect of various agents on prefrontal cortex inflammation (IL- 6, IL-10 and TNF-α levels) |
Results are mean ± standard deviation; two-way ANOVA followed by Bonferroni’s post-test.
a p<0.05 vs control rats, b p<0.05 vs Pre-VPA treated rats
IL-6 levels: a F1, 18 = 973.189; b F2, 18 = 84.89; IL-10 levels a F1, 18 = 219.928; b F2, 18 = 138.116; TNF-α levels a F1, 18 = 1105.281; b F2, 18 = 364.755.
Pre-VPA – Prenatal valproic acid; R Red – Ruthenium Red; D1- dose 1 (3 mg/kg); D2- dose 2 (6mg/kg), IL-6- interleukins-6; IL-10- interleukins 10; TNFα -Tumor necrosis factor-α
Blood brain barrier permeability and water content in the cerebellum
A considerably higher Evans blue concentration and water content were present in the cerebellum of Pre-VPA exposed rats in contrast to control rats. Treatment with R Red expressively mitigates Pre-VPA induced increased concentration of Evans blue and water content in the cerebellum (Figure 7), which suggest amelioration in Pre-VPA induced BBB dysfunction.
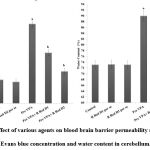 |
Figure 7: Effect of various agents on blood brain barrier permeability measured by Evans blue concentration and water content in cerebellum. |
Results are mean ± standard deviation, two-way ANOVA followed by Bonferroni’s post-test.
ap<0.05 Vs control rats, b p<0.05 Vs Pre-VPA treated rats.
A- a F1, 18 = 971.701; b F2, 18 = 163.993; B- a F1, 18 = 181.92; b F2, 18 = 94.856
Pre-VPA – Prenatal valproic acid; R Red – Ruthenium Red; D1- dose 1 (3 mg/kg); D2- dose 2 (6mg/kg).
Discussion
Oxidative stress is one of the sponsors for autistic characteristic in clinical as well pre-clinical findings 9,25,26. We found decreased levels of GSH along with increased TBARS in Pre-VPA exposed animals. In prior studies, pre-VPA results in increased levels of oxidative markers in rats 7. In the current study, Ruthenium Red successfully counteracted the oxidative stress produced by Pre-VPA. There are several pathways which are implicated in oxidative stress. In autism, Impaired glutathione associated antioxidant pathways such as methionine cycle, redox system, and transculturation has already been reported 27. Furthermore, previous research has suggested that autism manifests communication problems, repetitive behavior and social deficits due to chronic cerebellar damage, abnormal glutathione metabolism, and Wnt/β-catenin activation 28–30. The current study also found that repetitive and impaired social behavior was evident. Few previous research indicates ryanodine receptor antagonist reverse increased oxidative stress markers in different brain condition (31,32). Ryanodine receptor antagonist reverse Ca+ exito-toxicity associated endogenous oxidative stress markers, followed by lipid peroxidation products content
(TBARS) and therefore amelioration of oxidative stress18. It has been expressed that antagonism of ryanodine receptor lower the advanced glycation end products dependent generation of reactive oxygen species 33. Following the above discussion, it may be concluded that ruthenium red modulates the endogenous antioxidant pathways and results in reduced oxidative stress, which is followed by social and repetitive behavior corrections among rats exposed to pre-VPA.
TNF-α and IL-6 were increased, while IL-10 was decreased in Pre-VPA exposed animals, while R Red 3 mg/kg and 6 mg/kg counteracted the levels of neuroinflammatory markers in the current study. Previous research has also revealed that in Pre-VPA and autism, neuroinflammatory markers are higher, followed by neuroinflammation 5,26,34,35. Social behavior impairment can be caused by neuroinflammation followed by neuronal disorganization and reactive gliosis in frontal cortex, which has already been demonstrated 36. Additionally, we found impaired social behavior in the rats exposed to Pre-VPA. Social behavior impairments and repetitive behavior in autism are thought to be due to neuroinflammatory pathways. It has proposed that increased neuro-inflammatory markers like TNF-α and IL-6 may modulate social and repetitive behavior by altering the brain microvascular system (37) and equilibrium of excitatory and inhibitory state responsible for glutamate signaling 35,36,38. Earlier researchers have reported that antagonism of ryanodine receptor counter inflammatory marker viz. interleukin 2, interleukin-10, TNF-α 39,40. Further, ryanodine receptor antagonist administration effectively block the calcium mobilization and hinder cell death 41. It has been expressed that ryanodine receptor antagonism inhibits calcium entry via the NMDA receptors and backs glutamate-induced excite-toxicity 42,43. Because of this reason, we have tried to assess the permeability of blood brain barrier via Evans blue leakage. Ryanodine-mediated intracellular calcium discharge by the endoplasmic reticulum contributes to BBB distraction 33. On the above lines this may be suggested that increased levels of neuro-inflammatory markers could modulates social behavior, repetitive behavior along with BBB function in Pre-VPA induced autism in rats.
Ruthenium red increased CREB and BDNF levels in Pre-VPA treated rats in the current study. Researchers have found that CREB/BDNF signalling has a significant impact on synaptic plasticity, morphology, and behaviour 44,45, Therefore, this would be one of the major pathway in autism. It has already been established that the loss of BDNF causes abnormal learning, memory formation, and plasticity 46,47, As a consequence, Pre-VPA animals exhibited impaired social and repetitive behaviors, possibly due to BDNF/CREB-associated synaptic plasticity. BDNF and CREB have also been found to be decreased in animals exposed to Pre-VPA 23. It has suggested, CREB activity plays role in double cortin, dendritic development, expression of neuronal microtubule associated protein and BDNF that could result in autistic characteristic features such as altered synaptic plasticity and neuronal survival 48,49. CREB and BDNF levels were significantly reduced in the hippocampus of the rats exposed to Pre-VPA, which was in line with previous findings 50 and ruthenium red counter the effect of Pre- VPA associated BDNF and CREB. Ruthenium red might have increased levels of BDNF/CREB in the pre-VPA exposed rats through neuronal survival, calcium influx and discharge with activity-dependent calcium signals, synaptic versatility, and calcium signals that influence synaptic plasticity.
A previous study suggested BBB impairment in autistic condition, postmortem with ASD cerebellum exhibited altered gene expression responsible for BBB integrity 51. An interesting finding of our previous study was that rats pre-exposed to VPA had increased BBB permeability measured by Evans blue dye (7). In line with this, we found that in Pre-VPA exposed rats, Evans blue leakage and water content increased. The abnormal levels of cytokines, endogenous redox mechanisms, mitochondrial function, elevated levels of neurodermatitis, and oxidative stress may cause dysfunction in the BBB 52–54. Ruthenium red may affect cellular Ca+ levels in tight junctions through apoptosis and protein kinase C-alpha 55, Thus, in addition to being able to modulate water homeostasis, ruthenium red can also maintain Ca+ homeostasis in the brain in autistic individuals.
Conclusion
On the basic of the above line of discussion, ryanodine receptor antagonist; ruthenium red ameliorates Pre-VPA induced remarkable decrease in social behavior followed by GSH, IL-10, CREB and BDNF levels. Furthermore, ruthenium red was found to counter Pre-VPA associated increase in repetitive behavior, PFC IL-6, and TNF-α and TBARS. Pre-VPA has been found to increase in BBB permeability and water content, which was ameliorated by ruthenium red treatment in rats. Further studies can explore the molecular mechanisms of ryanodine receptors in autism.
Acknowledgement
Authors are thankful to Dr. A. K. Chauhan, Hon’ble Founder President, Ritnand Balve Education Foundation, India, and Dr. Atul Chauhan, Hon’ble Chancellor, Amity University Uttar Pradesh, India for providing all the necessary experimental facilities and motivation to conduct this research work. We are also thankful to Prof (Dr.). Nirmal Singh, Pharmacology Division, Department of Pharmaceutical Sciences and Drug Research, Faculty of Medicine, Punjab University, Patiala (Punjab), India, for his valuable suggestions.
Conflicts of Interest
There is no conflict of interest
Funding Source
There is no funding source.
References
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ [Internet]. 2018;67(6):1–23. Available from: http://www.cdc.gov/mmwr/volumes/67/ss/ss6706a1.htm?s_cid=ss6706a1_w
CrossRef - Markram K, Rinaldi T, La Mendola D, Sandi C, Markram H. Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology [Internet]. 2008;33(4):901–12. Available from: http://dx.doi.org/10.1038/sj.npp.1301453
CrossRef - Barrett CE, Hennessey TM, Gordon KM, Ryan SJ, McNair ML, Ressler KJ, et al. Developmental disruption of amygdala transcriptome and socioemotional behavior in rats exposed to valproic acid prenatally. Mol Autism. 2017;8(1).
CrossRef - Hirsch MM, Deckmann I, Santos-Terra J, Staevie GZ, Fontes-Dutra M, Carello-Collar G, et al. Effects of single-dose antipurinergic therapy on behavioral and molecular alterations in the valproic acid-induced animal model of autism. Neuropharmacology. 2020;
CrossRef - Kumar H, Sharma B. Memantine ameliorates autistic behavior, biochemistry & blood brain barrier impairments in rats. Brain Res Bull. 2016;124.
CrossRef - Kumar H, Sharma B. Minocycline ameliorates prenatal valproic acid induced autistic behaviour, biochemistry and blood brain barrier impairments in rats. Brain Res. 2016;1630.
CrossRef - Kumar H, Sharma BM, Sharma B. Benefits of agomelatine in behavioral, neurochemical and blood brain barrier alterations in prenatal valproic acid induced autism spectrum disorder. Neurochem Int. 2015;91.
CrossRef - Won H, Mah W, Kim E. Autism spectrum disorder causes, mechanisms, and treatments: focus on neuronal synapses. Front Mol Neurosci. 2013;6(August):1–26.
CrossRef - Frye RE, Rossignol DA. Mitochondrial dysfunction can connect the diverse medical symptoms associated with autism spectrum disorders. Pediatr Res. 2011;
CrossRef - Li J, You Y, Yue W, Jia M, Yu H, Lu T, et al. Genetic evidence for possible involvement of the calcium channel gene cacna1a in autism pathogenesis in Chinese han population. PLoS One. 2015;
CrossRef - Berridge MJ, Bootman MD, Lipp P. Calcium – A life and death signal. Nature. 1998.
CrossRef - Calì T, Ottolini D, Brini M. Mitochondrial Ca2+ and neurodegeneration. Cell Calcium. 2012 Jul;52(1):73–85.
CrossRef - Del Prete D, Checler F, Chami M. Ryanodine receptors: physiological function and deregulation in Alzheimer disease. Mol Neurodegener. 2014;9(1):21.
CrossRef - Suzuki M, Nagai Y, Wada K, Koike T. Calcium leak through ryanodine receptor is involved in neuronal death induced by mutant huntingtin. Biochem Biophys Res Commun. 2012 Dec;429(1–2):18–23.
CrossRef - Phillips KF, Deshpande LS, DeLorenzo RJ. Hypothermia reduces calcium entry via the N-methyl-D-aspartate and ryanodine receptors in cultured hippocampal neurons. Eur J Pharmacol. 2013 Jan;698(1–3):186–92.
CrossRef - Poole DP, Amadesi S, Veldhuis NA, Abogadie FC, Lieu TM, Darby W, et al. Protease-activated receptor 2 (PAR2) protein and transient receptor potential vanilloid 4 (TRPV4) protein coupling is required for sustained inflammatory signaling. J Biol Chem. 2013;
CrossRef - Schilling T, Eder C. Importance of the non-selective cation channel TRPV1 for microglial reactive oxygen species generation. J Neuroimmunol. 2009 Nov;216(1–2):118–21.
CrossRef - Jain S, Sharma B. Effect of ruthenium red, a ryanodine receptor antagonist in experimental diabetes induced vascular endothelial dysfunction and associated dementia in rats. Physiol Behav. 2016;164:140–50.
CrossRef - Hajisoltani R, Karimi SA, Rahdar M, Davoudi S, Borjkhani M, Hosseinmardi N, et al. Hyperexcitability of hippocampal CA1 pyramidal neurons in male offspring of a rat model of autism spectrum disorder (ASD) induced by prenatal exposure to valproic acid: A possible involvement of Ih channel current. Brain Res. 2019;
CrossRef - Kim KC, Kim P, Go HS, Choi CS, Yang S Il, Cheong JH, et al. The critical period of valproate exposure to induce autistic symptoms in Sprague-Dawley rats. Toxicol Lett. 2011;201(2):137–42.
CrossRef - Kumar H, Ranjan RK, Ramanathan AL. Hydrogeochemistry and arsenic distribution in the gorakhpur district in the middle gangetic plain, india. Safe and Sustainable Use of Arsenic-Contaminated Aquifers in the Gangetic Plain: A Multidisciplinary Approach. 2015.
CrossRef - Chauhan A, Gu F, Essa MM, Wegiel J, Kaur K, Brown WT, et al. Brain region-specific deficit in mitochondrial electron transport chain complexes in children with autism. J Neurochem. 2011;117(2):209–20.
CrossRef - Luhach K, Kulkarni GT, Singh VP, Sharma B. Vinpocetine amended prenatal valproic acid induced features of <scp>ASD</scp> possibly by altering markers of neuronal function, inflammation, and oxidative stress. Autism Res [Internet]. 2021 Aug 20 [cited 2021 Oct 20];aur.2597. Available from: https://onlinelibrary.wiley.com/doi/10.1002/aur.2597
- Manaenko A, Chen H, Kammer J, Zhang JH, Tang J. Comparison Evans Blue injection routes: Intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. J Neurosci Methods. 2011;195(2):206–10.
CrossRef - Nadeem A, Ahmad SF, Attia SM, Bakheet SA, Al-Harbi NO, AL-Ayadhi LY. Activation of IL-17 receptor leads to increased oxidative inflammation in peripheral monocytes of autistic children. Brain Behav Immun. 2018;
CrossRef - Rossignol DA, Frye RE. Evidence linking oxidative stress, mitochondrial dysfunction, and inflammation in the brain of individuals with autism. Front Physiol [Internet]. 2014;5:150. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24795645
CrossRef - James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, et al. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;
CrossRef - Frye RE, Melnyk S, Macfabe DF. Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl Psychiatry. 2013 Jan;3(1):e220.
CrossRef - Morakotsriwan N, Wattanathorn J, Kirisattayakul W, Chaisiwamongkol K. Autistic-Like Behaviors, Oxidative Stress Status, and Histopathological Changes in Cerebellum of Valproic Acid Rat Model of Autism Are Improved by the Combined Extract of Purple Rice and Silkworm Pupae. Oxid Med Cell Longev. 2016;
CrossRef - Zhang Y, Sun Y, Wang F, Wang Z, Peng Y, Li R. Downregulating the canonical wnt/b-catenin signaling pathway attenuates the susceptibility to autism-like phenotypes by decreasing oxidative stress. Neurochem Res. 2012;
CrossRef - Crouzin N, de Jesus Ferreira MC, Cohen-Solal C, Aimar RF, Vignes M, Guiramand J. α-Tocopherol-mediated long-lasting protection against oxidative damage involves an attenuation of calcium entry through TRP-like channels in cultured hippocampal neurons. Free Radic Biol Med. 2007;
CrossRef - Singh P, Sharma B. Reversal in Cognition Impairments, Cholinergic Dysfunction, and Cerebral Oxidative Stress Through the Modulation of Ryanodine Receptors (RyRs) and Cysteinyl Leukotriene-1 (CysLT1) Receptors. Curr Neurovasc Res. 2016 Jan;13(1):10–21.
CrossRef - Kuhlmann CRW, Zehendner CM, Gerigk M, Closhen D, Bender B, Friedl P, et al. MK801 blocks hypoxic blood-brain-barrier disruption and leukocyte adhesion. Neurosci Lett. 2009;
CrossRef - Khalaj R, Hajizadeh Moghaddam A, Zare M. Hesperetin and it nanocrystals ameliorate social behavior deficits and oxido-inflammatory stress in rat model of autism. Int J Dev Neurosci [Internet]. 2018 Oct;69:80–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29966739
CrossRef - De Theije CGM, Koelink PJ, Korte-Bouws GAH, Lopes da Silva S, Korte SM, Olivier B, et al. Intestinal inflammation in a murine model of autism spectrum disorders. Brain Behav Immun [Internet]. 2014;37:240–7. Available from: http://dx.doi.org/10.1016/j.bbi.2013.12.004
CrossRef - Codagnone MG, Podestá MF, Uccelli NA, Reinés A. Differential Local Connectivity and Neuroinflammation Profiles in the Medial Prefrontal Cortex and Hippocampus in the Valproic Acid Rat Model of Autism. Dev Neurosci. 2015;
CrossRef - Pober JS, Sessa WC. Inflammation and the Blood Microvascular System. Cold Spring Harb Perspect Biol. 2015 Jan;7(1):a016345.
CrossRef - Wei H, Zou H, Sheikh AM, Malik M, Dobkin C, Brown WT, et al. IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflammation. 2011;
CrossRef - López-Neblina F, Toledo-Pereyra LH, Toledo AH, Walsh J. Ryanodine Receptor Antagonism Protects the Ischemic Liver and Modulates TNF-α and IL-10. J Surg Res. 2007;
CrossRef - Dadsetan S, Zakharova L, Molinski TF, Fomina AF. Store-operated Ca 2+ Influx Causes Ca 2+ Release from the Intracellular Ca 2+ Channels That Is Required for T Cell Activation. J Biol Chem. 2008 May;283(18):12512–9.
CrossRef - Luciani DS, Gwiazda KS, Yang TLB, Kalynyak TB, Bychkivska Y, Frey MHZ, et al. Roles of IP3R and RyR Ca2+ channels in endoplasmic reticulum stress and β-cell death. Diabetes. 2009;
CrossRef - Makarewicz D, Ziemińska E, Łazarewicz JW. Dantrolene inhibits NMDA-induced 45Ca uptake in cultured cerebellar granule neurons. In: Neurochemistry International. 2003.
CrossRef - Mody I. NMDA receptor-dependent excitotoxicity: the role of intracellular Ca2+ release. Trends Pharmacol Sci. 1995 Oct;16(10):356–9.
CrossRef - Chen DY, Bambah-Mukku D, Pollonini G, Alberini CM. Glucocorticoid receptors recruit the CaMKIIα-BDNF-CREB pathways to mediate memory consolidation. Nat Neurosci. 2012;
CrossRef - Wang B, Zhao J, Yu M, Meng X, Cui X, Zhao Y, et al. Disturbance of intracellular calcium homeostasis and CaMKII/CREB signaling is associated with learning and memory impairments induced by chronic aluminum exposure. Neurotox Res. 2014;
CrossRef - Uutela M, Lindholm J, Louhivuori V, Wei H, Louhivuori LM, Pertovaara A, et al. Reduction of BDNF expression in Fmr1 knockout mice worsens cognitive deficits but improves hyperactivity and sensorimotor deficits. Genes, Brain Behav. 2012;
CrossRef - Tye C, Bolton P. Neural connectivity abnormalities in autism: Insights from the Tuberous Sclerosis model. BMC Med. 2013 Dec;11(1):55.
CrossRef - Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, et al. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. J Neurosci [Internet]. 2009 Jun 24 [cited 2021 Oct 19];29(25):7966–77. Available from: https://pubmed.ncbi.nlm.nih.gov/19553437/
CrossRef - Jancic D, Lopez de Armentia M, Valor LM, Olivares R, Barco A. Inhibition of cAMP response element-binding protein reduces neuronal excitability and plasticity, and triggers neurodegeneration. Cereb Cortex [Internet]. 2009 Nov [cited 2021 Oct 19];19(11):2535–47. Available from: https://pubmed.ncbi.nlm.nih.gov/19213815/
CrossRef - Wu H, Wang X, Gao J, Liang S, Hao Y, Sun C, et al. Fingolimod (FTY720) attenuates social deficits, learning and memory impairments, neuronal loss and neuroinflammation in the rat model of autism. Life Sci. 2017;
CrossRef - Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, Buie TM, et al. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism. 2016 Dec;7(1):49.
CrossRef - Sorby-Adams AJ, Marcoionni AM, Dempsey ER, Woenig JA, Turner RJ. The role of neurogenic inflammation in blood-brain barrier disruption and development of cerebral oedema following acute central nervous system (CNS) injury. International Journal of Molecular Sciences. 2017.
CrossRef - Dong B, Yu Y, Xie K, Su L, Yang Y, Zhang Z. Hemopexin alleviates cognitive dysfunction after focal cerebral ischemia-reperfusion injury in rats. BMC Anesthesiol. 2019;
CrossRef - Lochhead JJ, McCaffrey G, Quigley CE, Finch J, Demarco KM, Nametz N, et al. Oxidative stress increases blood-brain barrier permeability and induces alterations in occludin during hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2010;
CrossRef - Rakkar K, Bayraktutan U. Increases in intracellular calcium perturb blood-brain barrier via protein kinase C-alpha and apoptosis. Biochim Biophys Acta – Mol Basis Dis. 2016;
CrossRef