Manuscript accepted on :12-01-2022
Published online on: 25-01-2022
Plagiarism Check: Yes
Reviewed by: Dr. Daya Shankar Gautam
Second Review by: Dr. Salman Ahmed
Final Approval by: Dr Patorn Piromchai
Prasad Konduri1 , Eswar Kumar Kilari2
, Eswar Kumar Kilari2 and Ravindra Babu Sajja1, 3*
and Ravindra Babu Sajja1, 3*
1Department of Pharmacology, Shri Vishnu College of Pharmacy, Bhimavaram
2Department of Pharmacology , College of Pharmaceutical Sciences, Andhra University, Visakhapatnam.
3Department of Pharmacology, Malla Reddy Institute of Pharmaceutical Sciences, Secunderabad.
Corresponding Author E-mail: ravicology@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2387
Abstract
Objective: The objective of the present work is to evaluate the petroleum ether, ethyl acetate and methanolic extracts of vanda spathulata on experimental models for in vitro antioxidant activity and mast cell stabilizing activity. Methods: Mast cell stabilization effect was assessed using compound 48/80 induced mast cell degranulation in rat peritoneal mast cell. The antioxidant activity was evaluated using 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay. Results and Discussion: Vanda spathulata methanolic extract (VSME) at doses of 200 and 400mg/kg exhibited significant protection (p<0.01) and petroleum ether (VSPE) and ethylacetate (VSEA) extracts at the dose of 400mg/kg showed significant protection (p<0.05) against mast cell degranulation. VSME revealed better DPPH radical scavenging activity (IC50 value 38.39 µg/ml) which was closely resembled to standard ascorbic acid (IC50 value 33.98 µg/ml) when compared to other extracts. Phytochemical study revealed the presence of alkaloids, saponins, flavonoids, tannins, steroids and glycosides. Conclusion: From the results Vanda spathulata shows mast cell stabilizing activity mainly due to phytochemical constituents and strong antioxidant property of plant extracts.
Keywords
Antioxidant; Comp 48/80; DPPH; Flavonoids; Mast cell degranulation; Vanda spathulata
Download this article as:| Copy the following to cite this article: Konduri P, Kilari E. K, Sajja R. B. In Vitro Antioxidant and Mast Cell Stabilizing Activity of Different Extracts of Vanda Spathulata Flowers. Biomed Pharmacol J 2022;15(1) |
| Copy the following to cite this URL: Konduri P, Kilari E. K, Sajja R. B. In Vitro Antioxidant and Mast Cell Stabilizing Activity of Different Extracts of Vanda Spathulata Flowers. Biomed Pharmacol J 2022;15(1) Available from: https://bit.ly/3nWsexw |
Introduction
Herbs used as medicinal purposes for many centuries. Now a days, herbal medicines are used globally as home remedies in treatment of various ailments. People rely mainly on herbal drugs to meet their primary health care needs in some developing countries. Herbal medicines are also gaining popularity as alternative and complementary therapies in many industrialized and developed countries. There has been an increase in scientific studies on herbal medicines, due to insufficient availability of reliable data still today Mast cells are found in mucosal and epithelial tissues throughout the body. In rodents, mast cells also reside in peritoneal and thoracic cavities1.Activated mast cells are an important source of histamine, cysteinyl leukotrienes, and prostaglandins. These mediators are central to bronchoconstriction, vasodilation, and the allergic inflammatory cascade2. ROS have been shown to be associated with the pathogenesis of asthma by evoking bronchial hyper-reactivity as well as directly stimulating histamine release from mast cells and mucus secretion from airway epithelial cells3.
Vanda spathulata is an epiphytic orchid species belongs to a family Orchidaceae with golden yellow flowers. It is found in southern india and srilanka. It favors to grow on small trees and bushes in open sun4. Flowering and fruiting occur between September and January5. It is also used in folk and ayurvedic medicine, dried flowers are powdered and used for a variety of illnesses such as asthma, depression and manic troubles. The leaf juice of plant used for temper the bile and frenzy abate. It is thought it may have some memory enhancing properties and its antioxidant activity has been investigated6. It is also used as liver tonic.
In spite of the traditional indications, marked free radical scavenging capacity, the mast cell stabilization potential of Vanda spathulata has not been investigated. In the present study, we have investigated different extracts of Vanda spathulata on DPPH free radical scavenging activity and compound 48/80-induced induced mast cell degranulation in rat peritoneal mast cells.
Materials and Methods
Plant Material
The flowers of Vanda spathulata was collected from the forest of tirumala region, Andhra pradesh, India. The plants have been taxonomically identified and authenticated by Dr. K.Madhava Chetty, Department of Botany, in S.V.University, Tirupathi, Andhra Pradesh, India. A voucher specimen No:2194 has been deposited in the herbarium of Malla Reddy Institute of Pharmaceutical Sciences, Hyderabad, India, for future reference.
Preparation of Extracts
Flowers of Vanda spathulata were washed thoroughly and cleaned with tap water, air dried and coarsely powdered. Powdered plant material (500g) was extracted successively with petroleum ether, ethyl acetate and methanol in a soxhlet extractor till the solvent in siphon tube of an extractor become colorless. The so obtained extract was kept in a desicator to remove moisture and stored in an amber-colored bottle at 40C. The extracts are abbreviated as VSPE, VSEA and VSME for petroleum ether, ethyl acetate and methanolic extract of Vanda spathulata, respectively. The yields of these extracts were found to be 5.7%, 11.1%, and 15.5% respectively.
Experimental animals
Adult male wistar albino rats (150–200 g) were procured from Sanzyme pvt Ltd, Hyderabad. All the Animals were maintained room temperature at 22 ± 1°C, relative humidity of 55 ± 5%, 12-hr light and dark cycle, and allowed free access to food (standard pellet diet, certified VRK laboratory animal feed, Sangli, Maharashtra, India.) and water ad libitum. All the experimental procedures and protocols were approved by the Institutional Animal Ethics Committee (Approval No:11/MRIPS/CPCSEA-IAEC-II/Hyd/2016) of Malla Reddy Institute of Pharmaceutical Sciences, Secunderabad (Reg. No: 1662/PO/Re/S/12/CPCSEA).
Preliminary Phytochemical Screening7-9
The preliminary phytochemical analysis of various extracts of vanda spathulata was performed for Alkaloids, Saponins, Tannins, Steroids and triterpenoids Flavonoids, Phenols, Glycosides, Carbohydrates, Proteins, Fixed oils & Fats, Gums & Mucilages according to published standard methods.
Acute toxicity study10
Healthy adult swiss albino mice weighing about 20-30 g were used in this study. Acute toxicity test was performed according to OECD guideline 423.
Compound 48/80 induced mast cell degranulation
This method was performed on healthy adult albino rats. Different extracts(pet ether, ethyl acetate and methanol) of Vanda spathulata at doses of 100, 200 and 400mg/kg, p.o and the standard drug, disodium cromoglycate (10 mg/kg, i.p) was administered to different groups of animals each consisting of 6 animals for 4 days. On the 5thday, 2 h after the last treatment, 10 ml/kg of normal saline was injected into the peritoneal cavity of rats. After gentle abdominal massage for 90s, the peritoneal fluid containing mast cells was collected and transferred into the eppendorf test tubes containing 7-10ml of RPMI-1640 media (pH 7.2–7.4). Then the mast cells were washed three times with RPMI-1640 media by centrifugation at low speed (500–600 rpm), discarding the supernatant and re suspending the pellets of mast cells in the medium. Mast cells from the treated and control groups were incubated with 0.1ml of compound 48/80 (10µg/ml) at 37 ◦C for 10 min in a water bath. After incubation, mast cells were stained with 1% toluidine blue solution and percent of protection against degranulation was counted under high power microscope (45X)11. Percentage protection of the mast cells in the control group and the treated groups were calculated by counting the number of degranulated mast cells from total of at least 100 mast cells counted. Percent inhibition of mast cell degranulation for each treatment was calculated by following formula:
In vitro antioxidant activity
Determination of 1, 1- Diphenyl-2-Picrylhydrazyl (DPPH) Radical scavenging Activity
The scavenging activity for DPPH free radicals was measured according to the procedure described by Braca et al., 200312. An aliquot of 3ml of 0.004% DPPH solution in methanol and 0.1 ml of plant extract at various concentrations (5-125µg/ml) were mixed. The mixture was shaken vigorously and allowed to reach a steady state at room temperature for 30 min. Decolorization of DPPH was determined by measuring the absorbance at 517 nm. A control was prepared using 0.1 ml of respective vehicle in the place of plant extract/ascorbic acid. The percentage inhibition activity was calculated as [(A0-A1)/A0] ×100, where A0 was the absorbance of the control, and A1 was the absorbance of the plant extract/ ascorbic acid. IC50 values denote the concentration of sample required to scavenge 50% DPPH free radicals. The IC50 values for different extracts were determined graphically from the graph with percentage inhibition plotted on y-axis and concentration on x-axis.
Statistical analysis
The results obtained were expressed as mean ± SEM. Statistical analysis was performed using a one-way analysis of variance (ANOVA). Data was considered statistical significant at p <0.05. When data was found to be very (p<0.01) or highly (p< 0.001) significant, this was indicated in the results. All statistical analyses were performed using Graph Pad prism 8 software (San Diego, CA).
Results
Preliminary Phytochemical Screening
The results obtained from the phytochemical tests are presented in Table 1. Preliminary phytochemical screening of different extract of Vanda spathulata showed the presence of alkaloids, flavonoids, glycosides, tannins, steroids & terpenoids and saponins.
Acute toxicity studies
Various extract of Vanda spathulata at a dose of 2000mg/kg administered orally did not showed any toxicity in tested animals. No signs of observable toxicity and no mortality was seen during the period of 14 days study. Hence there is no LD50 and tested plant extracts are considered safe and nontoxic. There by the therapeutic doses for the pharmacological evaluation was 1/20th, 1/10th and 1/5th of the maximum tolerated which was then fixed to be 100mg/kg, 200mg/kg and 400mg/kg p.o of the experimental animals.
Compound 48/80 induced mast cell degranulation
Effect of different extracts of Vanda spathulata on compound 48/80 induced rat peritoneal mast cell degranulation
The group administered with compound 48/80 showed (79.50 ±3.4%) degranulation of mast cells while groups treated with disodium cromoglycate (10mg/kg), a reference standard drug significantly protect (23.00±1.8%) degranulation of mast cells. The negative control group showed 3.50 ±0.43% mast cell degranulation.
VSME at doses (100, 200 and 400mg/kg) showed an inhibition of 18.86%, 44.95% and 68.15% of mast cell degranulation respectively. VSME at concentrations 200 and 400mg/kg showed significant (p<0.01) protection against compound 48/80 induced mast cell degranulation (Table. 1and Fig.1). VSEA at doses 200 and 400mg/kg showed significant protection (15.59%, 26.00%), but lower concentration (100mg/kg) did not show any protection (11.53%) against mast cell degranulation (Table.1 and Fig.1). VSPE showed significant protection (23.96%) at 400mg/kg concentration. However, it did not show any protection at lower concentrations (100 and 200mg/kg). Histopathological changes were shown in Fig.3.
In-vitro Antioxidant activity
In the present study, the antioxidant activity of different extracts of Vanda spathulata was evaluated by determining their DPPH radical scavenging abilities and results were presented as IC50 (μg/ml). The scavenging effect of Ascorbic acid, methanolic (VSME), ethyl acetate (VSEA) and petroleum ether (VSPE) extracts of Vanda spathulata showed concentration dependent scavenging activity on DPPH radicals. The results of scavenging activity on DPPH radicals were given in Table.2 and Fig.2. The lower the IC50 value indicated a higher antioxidant activity. The mean IC50 values for hydroxyl radical of VSME, VSEA and VSPE were found to be 38.39µg/ml, 61.47 µg/ml and 86.07µg/ml, respectively. The mean IC50 value of ascorbic acid was found to be 33.98µg/ml. IC50 value of VSME was closely resembled to that of standard ascorbic acid. The order of free radical scavenging activity of different extracts of Vanda spathulata against DPPH radical was Ascorbic acid > Methanolic extract> Ethyl acetate extract >Petroleum ether extract.
Table 1: Effect of different extracts of Vanda spathulata on compound 48/80 induced rat peritoneal mast cell degranulation.
| Treatment | Concentration(mg/kg) | % Mast cells degranulation ± SEM | % inhibition of degranulation |
| Negative control | Normal saline 1ml/kg | 3.50 ±0.43 | _ |
| Positive control (Compound 48/80) | 10μg/ml | 79.50 ±3.4# | _ |
| Disodium cromoglycate | 10mg/kg | 23.00±1.8** | 71.06 |
| VSPE | 100mg/kg | 73.54± 3.9ns | 7.49 |
| VSPE | 200mg/kg | 69.62±2.05ns | 12.42 |
| VSPE | 400mg/kg | 60.45± 2.34* | 23.96 |
| VSEA | 100mg/kg | 70.33 ± 3.1ns | 11.53 |
| VSEA | 200mg/kg | 67.10± 2.7* | 15.59 |
| VSEA | 400mg/kg | 58.83 ± 1.98** | 26.00 |
| VSME | 100mg/kg | 64.50 ±2.64* | 18.86 |
| VSME | 200mg/kg | 43.76±1.67** | 44.95 |
| VSME | 400mg/kg | 25.32±2.48 ** | 68.15 |
Values are expressed as mean ± SEM. n=6 rats/goup. Negative control: saline, Positive control: compound 48/80 (l0μg/ml), Standard: disodium cromoglycate (10mg/kg), VSPE: Vanda spathulata petroleum ether extract, VSEA: Vanda spathulata ethyl acetate extract, VSME: Vanda spathulata methanolic extract. #p<0.01: Significantly different from negative control. ** p< 0.01, *p< 0.05: Significantly different from positive control. ns: Not significant.
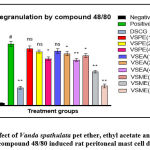 |
Figure 1: Effect of Vanda spathulata pet ether, ethyl acetate and methanolic extracts on compound 48/80 induced rat peritoneal mast cell degranulation. |
All bars represent the mean ± SEM (n=6). Negative control: saline, Positive control: compound 48/80 (l0μg/ml), DSCG (standard): disodium cromoglycate (10mg/kg), VSPE: Vanda spathulata petroleum ether extract, VSEA: Vanda spathulata ethyl acetate extract, VSME: Vanda spathulata methanolic extract. #p<0.01: Significantly different from negative control. ** p< 0.01, *p< 0.05: Significantly different from positive control. ns: Not significant.
In vitro antioxidant studies
Table 2: Effect of different extracts of flowers of Vanda spathulata on DPPH scavenging
| Concentration
(µg/ml) |
Percentage inhibition of DPPH radical | |||
| Ascorbic acid | VSME | VSEA | VSPE | |
| 5 | 21.67±0.67 | 17.16±0.13 | 11.2±0.81 | 6.79±0.14 |
| 10 | 33.71±0.58 | 38.12±0.67 | 16.33±0.79 | 13.43±0.269 |
| 25 | 54.89±0.88 | 47.81±1.09 | 35.48±0.82 | 25.62±0.321 |
| 50 | 64.97±0.68 | 62.30±0.54 | 47.67±0.67 | 34.88±0.11 |
| 75 | 78.95±0.33 | 76.28±1.45 | 53.85±0.76 | 48.60±0.31 |
| 100 | 88.66±0.56 | 85.56±0.88 | 76.33±0.93 | 53.31±0.54 |
| 125 | 92.40±0.92 | 89.23±0.58 | 85.10±0.43 | 67.30±0.25 |
| R2 Value | 0.909 | 0.906 | 0.969 | 0.974 |
| IC50 Value | 33.98 | 38.39 | 61.47 | 86.07 |
Results are expressed as mean ± SEM (n=3) of triplicate observations. IC50 Values were obtained from the figure and R2 Values are mentioned in the table. VSME: Vanda spathulata methanolic extract. VSEA: Vanda spathulata ethyl acetate extract, VSPE: Vanda spathulata petroleum ether extract.
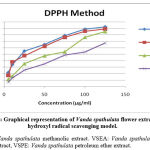 |
Figure 2: Graphical representation of Vanda spathulata flower extracts on hydroxyl radical scavenging model. |
 |
Figure 3: Histopathological studies of effect of various extracts of Vanda spathulata on mast cell degranulation. |
Microscopic photographs of rat peritoneal mast cells. (A) Normal control group shows normal mast cells histology, (B) Compound 48/80 (10µg/ml) treated group shows significant mast cell degranulation, (C) Disodium cromoglycate (10 mg/kg) treated group shows significant mast cell stabilization, (D) VSME (100mg/kg) treated group shows less mast cell stabilization, VSME(200mg/kg) treated group shows moderate mast cell stabilization, (F)VSME(400mg/kg) treated group shows significant mast cell stabilization, (G) VSEA (100mg/kg) treated group did not show mast cell stabilization, (H) VSEA(200mg/kg) treated group shows moderate mast cell stabilization, (I)VSEA(400mg/kg) treated group shows significant mast cell stabilization, (J) VSPE (100mg/kg) treated group did not show mast cell stabilization, (K) VSPE(200mg/kg) treated group shows less mast cell stabilization, (L)VSPE(400mg/kg) treated group shows significant mast cell stabilization.
Discussion
Mast cells are present virtually in all organs and are well known participants in allergic diseases13. Mast cell activation occurs when the FcεR-bound IgE is cross linked by binding to multivalent antigen. Signalling in this way stimulates release of both pre-formed mediators and the production of new inflammatory mediators. Their activation and subsequently degranulation can be elicited by not only the aggregation of cell surface-specific receptor for IgE, FcεRI, but also by the number of positively charged substances like compound 48/8014. Compound 48/80 increases intracellular calcium level and generates ROS endogenously which results in mast cell disruption to produce proinflammatory mediators15. Numerous reports established that stimulation with compound 48/80 or IgE initiates the activation of signal transduction pathway which leads to histamine release. Degranulated mast cells release number of mediators including histamine, a potent vasoactive mediator, which may precipitate hypersensitive reactions16. Compound 48/80 was used to induce mast cell degranulation and histamine release from RPMC. Decrease in histamine release from RPMC was taken as an index of mast cell stabilization
Phytochemical screening of Vanda spathulata showed the presence of saponins, flavonoids, tannins, steroides and glycosides. Saponins are reported to possess mast cell stabilizing, anti allergic and antihistaminic activities17-19. Tannins are reported to possess mast cell stabilizing, anti-allergic and anti-histaminic activities20. The flavonoids also inhibited the histamine release induced by compound 48/8021. Thus, the presence of flavonoids and Tannins in the plant extracts might be responsible for the mast cell stabilizing activity.
The extracts of Vanda spathulata showed the attenuation of compound 48/80 induced mast cell degranulation in dose dependent fashion. The methanolic extract of Vanda spathulata showed most significant mast cell stabilizing activity. It was found that methanolic extract of Vanda spathulata was potent inhibitor of DPPH free radical while petroleum ether extract was least active.
Conclusion
It may be concluded that vanda spathulata possess mast cell stabilization property mediated through their phytochemical constituents and antioxidant capacity. In addition, further studies are required to clear molecular mechanisms of these plant extracts to investigate for the successful development of the drug for clinical use.
Acknowledgment
The authors are thankful to the Shri Vishnu College of Pharmacy, Bhimavaram, AU College of Pharmaceutical Sciences, Vishakhapatnam and Malla reddy Institute of Pharmaceutical Sciences, Hyderabad for providing necessary facilities to carry out the work.
Conflict of interest
The authors declare that they have no conflict of interest.
Founding Sources
There is no funding Source.
References
- Da Silva EZ, Jamur MC, Oliver C. Mast cell function: a new vision of an old cell. J Histochem 2014; 62(10):698–738.
CrossRef - Pavithra H. Dave, Preetha. Pathogenesis and Novel Drug for Treatment of Asthma – A Review. Research J. Pharm. and Tech 2016; 9(9):1519-1523. doi: 10.5958/0974-360X.2016.00297.3
CrossRef - Krishna MT, Madden J, Teran LM, Biscione GL, Lau LC, Withers NJ, Sandstrom T, Mudway I, Kelly FJ, Walls A, Frew AJ, Holgate ST. Effects of 0.2 ppm ozone on biomarkers of inflammation in bronchoalveolar lavage fluid and bronchial mucosa of healthy subjects. Eur Respir J. 1998 Jun; 11(6):1294-1300. doi: 10.1183/09031936.98.11061294.
CrossRef - Kottaimuthu R, Ganesan R, Ganesan V, Sundaram VM. Enumeration of Orchids of Sirumalai Hills (Eastern Ghats), Tamil Nadu, India. Ethnobotanical Leaflets 2008; 12: 506-512.
- Decruse SW, Gangaprasad A, Seeni S. Menon VS. Micropropagation and ecorestoration of Vanda spathulata, an exquisite orchid. Plant Cell, Tissue and Organ Culture 2003; 72(2):199-202.
CrossRef - Rajaneekar Dasari D. Sathyavati D, Sampath Kumar B, Jayachandra Reddy P, Abbulu K. Evaluation of antioxidant activity of two important memory enhancing medicinal plants Celtis timorensis and Vanda spathulata. Asian Journal of Pharmaceutical and Clinical Research 2013; 6(2):153-155.
- Peach D, Tracey MV. Modem Methods of Plant Analysis. Berlin: Springer-Verlog; 1955. p.373-374
- Kokate CK, Purohit AP, Gokhale SB. In-Pharmacognosy, alkaloid, 30th ed. Pune: Nirali Prakashan; 1996. p. 447.
- Evans WC. Trease and Evans’ Pharmaconosy. 15th ed. London: WB Sounders Company Ltd; 2005.p. 224, 230, 336, 541.
- OECD guidelines for testing of chemicals. Acute Oral Toxicity-Acute Toxic Class Method. 2001; p 1-14.
- Gupta PP, Srimal RC, Srivastava M, Singh KL and Tandon JS. Antiallergic activity of Arbortristosides from Nyctanthus arbortristis. International Journal of Pharmacognosy. 1995; 33(1):70–72.
CrossRef - Braca A, Fico G, Morelli I, De Simone F, Tome F, De Tommasi N. Antioxidant and free radical scavenging activity of flavonol glycosides from different Aconitum species. J Ethnopharmacol. 2003; 86(1): 63-67. doi: 10.1016/s0378-8741(03)00043-6.
CrossRef - Singh B, Nadkarni JR, Vishwakarma RA, Bharate SB, Nivsarkar M, Anandjiwala S. The hydroalcoholic extract of Cassia alata (Linn.) leaves and its major compound rhein exhibits antiallergic activity via mast cell stabilization and lipoxygenase inhibition. Journal of Ethnopharmacology. 2012; 141(1):469-73. DOI: 10.1016/j.jep.2012.03.012
CrossRef - Lim KT. Inhibitory effect of glycoprotein isolated from Opuntia ficus-indica var. saboten MAKINO on activities of allergy-mediators in compound 48/80-stimulated mast cells. Cellular Immunology. 2010; 264:78-85.
CrossRef - Chandrashekhar VM, Halagali KS, Nidavani RB, Shalavadi MH, Biradar BS, Biswas D et al. Anti-allergic activity of German chamomile (Matricaria recutita L.) in mast cell mediated allergy model. Journal of Ethnopharmacology. 2011; 137:336-340.
CrossRef - Petersen LJ, Mosbech H, Skov PS. Allergen-induced histamine release in intact human skin in vivo assessed by skin microdialysis technique: characterization of factors influencing histamine releasability. Journal of Allergy and Clinical Immunology. 1996; 97(2):672-679.
CrossRef - John RK, Zutshi U, Kameshwaran L, Atal CK. Effect of quercetin and Albizzia saponins on rat mast cell. Indian J Physiol Pharmacol 1985 Jan-Mar; 29(1): 43-6.
- Gupta SS, Tripathi RM. Effect of chronic treatment of the saponin of Clerodendron serratum on disruption of the mesenteric mast cells of rats. Asp Allergy Appl Immunol 1973; 4: 177-88.
- Gupta SS. Development of antihistamine and antiallergic activity after prolonged administration of a plant saponin from Clerodendron serratum. J Pharm Pharmacology 1968; 20(10):801-802. DOI: 10.1111/j.2042-7158.1968.tb09644.x
CrossRef - Gupta SS. Development of antihistamine and antiallergic activity after prolonged administration of a plant saponin from Clerodendron serratum. J Pharm Pharmacology 1968; 20(10):801-802. DOI: 10.1111/j.2042-7158.1968.tb09644.x
CrossRef - Bellanti JA. Mechanism of tissue injury produced by immunologic reactions. In: Immunology. Asian ed. Tokyo: W.B Saunders Co; 1971. p. 184.







