Manuscript accepted on :20-01-2022
Published online on: 28-01-2022
Plagiarism Check: Yes
Reviewed by: Dr. V. Sivamurugan
Second Review by: Dr. Amarendranath Choudhury
Final Approval by: Dr. Pallav Sengupta
Ni Wayan Bogoriani1* , Komang Ariati1
, Komang Ariati1 and I Gusti Ayu Putu Eka Pratiwi2
and I Gusti Ayu Putu Eka Pratiwi2
1Department of Chemistry, Faculty of Mathemathic and Natural Science, Udayana University, Badung, Bali, Indonesia, 80361
2Doctor Education Study Program, Medical Faculty, Udayana University, Indonesia Bali, 80232,
Corresponding Author E-mail: bogi_wayan@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2372
Abstract
Kecombrang is a plant that contains many antioxidants such as polyphenols, tannins, saponins, flavonoids and steroids. The purpose of this study was to observe the potential of the ethanolic extract of kecombrang flowers and stems as an antioxidant on the activity of superoxide dismutase (SOD),glutathione (GSH)and fatty liver of obese wistar rats. This study used 24 rats divided into 4 treatment groups, namely the normal group (standard diet), group 1 (high fat diet), group 2 (high fat diet + 100 mg / kg bw kecombrang flowers extract) and group 3 (high fat diet + 100 mg / kg bw kecombrang stems extract). The treatment duration was 30 days and on the last treatment day, the rats were fasted for 14 hours and then their blood was taken and dissected for measurement of SOD, and GSH activity. Liver was taken for fatty liver analysis. The results showed that the extracts intake of kecombrang flowers and stems gave increase SOD and GSH concentrations and decrease fatty liver with significant differences (p <0.05).It can be concluded that the intake of kecombrang flower and stem extracts have the potential as an antioxidant against SOD, GSH activity, and reduce fatty liver.
Keywords
Antioxidants; Fatty liver; GSH; Kecombrang; SOD
Download this article as:| Copy the following to cite this article: Bogoriani N. W, Ariati K, Pratiwi I. G. A. P. E. Potency of Balinese Kecombrang (Etlingeraelatior) Extract As Antioxidant Against The Activity of Superoxide Dismutase (SOD), Glutathione (GSH) And Fatty liver in Obese rats. Biomed Pharmacol J 2022;15(1) |
| Copy the following to cite this URL: Bogoriani N. W, Ariati K, Pratiwi I. G. A. P. E. Potency of Balinese Kecombrang (Etlingeraelatior) Extract As Antioxidant Against The Activity of Superoxide Dismutase (SOD), Glutathione (GSH) And Fatty liver in Obese rats. Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3o5UOfW |
Introduction
Obesity is condition of being overweight due to the amount of fat in the body that is stored in adipose tissue. The cause of obesity is excessive intake of nutrients compared to body’s needs so that excess intake will be stored as energy reserves in the form of fat which in the long term will make more fat accumulative in the body.1,2
In obesity there is an increase in adipose mass in the body. The increase in adipose mass in obesity causes changes in adipokines which will also cause a decrease in insulin sensitivity. Adipokines are specific structural proteins that are secreted by adipose into the circulation.1,3,4
Decreased insulin sensitivity will increase triglyceride lipolysis in adipose tissue. This leads to excessive production of free fatty acids in obesity which in turn will increase the production of small dance LDL which is easily oxidized. LDL oxidation will produce Reactive Oxygen Species (ROS), so that in hyperlipidemic conditions excessive ROS are formed due to the amount of oxidized LDL.1–3 ROS are highly reactive and capable of oxidizing surrounding molecules. ROS include superoxide radicals (O2-), hydrogen peroxide (H2O2), hydroxyl radicals (OH–) and various other compounds. ROS and free radicals will always be fought by the body’s defense system known as antioxidants.3,5
Antioxidants are able to slow down, delay and prevent damage to cellular components due to free radicals. Antioxidants consist of endogenous and exogenous antioxidants. Endogenous antioxidants are the main defense system in the body including superoxide (GSH), and glutathione peroxide (GPX). SOD enzyme will convert O2– into H2O2 will be catalyzed by catalase and gluthione peroxide enzymes and together with other enzymes. Catalase and other antioxidant enzymes will work together to neutralize ROS that are formed in normal amounts so that the balance between free radicals and antioxidants is maintained, but in obesity there is an increase in ROS which causes the balance of oxidation-reduction (redox) reactions to disturbed, resulting in a decrease in enzyme activity both SOD and GSH in the body which is called oxidative stress. High oxidative stress can be indicated by low cellular antioxidant status. Decrease endogenous enzyme activity and increased production of ROS that occur in obese patients will be the beginning of the development of various degenerative diseases.6–8
The occurrence of oxidative stress due to excess Reactive Oxygen Species (ROS) means that these endogenous antioxidants must receive additional exogenous antioxidants from food and beverage intake that is consumed every day. Antioxidants have a very important role for the health of the human body, because they function to inhibit and neutralize oxidation reactions that involve free radicals. Antioxidants in food and drinks can be natural and synthetic antioxidants.5,6 Amarowiczet al. (2000) reported that the use of synthetic antioxidants for a long time can cause side effects in the form of inflammation to liver damage and increase the risk of carcinogenesis in experimental animals.7 Therefore it is necessary to consume natural antioxidants which are found in fruits, vegetables, flowers and other parts of plants that contain vitamins A, C, E, folic acid, carotenoids, anthocyanins, phenolic compounds, flavonoids, saponins and tannins to prevent obesity so that body fitness is maintained. 8–12
Kecombrang (Etlingera elatior) is one of the local plants that is a typical Balinese food which contains a lot of antioxidant compounds, namely vitamin C, flavonoids, phenol groups, steroids and essential oils. Antioxidant compounds can increase enzyme activity of superoxide dismutase (SOD), glutathione (GSH), catalase (CAT) and reduce oxidative stress (MDA).13,14
Based on the above background, the researcher intends to observe the potency of kecombrang extract on SOD and GSH activity and fatty liver in obese wistar rats.
Materials and Methods
Materials
The samples used were kecombrang flowers and stems (Etlingera elatior) obtained in Sukawati, Gianyar, Bali, Indonesia.
Lard
Duck egg yolk
Female wistar rats
Superoksida Dismutase (SOD)Assay kit (Elabscience, E-BC-K020)
Glutathione (GSH) Elisa Kit (Elabscience, E-EL-0026)
Collection and determination of plants
Kecombrang flowers and stems were collected from the Sukawati area, Gianyar, Bali, in February 2020, determined by the Head ofthe Plant Conservation Center of the Botanical Garden ’Eka Karya’ Bali-LIPI. The flowers and stems obtained were thencleaned with running water, and cut into small pieces then dried at room temperature in an open room to amoisture content of ± 8%, then dried flowers and stems were ground in a blender flask and filtered to 100 mesh fineness.Powder material was extracted by maceration using ethanol solvent 12.
Kecombrang flowers and stems extraction
Five hundred grams of powder from the flowers and stems of kecombrang were put in a 4.5liter beaker separately then extracted with 3 liters of ethanol solvent for 24 hours at room temperature. Maseration can be repeated five times. The filtrate collected, combined and evaporated.15 This Kecombrang flower and stem extracts were used for phytochemical test, and the invivo assay.
Phytochemical Test
The phytochemical tests were carried out qualitatively according to Bogoriani et al.(2021).12
High Fat Composition
A high fat diet (HFD) composition was done by mixing 60% standard diets/CP 550 and 20% lard and 20% duck egg yolks. Diet is provided in pellet form and given for 60 days.11
Animals of Experiment
White wistar rats as a research protocol were taken from the laboratory of the Center for Study of Animal Diseases (CSAD) of Veterinary Medicine Faculty of Udayana University (No : 26/UN14.2.9/PT.01.04/2020). Twenty-four female wistar rats, 11-12 weeks old and 100-150 g of weight were divided into 4 groups, one control group (6 normal rats with standard diet Cp 550), 3 treatment groups (18 rats were obese to the Lee obesity index > 0.3) by a calculation of Bogorianiet al., 2020 with a high-fat diet for 8 weeks.10 After the rats became obese and then divided into 3 groups. The treatment group I was rats fed only high_ fat, the treatment group II was rats fed high_ fat and added kecombrang flowers extract 100 mg/kg bw/day, the treatment III is equal to the treatment group I, and added kecombrang stems extract with dose 100 mg/kgbw/day, each group of 6 rats. Rats from each group were individually caged at room temperature, with a bright cycle: dark 12:12 hours. Rats were treated for 30 days and free to drink ad libitum. After 30 days of treatment, the rats were fasted by withdrawing all food and drink for 14 hours. All drugs given to rats through oral once daily. At the end of the study the rats were anesthetized with Chloroform. Blood was taken through the orbital sinus, collected in a blood tube and centrifuged at 5000 g for 15 minutes at 4 ° C to obtain serum and then frozen until analysis. After the blood collection, the rats were dissected and parameters were measured: SOD, GSH and fatty liver by histopathology.
Design of Experiment
After acclimatization to the laboratory conditions, rats of experiment were randomly divided in to four groups (6 rats each) placed in individual cages and classified as follow:
Group I ( the group of normal control): rats fed standard diet.
Group II (the group of obesity induced) : rats fed high-fat diet (HFD)
Group III (the group of obesity induced + Kecombrang flowers extract 100 mg/kg bw/day)
Group IV (the group of obesity induced + Kecombrang stems extract 100 mg/kg bw/day)
Biochemical analysis
SOD and GSH activity test
Determination of serum SOD activity using the WST-1 method of the Superoxide Dismutase (SOD) Assay kit (Elabscience, E-BC-K020). Observe the serum samples, certrifuge for 10 min at 2000 g if it’s muddy. Collect the supernatant and carry out the assay immediately. The supernatant is diluted into difference concentration with normal saline, then take the pre-experiment.
Operation steps
Control well: add 20 μL of double distilled water and 20 μL of enzyme working solution. Blankcontrol well: add 20 μL of double distilled water and 20 μL of enzyme diluents. Sample well: add 20 μL of sample and 20 μL of enzyme working solution. Blanksample well: add 20 μL of enzyme diluents
Add μL of substrate application solution with a multi-channel pipettor into each well and mix fully.
Incubate at 37oC for 20 min. Measure OD values of each well with Microplate Reader.
Determination of serum GSH activity using GSH (Glutathione) ELISA Kit. Each well which already contains 50μL sample/standard is then added 50μL Biotinylated Detection and Incubate for 45 minutes at 37oC. Then aspirated and washed 3 times. 3. Each wall Added 100μL Horseradish Peroxidase (HRP) conjugated and incubated for 30 minutes at 37oC, then aspirated and washed 5 times. Add 90μL substrate reagent and incubated 15 minute at 37oC, add 50μL of stop solution to each well. Read at 450nm immediately and calculation of results.
Histopathological Observation
A portion of liver tissue of normal rats group, High-Fat Diet group, and the group of rats with kecombrang flower and stem extracts with a dose of 100 mg/kg/day each were stored in containers in15% formalin solution and subjected to histopathological study Observed microscopically for histopathological changes that is normal liver, HFD liver, and recovered liver was studied and compared.
Statistical Calculation
All the values were expressed as mean ± standard deviation . The data of results were analyzed by one-way ANOVA, and the difference among the treatments groups was determined with LSD. Values p <0.05 was used to consider to be significant.10
Results and Discussion
Kecombrang Flowers and Stems Ethanol Extract with Maceration Method
Maceration results from 500 grams of dry powder of flowers and stems using ethanol solvent obtained thick extracts of 85 grams and 80 grams, respectively. The yield of powder extract were 17% and 16%, respectively. The calculation of extraction yield according to the formula from Bogorianiet al., 2021.12
Phytochemical Test
The results of phytochemical screening on the extract of kecombrang flowers and stems are presented in Table 1
Table 1: Result of phytochemical test from methanol extract of kecombrang flower and stem
| No | Phytochemical compound | Test | Result | |
| Flower | Stem | |||
| 1 | Polyphenols | FeCl3 test | + | + |
| 2 | Flavonoids | Mg and HCl | + | + |
| 3 | Saponins | Foam test | + | + |
| 4 | Alkaloids | Mayer test | + | + |
| 5 | Steroids | Liebermann-burchard test | + | + |
| 6 | Tannins | FeCl3 test | + | + |
| 7 | Phytosterols | Liebermann-burchard test | + | + |
| 8 | Amino acids | Ninhydrin reagent | + | + |
+ = presence; – = absence
Table 1 shows that ethanol extract of kecombrang flowers and stems contained all of the tested metabolites such as polyphenols, flavonoids, saponins, alkaloids, steroids, tannins, phytosteroids and amino acids. All these metabolites have been reported to have activity as drugs such anti glycemia, antioxidant, anticancer, anti-lipidemia, anti obesity, anti microba and immunomodulatory activities. 12,16 Ethanol extracts of kecombrang flowers and stems were found to be quite effective solvent in extraction. Ethanol extracts ofkecombrang flowers and stems proved exhibited the positive reaction in all the assays. The results of phytochemical screening are almost the same as those conducted by Bogoriani et al. (2021)12.
Effect of Kecombrang flower and stem extracts administration on Lee Obesity index, SOD and GSH concentrations and Fatty liver in Obesity induced in female rats.
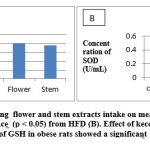
After 30 days of treatment, the blood of the rats was taken for analysis of the SOD and GSH concentrations and was dissected for histophatological determination. The concentrations of SOD and GSH can be seen in Table 2.
Table 2 shows that the extracts of kecombrang flowers and stems have an effect on increasing serum SOD and GSH concentrations with a significant difference (p <0.05)when compared to the high-fat diet group. The effect of kecombrang flowers extract on GSH concentration was significantly different (p <0.05), while the stems extract was not significantly different (p> 0.05) than the normal control group. The effect of 100 mg / kg bw of kecombrang flower and stem extracts intake on SOD activity of obesity-induced rats were significantly different (p < 0.05) compared to the normal control group. An increase in SOD and GSH concentrations and reduce fatty liver because kecombrang flower and stem extracts contain antioxidant compounds such as tannins, flavonoids, vitamin C and phenolic compounds. Maimulyanti and Prihadi, 2015 reported that flowers and stems contain tannin compounds, flavonoids, saponins and steroids. Micronutrients contained in plants such as vitamins A, C, E, folic acid, carotenoids, anthocyanins, and polyphenols have the ability to scavenge free radicals so that they can be used as a substitute for consumption of synthetic antioxidant. 4,16–18
Table 2: The effect of kecombrang extracts intake on mean Lee Obesity Index, concentration of SOD and GSH and Fatty liver in obesity-induced female rats
| Parameter | Control | HFD | Flower | Stem |
| Lee Obesity Index | 0.290±0.006 | 0.355±0.003 | 0.216±0.008a,b, | 0.231±0.016a,b |
| GSH (μg/mL) | 37.82 ± 0.03 | 13.64 ±0.17 | 39.78 ± 0.14a,b | 37.76 ± 0.17b |
| SOD (U/mL)
|
0.49± 0.01 | 0.14 ±0.01 | 0.43 ±0.02a,b | 0.41 ± 0.51a,b |
| Fatty liver (cells in five points of view) | 0.00 ±0.00 | 68 ± 0.00 | 4.33 ± 0.51a,b | 20 ± 0.00a,b |
Results are expressed in Mean ± SD. SD = standard deviation; a exhibits a significant difference from normal group ( p < 0.05); b exhibits a significant difference from High -Fat Diet (HFD) (p < 0.05)
Obese rats with a high-fat diet showed a significant reduction in SOD and GSH concentrations and there was an increase Lee obesity index and fatty liver (p <0.05). This is because of obesity is one of the metabolic syndromes which is a risk factor for cardiovascular disease and is the main cause of morbidity and mortality in the world. 2,18 Noronha et al., 2001 reported that Sindrum metabolic (SM) is affected by high levels of oxidative stress, which gradually develops into a disease vascular.19 Volpato et al. 2004 added that the risk of SM is higher in women than in men.20
In fact, under SM conditions, there is an increase in glucose delivery to the adipose tissue. Endothelial cells in adipose tissue stimulate an increase in glucose uptake via glucose transporter, so that it increases the activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and the production of mitochondrial reactive oxygen compounds. An increase in reactive oxygen species (ROS) causes oxidative stress and activates inflammatory signals, so that the activated endothelium attracts proinflammatory macrophages. 3 Weisberg et al. (2003) reported that macrophages infiltrating the adipose tissue of obese people are the main source of inflammatory cytokines. Since then macrophages have been known to produce ROS, because macrophages infiltrated into adipose also contribute to increasing NADPH oxidase activity and ROS production in these tissues. 21 Oxidative stress is defined as an imbalance in ROS production and endogenous antioxidant status. This condition is a comorbidity of obesity, diabetes mellitus, and impaired kidney function. The low activity of SOD proves the high level of oxidative stress in the body, so it is unable to eliminate the amount of oxidants (free radical) 1,3,22
High oxidative stress is also associated with the condition of obese sufferers. In the condition of obesity, the wider adipose tissue can lead to hypoxic conditions (lack of O2). Debevecet al. 2017 explained that chronic hypoxia increases oxidative stress by producing excessive ROS without compensating for antioxidant enzyme activity. Several studies have shown that during hypoxia, the production of ROS increases so that it suppresses the action of the SOD enzyme. 23 This occurs because hypoxia is a partial inhibition of the activity of the electron transport chain due to the leakage of electrons from complex I, resulting in the formation of ROS. Yuan et al. (2008) added that during the hypoxia / reoxygenation cycle, ROS is formed enzymatically through the xanthine oxidase pathway. On the other hand, ROS is also formed during ischemia / reperfusion, therefore the level of oxidative stress in obese patients is higher which results in lower activity of the SOD and GSH enzymes. 24
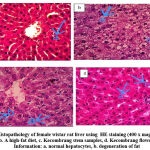
Liver Histopathology
The results of the study of liver histopathology are shown in Fig. 2 and Table 2. Intracellular fat examination was carried out in five fields of view with Hematoxylin Eosin (HE) staining using 400 x magnification can be seen in each image. It shows that there is a decrease fatty liver in the group treated with 100 mg / kg bw flower and stem extracts with a significant difference (p< 0.05) compared to the a high-fat diet group. Obese rats show an increase in fatty liver. Characteristics of fatty liver is characterized by the accumulation of fat in liver cells which is almost equal to 5% of hepatocytes.25 An increase in fat in the liver of rats with a high-fat diet triggers liver cell damage.26 Fatty liver is often associated with a high-fat diet, obesity, and insulin resistance which will lead to impaired lipolysis in the periphery, thus increasing fat uptake to the liver. One of the causes of fatty liver is the high consumption of food sources of fat (atherogenic) so that it accumulates free fatty acids in the liver which are then esterified into triglycerides. The pathophysiology of fatty liver is affected by an imbalance between the synthesis and β-oxidation of triglycerides from fatty acids in the liver due to the accumulation of free fatty acids in the blood circulation of a high-fat diet.25
Conclusion
The results of this study concluded that the extract of kecombrang flowers and stems have activity as an antioxidant in vivo so that it has the potential to increase body immunity.
Acknowledgment
This research was supported by PNBP funds from Udayana University. We also thank students and all research members and PUIPAR who have helped a lot.
Conflict of Interest
There are no conflict of interest.
Funding Sources
There are no funding source.
References
- Tripathi S, Srivastava S, Tripathi Y. Obesity and Its complication: Role of autophagy. J Pharm Sci Res. 2018;9(8):3100-3113. doi:10.13040/IJPSR.0975-8232.9(8).3100-13
- Sasidharan S., Joseph J., Anandakumar S, et al. Ameliorative Potential of Tamarindus indica on High Fat Diet Induced Nonalcoholic Fatty Liver Disease in Rats. Sci World J. 2014;2014:1-10.
CrossRef - Sanchez A., Santillan E., Bautista M, et al. Inflammation , Oxidative Stress , and Obesity. 2011;6:3117-3132. doi:10.3390/ijms12053117
CrossRef - Hossain M., Dayem A., Han J, et al. Molecular Mechanisms of the Anti-Obesity and Anti-Diabetic Properties of Flavonoids. Int J Mol Sci. 2016;17(569):1-32. doi:10.3390/ijms17040569
CrossRef - Adly AA. Oxidative stress and disease: An updated review. 2010;3(2):129-145. https://scialert.net/fulltext/?doi=rji.2010.129.145&org=11.
CrossRef - Muhson I, Mashkor A. Evaluation of antioxidant activity of clove (Syzygium aromaticum). Int J Sci. 2015;13(1):23-30. https://www.tsijournals.com/articles/evaluation-of-antioxidant-activity-of-clove-syzygium-aromaticum.pdf.
- Amarowicz R, Naczk M, Shahidi F. Antioxidant Activity of Crude Tannins of Canola and Rapeseed Hulls. J Am Oil Chem Soc. 2000;77(9):957-961. doi:10.1007/s11746-000-0151-0
CrossRef - Askin H, Yilmaz B, Gulcin İ, et al. Antioxidant Activity of the Aqueous Extract of Iris taochia and Identification of its Chemical Constituents. Indian J Pharm Sci. 2018;80(5):802-812. doi:10.4172/parmaceutical-sciences. 1000425
CrossRef - Bogoriani NW, Laksmiwati AAIM, Putra AA., Heltyani WE, Lestari KDP, Mahayani PA. Saponins Role of Bali Andong Leaf as Antiobesity In Rats. Int J Pharm Res. 2019;11(2):382-389. https://erepo.unud.ac.id/id/eprint/27843.
CrossRef - Poorassar A, Reza M, Ardekani S, Hajhashemi V. Antiobesity effects of seedlac and shellac in rats fed with a high-fat diet. Res Pharm Sci. 2020;15(1):57-65. doi:10.4103/1735-5362. 278715
CrossRef - Bogoriani N., Suaniti N., Bawa AA., Pradnya Lestari K., Heltyani W. The Effect of Cordyline terminalis ’ s Leaf Extract on Lipid Profile , Obesity and Liver Function in Obese Induced Rats. Sys Rev Pharm. 2020;11(11):1080-1086. https://www.sysrevpharm.org/articles/ the-effect-of-cordyline-terminaliss-leaf-extract-on-lipid-profile-obesity-and-liver-function-in-obese-induced-rats.pdf.
- Bogoriani NW, Putra AAB, Wahjuni S, Heltyani WE, Dewi NPPMS, Sadin VY. The effect of Andong ( Cordyline terminalis ) leave , one of the traditional plants in Bali as antioxidant and antibacterial The effect of Andong ( Cordyline terminalis ) leave , one of the traditional plants in Bali as antioxidant and antibacterial. IOP Conf J. 2021;724:1-14. doi:10.1088/1755-1315/724/1/012018
CrossRef - Singh RS., Negi P., Radha C. Phenolic composition , antioxidant and antimicrobial activities of free and bound phenolic extracts of Moringa oleifera seed flour. J Funct Foods. 2013;5(4):1883-1891. doi:10.1016/j.jff.2013.09.009
CrossRef - Badariah C, Aziz A, Qusyasyiah S, et al. Effects of Tualang honey in modulating nociceptive responses at the spinal cord in offspring of prenatally stressed rats. J Integr Med. 2018;17(1):66-70. doi:10.1016/j.joim.2018.12.002
CrossRef - Bogoriani N., Ariati N. The Activity of Bali Andong Rhizome Extract of Cordyline Terminalis Kunth as Hypolipidemia Agent in Wistar Rats with High-Cholesterol Diet. Intern J Pharm Phytopharm Res. 2018;8(1):75-80. https://eijppr.com/en/article/the-activity-of-bali-andong-rhizome-extract-of-cordyline-terminalis-kunth-as-hypolipidemia-agent-in-wistar-rats-with-high-cholesterol-diet.
- Ozcan T, Akpinar-Bayizit L, Yilmaz-Ersan, B D. Phenolics in Human Health. Int J Chem Eng Appl. 2014;5(5):393-396. doi:10.7763/IJCEA.2014.V5.416
CrossRef - Maimulyanti A, Prihadi A. Chemical composition , phytochemical and antioxidant activity from extract of Etlingera elatior flower from Indonesia. J Pharmacogn Phytochem.2015;3(6):233-238.https://www.phytojournal.com/archives/2015/vol3issue6/PartE/4-1-34.1.pdf.
- Gill M., Tomas-Barberan F., Hess-Pierce B, Kader A. Vitamin C Contents of Nectarine , Peach , and Plum Cultivars. J Agric Food Chem. 2002;50(17):4976-4982. doi:10.1021/jf020136b
CrossRef - Noronha B., Li J, Wheatcroft S., Shah A., Kearney M. on Vascular and Metabolic Function in Obesity. Diabetes. 2005;54(April):1-8. doi:10.2337/diabetes.54.4.1082
CrossRef - Volpato S, Guralnik J., Ferrucci L, et al. Cardiovascular disease, interleukin-6, and risk of mortality in older women: the women’s health and aging study. Circulation. 2001;103(7):947-954. doi:10.1161/01.cir.103.7.947
CrossRef - Weisberg S., Mccann D, Desai M, Rosenbaum M, Leibel R., Ferrante A. Obesity is associated with macrophage accumulation. J Clin Invest. 2003;112(12):1785-1808. doi:10.1172/JCI200319246.Introduction
CrossRef - Valko M, Izakovic M, Mazur M, Christopher J, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266(1-2):37-56. doi:10.1023/b:mcbi.0000049134.69131.89
CrossRef - Debevec T, Millet G., Pialoux V. Hypoxia-Induced Oxidative Stress Modulation with Physical Activity. Front Physiol. 2017;8(February):1-9. doi:10.3389/fphys.2017.00084
CrossRef - Yuan G, Nanduri J, Khan S, Semenza G. Induction of HIF-1 a Expression by Intermittent Hypoxia : Involvement of NADPH Oxidase , Ca 2 R Signaling , Prolyl Hydroxylases , and mTOR. J Cell Physiol. 2008;217(3):674-685. doi:10.1002/jcp.21537
CrossRef - Fabbrini E, Sullivan S, Klein S. REVIEWS Obesity and Nonalcoholic Fatty Liver Disease: Biochemical, Metabolic, and Clinical Implications. Hepatology. 2010;51(2):679-689. doi:10.1002/hep.23280
CrossRef - Reddy S., Aveti S, Anjum M, Raju G. Anti-hyperlipidemic Activity of Methanolic Extract of Syzygium Alternifolium Bark Against High-Fat Diet and Dexamethasone-Induced Hyperlipidemia in Rats. Asian J Clin Pharm res. 2015;8(6):165-168. https://innovareacademics.in/journals/index.php/ajpcr/article/view/8107.